A Longitudinal Study on Feeding Behaviour and Activity Patterns of Released Chimpanzees in Conkouati-Douli National Park, Republic of Congo
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Study Subjects
2.1. Study Site
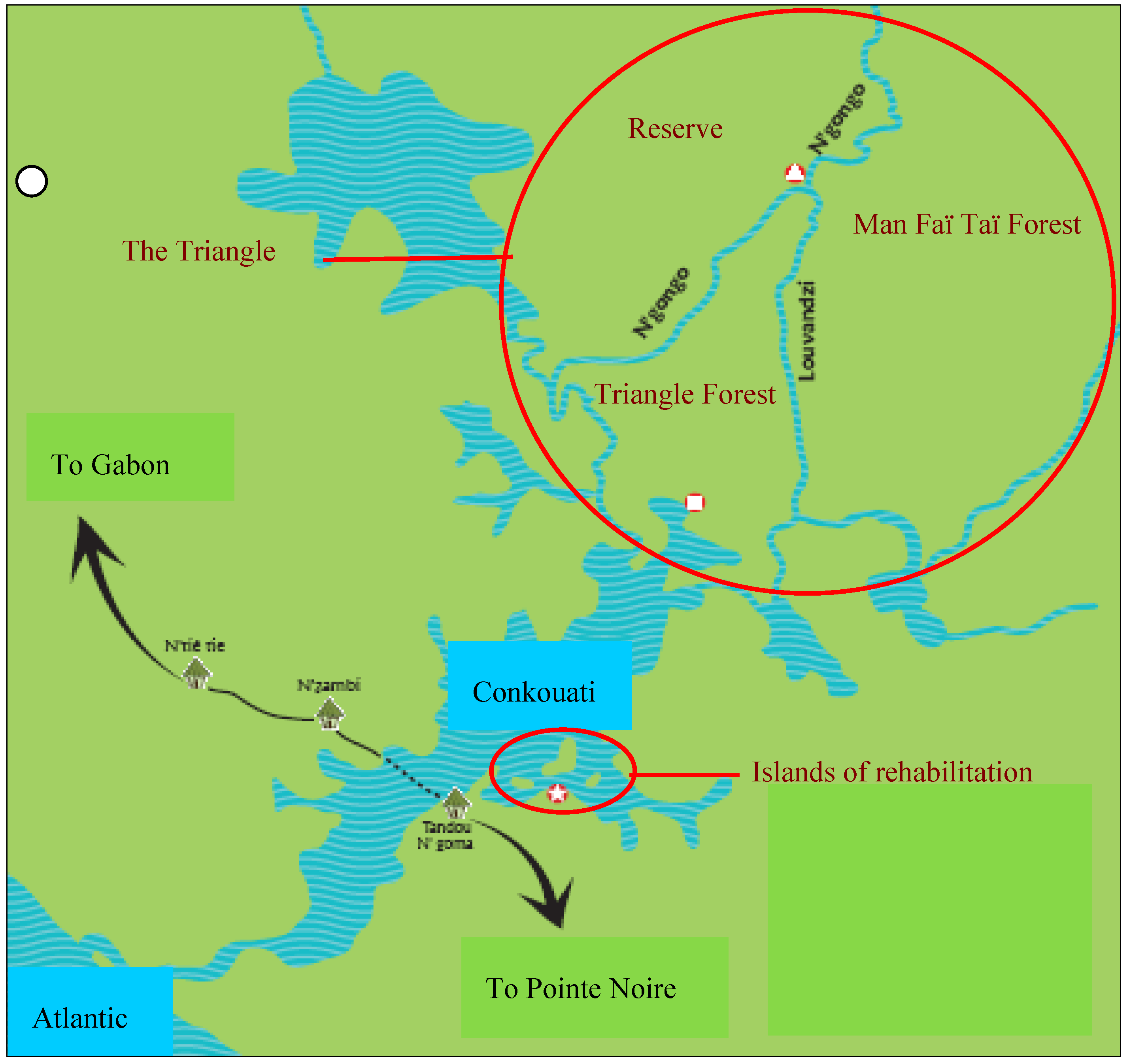
2.2. Study Animals, Data Collection and Analyses
| ID | Sex | Arrival age (years)/ Estimated date of birth/Date arrived sanctuary) | Release age (years)/ Date release | Number data blocks (a) (dates data collection) | Date death (cause) | Time since release to 2009 or death (years) | Offspring born in Triangle (b) | No. infants known up to 2009 |
|---|---|---|---|---|---|---|---|---|
| Bounie | F | 5.0 Jan 1987–Jan 1992 | 9.8 Nov 1996 | 4 (1997–1998) | – | 13 | Yes | 1 |
| Choupette | F | 1.8 Nov 1989–Aug 1991 | 6.9 Nov 1996 | 10 (1997–1999, 2003–2004) | – | 13 | Yes | 3 |
| Jeanette | F | 3.2 Jun 1988–Aug 1991 | 8.3 Nov 1996 | 10 (1997–1999, 2003–2004) | – | 13 | Yes | 1 |
| Mekoutou | M | 2.0 Jun 1990–Jun 1992 | 6.3 Nov 1996 | 11 (1997–1999, 2003–2005) | Apr 2006 (unknown) | 10 | Yes | 1 |
| Rosette | F | 1.5 Feb 1990–Aug 1991 | 6.9 Feb 1997 | 6 (1997–1999) | – | 12 | – | – |
| Koutou | M | 2.2 Jun 1989–Aug 1991 | 9.6 Feb 1999 | 12 (1999, 2001–2005) | Mar 2009 (Wild attack) | 10 | – | – |
| Chinois | M | 2.2 Jun 1989–Aug 1991 | 11.0 Jun 2000 | 10 (2001–2005) | – | 9 | – | – |
| Bilinga | M | 0.4 Jun 1993–Nov 1993 | 8.0 Jun 2001 | 4 (2002–2004) | Oct 2004 (Oesopha-gostomum infection) | 3 | – | – |
| Activity budget | Definition |
|---|---|
| Travel | Moving (walking, running, jumping) on the ground or in the trees, climbing or descending |
| Allogrooming | Grooming another chimpanzee |
| Rest | Lying, sleeping, sitting, not moving |
| Feed | Removing food items from a substrate, eating, chewing, swallowing |
| Other | All the activities that do not correspond to those listed above, e.g., aggression, play, copulation, veterinary interventions, displays, hunting, nest building and coprophagy |
| Dietary items | Items included |
