Behavioural Profiles of Brown and Sloth Bears in Captivity
Abstract
:Simple Summary
Abstract
1. Introduction
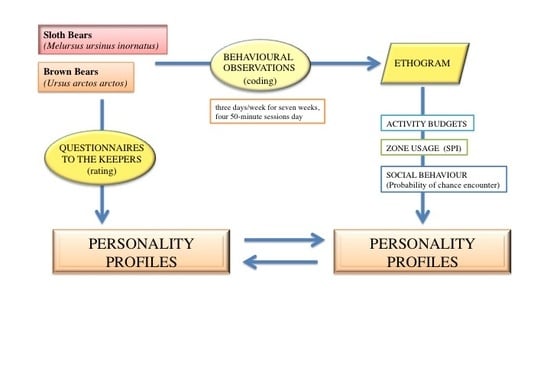
2. Materials and Methods