| Plant | Fruit (Fr.), leaf (L.), flower (Fl.), seed (Se.), and stem (St.) |
| Insect | Ants, larvae, caterpillars, termites and grasshoppers |
| Meat | Birds, pangolins, and squirrels |
| Other food items | Sap (exudates eaten from tree trunks/branches), bark, soil, clay and honey |
| Genus/Species | Family | % diet | Local name | Part consumed | Plant type | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fl. | Fr. | L. | Se. | St. | |||||
| Irvingia gabonensis | Irvingiaceae | 15 | x | x | Tree | ||||
| Vitex | Verbenaceae | 14 | x | x | x | x | Tree | ||
| Staudtia | Myristicaceae | 10 | Niove | x | x | Tree | |||
| Nauclea | Rubiaceae | 9 | Bilinga | x | Tree | ||||
| Elaeis guineensis | Palmae | 8 | x | x | Tree | ||||
| Millettia | Papilionaceae | 6 | x | x | Tree | ||||
| Aframomum | Zingiberaceae | 6 | Ntoundou | x | x | x | x | Herb | |
| Dialium | Caesalpiniaceae | 5 | Dakar | x | x | Tree | |||
| Grewia | Tiliaceae | 5 | x | x | Tree | ||||
| Landolphia | Apocynaceae | 5 | Malombo | x | x | Liana | |||
| Scytopetalum | Scytopetalaceae | 4 | x | Shrub | |||||
| Santiria | Burseraceae | 3 | x | Tree | |||||
| Hexalobus | Annonaceae | 2 | Vadou | x | Tree | ||||
| Ipomoea | Convolvulaceae | 2 | x | x | Herb | ||||
| Warneckea | Melastomataceae | 2 | x | x | x | Herb | |||
| Diospyros | Ebenaceae | 1 | x | x | x | Tree | |||
| Ficus | Moraceae | 1 | x | x | Shrub | ||||
| Saccoglottis gabonensis | Humiriaceae | 1 | Ozouga | x | Tree | ||||
| Urera | Urticaceae | 1 | x | Woody liana | |||||
| Afzelia | Caesalpiniaceae | <1 | x | Tree | |||||
| Agelaea | Connaraceae | <1 | x | x | Woody liana | ||||
| Alchornea | Euphorbiaceae | <1 | Mukukusa | x | x | Shrub | |||
| Allanblackia | Clusiaceae | <1 | x | Tree | |||||
| Ancistrophyllum | Palmae | <1 | Mukawa | x | Liana | ||||
| Annona | Annonaceae | <1 | x | Shrub | |||||
| Asplenium | Aspleniaceae | <1 | x | Herb | |||||
| Berlinia | Caesalpiniaceae | <1 | Ebiara | x | x | Tree | |||
| Bridelia | Euphorbiaceae | <1 | x | Tree | |||||
| Canthium | Rubiaceae | <1 | x | Shrub | |||||
| Chytranthus | Sapindaceae | <1 | x | Tree | |||||
| Cissus | Vitaceae | <1 | x | Liana | |||||
| Citrus | Rutaceae | <1 | x | Tree | |||||
| Cleistopholis patens | Annonaceae | <1 | x | Tree | |||||
| Cola | Sterculiaceae | <1 | x | x | x | Tree | |||
| Costus | Zingiberaceae | <1 | Mussangavulu | x | x | x | Herb | ||
| Dacryodes | Burseraceae | <1 | Safou | x | Tree | ||||
| Desplatsia | Tiliaceae | <1 | x | x | Tree | ||||
| Diogoa | Olacaceae | <1 | x | Tree | |||||
| Eremospatha | Palmae | <1 | Mbamba | x | x | Liana | |||
| Enantia | Annonaceae | <1 | x | Tree | |||||
| Gnetum africanum | Gnetaceae | <1 | Mfumbu | x | Tree | ||||
| Guibourtia | Caesalpiniaceae | <1 | x | x | Tree | ||||
| Haumania | Marantaceae | <1 | x | Herb | |||||
| Hibiscus | Malvaceae | <1 | x | Herb | |||||
| Hypselodelphis | Marantaceae | <1 | Mangongolo | x | Herb | ||||
| Klainedoxa | Irvingiaceae | <1 | x | Tree | |||||
| Macaranga | Euphorbiaceae | <1 | Msamsa | x | Tree | ||||
| Manihot | Euphorbiaceae | <1 | Manioc | x | Tree | ||||
| Mapania | Cyperaceae | <1 | x | x | Herb | ||||
| Marantochloa | Marantaceae | <1 | x | Herb | |||||
| Milicia excelsa | Moraceae | <1 | Iroko | x | Tree | ||||
| Mimosa | Mimosaceae | <1 | x | x | Liana | ||||
| Musanga | Moraceae | <1 | Musenga | x | Tree | ||||
| Myrianthus | Moraceae | <1 | x | Tree | |||||
| Palisota | Commelinaceae | <1 | x | x | Herb | ||||
| Parkia | Mimosaceae | <1 | x | x | x | Tree | |||
| Pentaclethra | Mimosaceae | <1 | Tibossi | x | x | Tree | |||
| Pentadesma butyracea | Clusiaceae | <1 | x | Tree | |||||
| Polyalthia | Annonaceae | <1 | x | Tree | |||||
| Pseudospondias | Anacardiaceae | <1 | Ngarila | x | x | Tree | |||
| Salacia | Celastraceae | <1 | x | x | Tree | ||||
| Strychnos | Loganiaceae | <1 | x | Woody liana | |||||
| Symphonia globulifera | Clusiaceae | <1 | x | Tree | |||||
| Trachyphrynium | Marantaceae | <1 | x | Herb | |||||
| Trichoscypha | Anacardiaceae | <1 | Mvuta | x | Tree | ||||
| Uapaca | Euphorbiaceae | <1 | x | Tree | |||||
| Uvaria | Annonaceae | <1 | x | Tree | |||||
| Uvariastrum pierreanum | Annonaceae | <1 | x | x | Tree | ||||
| Xylopia | Annonaceae | <1 | Moukana | x | Tree | ||||
| Treculia | Moraceae | <1 | x | Tree | |||||
| Pycnanthus | Myristicaceae | <1 | Mulomba | x | Tree | ||||
| Ongokea gore | Olacaceae | <1 | x | Tree | |||||
| Podococcus | Palmae | <1 | x | Tree | |||||
| Raphia | Palmae | <1 | Mahouhou | x | x | x | Tree | ||
| Panda oleosa | Pandaceae | <1 | x | Tree | |||||
| Leptoderris | Papilionaceae | <1 | x | x | Shrub | ||||
| Pterocarpus | Papilionaceae | <1 | x | Tree | |||||
| Passiflora | Passifloraceae | <1 | x | Herb | |||||
| Oxytenanthera abyssinica | Poaceae | <1 | Bambou | x | Herb | ||||
| Porterandia | Rubiaceae | <1 | x | Tree | |||||
| Sherbournia | Rubiaceae | <1 | x | Tree | |||||
| Pancovia | Sapindaceae | <1 | x | Tree | |||||
| Baillonella | Sapotaceae | <1 | Moabi | x | Tree | ||||
| Sterculia | Sterculiaceae | <1 | Ikirulanga | x | x | x | x | Tree | |
| Glyphaea brevis | Tiliaceae | <1 | x | Tree | |||||
2.3. Statistics
3. Results and Discussion
3.1. Results
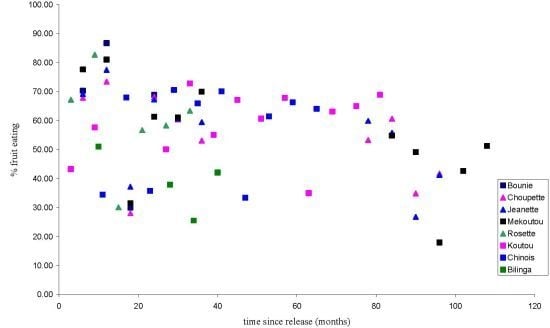
3.1.1. Feeding Patterns
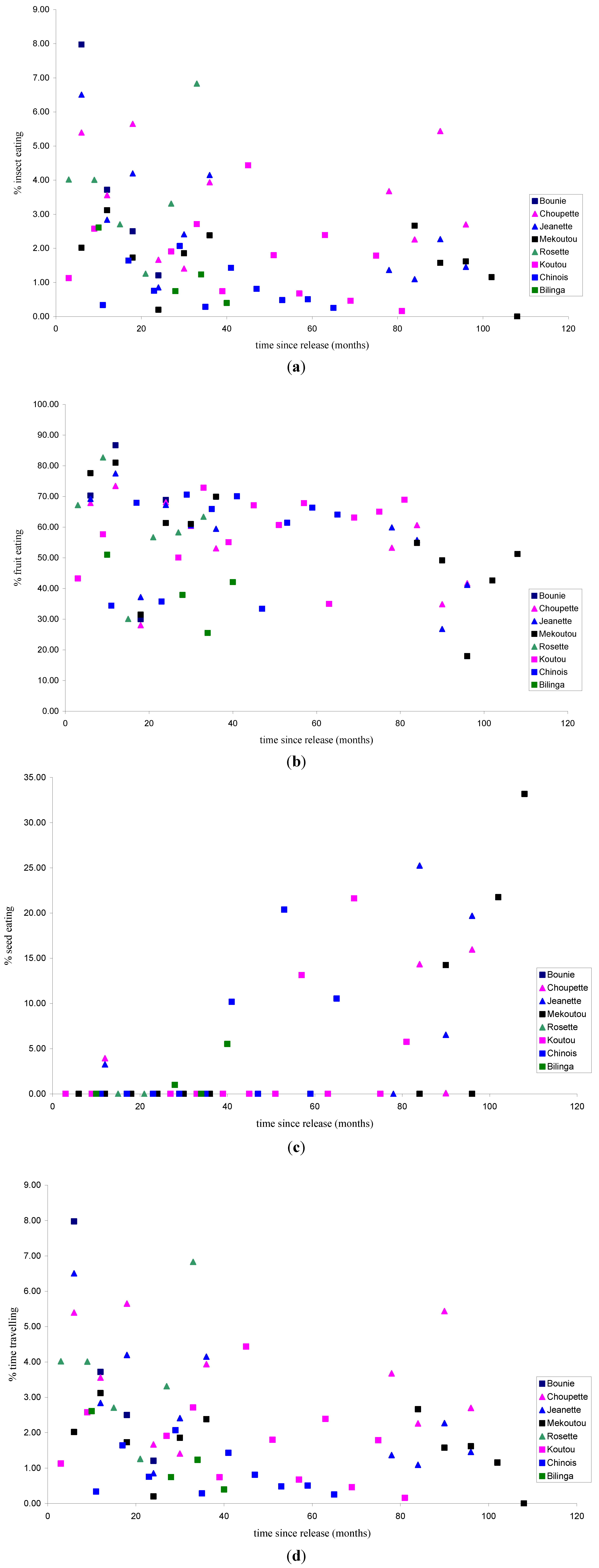
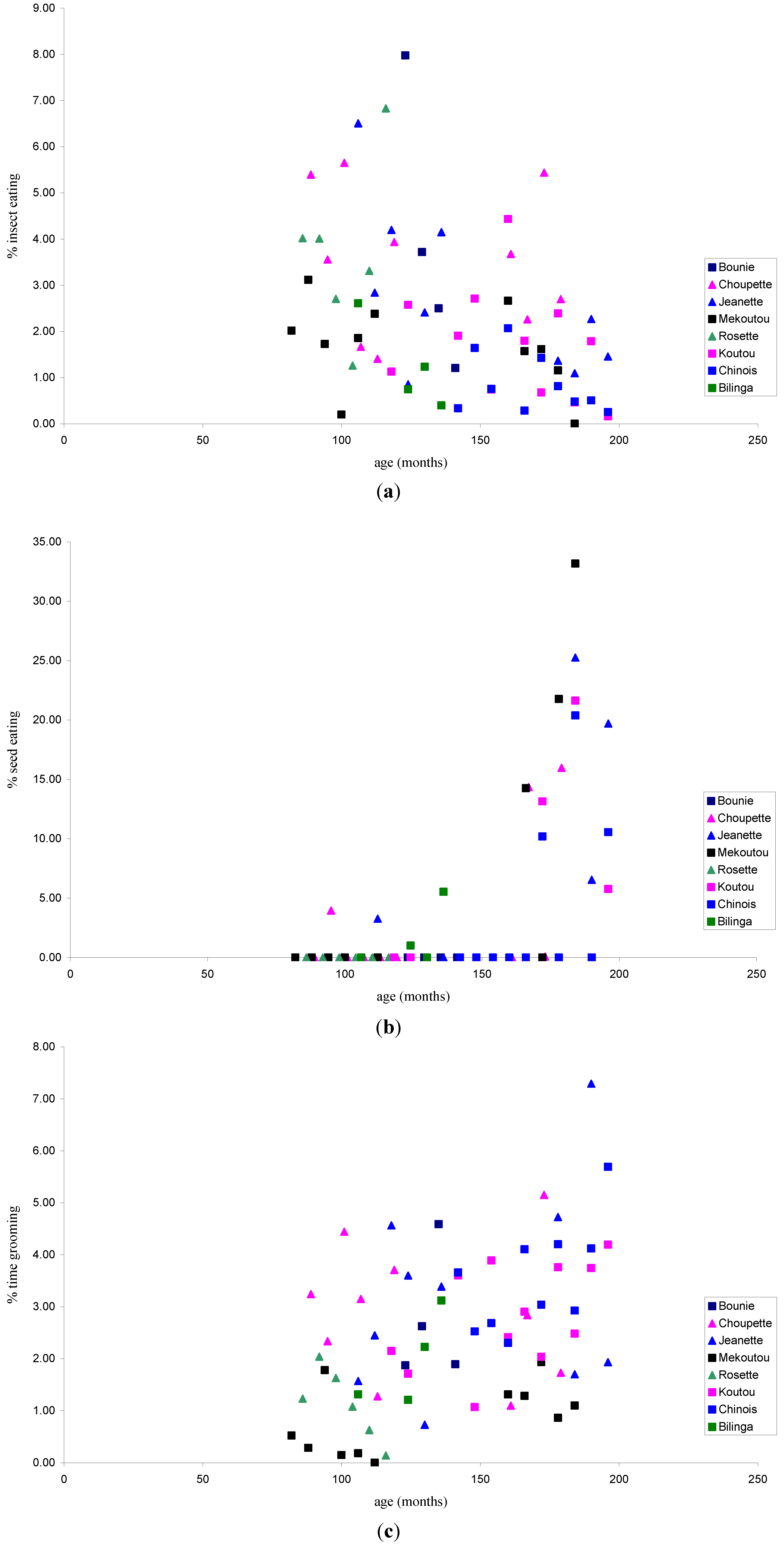
3.1.2. Activity Patterns
3.2. Discussion
4. Conclusions
Acknowledgments
Conflict of Interest
References
- IUCN Red List of Threatened Species. 2013. Available online: www.iucnredlist.org (accessed on 4 June 2013).
- Beck, B.; Walkup, K.; Rodrigues, M.; Unwin, S.; Travis, D.; Stoinski, T. Best Practice Guidelines for the Re-Introduction of Great Apes; Occasional Paper of the IUCN Species Survival Commission No. 35; UICN/SSC Primate Specialist Group: Gland, Switzerland, 2007; pp. 7–8. [Google Scholar]
- Borner, M. The rehabilitated chimpanzees of Rubondo Island. Oryx 1985, 19, 151–154. [Google Scholar] [CrossRef]
- Hannah, A.; McGrew, W. Rehabilitation of captive chimpanzees. In Primate Responses to Environmental Change; Box, H.O., Ed.; Chapman & Hall: London, UK, 1991; pp. 167–186. [Google Scholar]
- Yaeger, C.P. Orangutan rehabilitation in Tanjung Putting National Park, Indonesia. Conserv. Biol. 1997, 11, 802–805. [Google Scholar]
- Humle, T.; Colin, C.; Laurans, M.; Raballand, E. Group Release of Sanctuary Chimpanzees (Pan troglodytes) in the Haut Niger National Park, Guinea, West Africa: Ranging Patterns and Lessons So Far. Int. J. Primatol. 2011, 32, 456–473. [Google Scholar] [CrossRef]
- Custance, D.M.; Whiten, A.; Fredman, T. Social learning and primate reintroduction. Int. J. Primatol. 2002, 23, 479–499. [Google Scholar] [CrossRef]
- Seddon, P.J. Persistence without intervention: Assessing success in wildlife reintroductions. Trends Ecol. Evol. 1999, 14, 503. [Google Scholar] [CrossRef]
- Paredes, J. The Adaptation Process of Seven Released Chimpanzees (Pan t. troglodytes) in the Reserve of Conkouati, Congo; Unpublished Report; H.E.L.P., Congo: Pointe Noire, Congo, 1997. [Google Scholar]
- Brewer, S. The Chimpanzees of Mt Assirik; A. Knopf: New York, NY, USA, 1978. [Google Scholar]
- Marsden, S.B.; Marsden, D.; Thompson, M.E. Demographic and female life history parameters of free-ranging chimpanzees at the chimpanzee rehabilitation project, river Gambia National Park. Int. J. Primatol. 2006, 27, 391–409. [Google Scholar] [CrossRef]
- Farmer, K.H.; Buchanan-Smith, H.M.; Jamart, A. Behavioural adaptation of Pan troglodytes troglodytes. Int. J. Primatol. 2006, 27, 747–767. [Google Scholar] [CrossRef]
- Moscovice, L.R.; Issa, M.H.; Petrzelkova, K.J.; Keuler, N.S.; Snowdon, C.T.; Huffman, M.A. Fruit availability, chimpanzee diet, and grouping patterns on Rubondo Island, Tanzania. Am. J. Primatol. 2007, 69, 487–502. [Google Scholar] [CrossRef]
- Goossens, B.; Setchell, J.M.; Tchidongo, E.; Dilambaka, E.; Vidal, C.; Ancrenaz, M.; Jamart, A. Survival, interactions with conspecifics and reproduction in 37 chimpanzees released into the wild. Biol. Conserv. 2005, 123, 461–475. [Google Scholar] [CrossRef]
- Hladik, C.M. Alimentation et activité d’un groupe de chimpanzés réintroduits en forêt Gabonaise. La Terre et la Vie 1973, 27, 343–413. [Google Scholar]
- Hladik, C.M. Chimpanzees of Gabon and chimpanzees of Gombe: Some comparative data on the diet. In Primate Ecology: Studies of Feeding and Ranging in Lemurs, Monkeys, and Apes; Clutton-Brock, T.H., Ed.; Academic Press: New York, NY, USA, 1977; pp. 480–501. [Google Scholar]
- Goossens, B.; Funk, S.M.; Vidal, C.; Latour, S.; Jamart, A.; Ancrenaz, M.; Wickings, E.J.; Tutin, C.E.G.; Bruford, M.W. Measuring genetic diversity in translocation programs: Principles and application to a chimpanzee release project. Anim. Conserv. 2002, 5, 225–236. [Google Scholar] [CrossRef]
- Le Hellaye, Y.; Goossens, B.; Jamart, A.; Curtis, D.J. Acquisition of fission-fusion social organization in a chimpanzee (Pan troglodytes troglodytes) community released into the wild. Behav. Ecol. Sociobiol. 2010, 64, 349–360. [Google Scholar] [CrossRef]
- Moscovice, L.R.; Mbago, F.; Snowdon, C.T.; Huffman, M.A. Ecological features and ranging patterns at a chimpanzee release site on Rubondo Island, Tanzania. Biol. Conserv. 2010, 143, 2711–2721. [Google Scholar] [CrossRef]
- Tutin, C.E.G.; Ancrenaz, M.; Paredes, J.; Vacher-Vallas, M.; Vidal, C.; Goossens, B.; Bruford, M.W.; Jamart, A. Conservation biology framework for the release of wild-born orphaned chimpanzees into the Conkouati Reserve, Congo. Conserv. Biol. 2001, 15, 1247–1257. [Google Scholar] [CrossRef]
- Farmer, K.H. Pan-African Sanctuary Alliance: Status and Range of Activities for Great Ape Conservation. Am. J. Primat. 2002, 58, 117–132. [Google Scholar] [CrossRef]
- Tutin, C.E.G. Expertise sur les Possibilités de Réintroduction de Chimpanzés en Forêt Naturelle dans la Réserve de Conkouati, Congo; Unpublished Report; H.E.L.P., Congo: Pointe Noire, Congo, 1996. [Google Scholar]
- Goossens, B.; Setchell, J.M.; Vidal, C.; Dilambaka, E.; Jamart, A. Successful reproduction in wild-released orphan chimpanzees (Pan troglodytes troglodytes). Primates 2003, 44, 67–69. [Google Scholar]
- Altmann, J. Observational Study of Behaviour: Sampling Methods. Behaviour 1973, 48, 1–41. [Google Scholar]
- Letouzey, R. Manual of Forest Botany: Tropical Africa; Centre Technique Forestier Tropical, Nogent-sur-Marne, France; Ministère de l'Enseignement Supérieur et de la Recherche Scientifique: Yaounde, Cameroon, 1986; Volumes 1, 2A, 2B. [Google Scholar]
- Head, J.; Boesch, C.; Makaga, L.; Robbins, M.M. Sympatric Chimpanzees (Pan troglodytes troglodytes) and Gorillas (Gorilla gorilla gorilla) in Loango National Park, Gabon: Dietary Composition, Seasonality, and Intersite Comparisons. Int. J. Primates 2011, 32, 755–775. [Google Scholar] [CrossRef]
- Nkurunungi, J.B.; Ganas, J.; Robbins, M.M.; Stanford, C.B. A comparison of two mountain gorilla habitats in Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 2004, 42, 289–297. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Koman, J. The flora of Bossou: Its utilisation by chimpanzees and humans. Afr. Study Monogr. 1992, 13, 127–169. [Google Scholar]
- Tutin, C.E.G.; Fernandez, M. Composition of the diet of chimpanzees and comparisons with that of sympatric lowland gorillas in the Lope Reserve, Gabon. Am. J. Primatol. 1993, 30, 195–211. [Google Scholar] [CrossRef]
- Wrangham, R.W. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In Primate Ecology: Studies of Feeding and Ranging on Lemurs, Monkeys and Apes; Clutton-Brock, T., Ed.; Academic Press: New York, NY, USA, 1977; pp. 503–538. [Google Scholar]
- McGrew, W.; Baldwin, P.J.; Tutin, C.E. Diet of wild chimpanzees (Pan troglodytes verus) at Mt Assirik, Senegal: I. Composition. Am. J. Primatol. 1988, 16, 213–226. [Google Scholar] [CrossRef]
- Nishida, T.; Uehara, S. Natural diet of chimpanzees (Pan troglodytes schweinfurthii): Longterm record from the Mahale Mountains, Tanzania. Afr. Study Monogr. 1983, 3, 109–130. [Google Scholar]
- Hunt, K.D.; McGrew, W. Chimpanzees in the dry habitats of Assirik, Senegal and Semliki Wildlife Reserve, Uganda. In Behavioural Diversity in Chimpanzees and Bonobos; Boesch, C., Hohmann, G., Marchant, L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 35–51. [Google Scholar]
- Boesch, C.; Boesch-Achermann, H. The Chimpanzees of the Taï Forest—Behavioural Ecology and Evolution; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Boesch, C.; Head, J.; Robbins, M.M. Complex tool sets for honey extraction among chimpanzees in Loango National Park, Gabon. J. Hum. Evol. 2009, 56, 560–569. [Google Scholar] [CrossRef]
- Strier, K.B. Primate Behavioral Ecology; University of Wisconsin-Madison: Madison, WI, USA, 2003. [Google Scholar]
- Nishida, T. The Social Group of Wild Chimpanzees in the Mahale Mountains. Primates 1968, 9, 167–224. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Ganzhorn, J.U. Nested patterns of species composition and its implications for lemur biogeography in Madagascar. Folia Primatol. 1998, 69, 332–341. [Google Scholar] [CrossRef]
- Reynolds, V.; Plumptre, A.J.; Greenham, J.; Harborne, J. Condensed tannins and sugars in the diet of chimpanzees (Pan troglodytes schweinfurthii) in the Budongo Forest, Uganda. Oecologica 1998, 115, 331–336. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Renaud, A.; Jamart, A.; Goossens, B.; Ross, C. A Longitudinal Study on Feeding Behaviour and Activity Patterns of Released Chimpanzees in Conkouati-Douli National Park, Republic of Congo. Animals 2013, 3, 532-550. https://doi.org/10.3390/ani3020532
Renaud A, Jamart A, Goossens B, Ross C. A Longitudinal Study on Feeding Behaviour and Activity Patterns of Released Chimpanzees in Conkouati-Douli National Park, Republic of Congo. Animals. 2013; 3(2):532-550. https://doi.org/10.3390/ani3020532
Chicago/Turabian StyleRenaud, Amandine, Aliette Jamart, Benoit Goossens, and Caroline Ross. 2013. "A Longitudinal Study on Feeding Behaviour and Activity Patterns of Released Chimpanzees in Conkouati-Douli National Park, Republic of Congo" Animals 3, no. 2: 532-550. https://doi.org/10.3390/ani3020532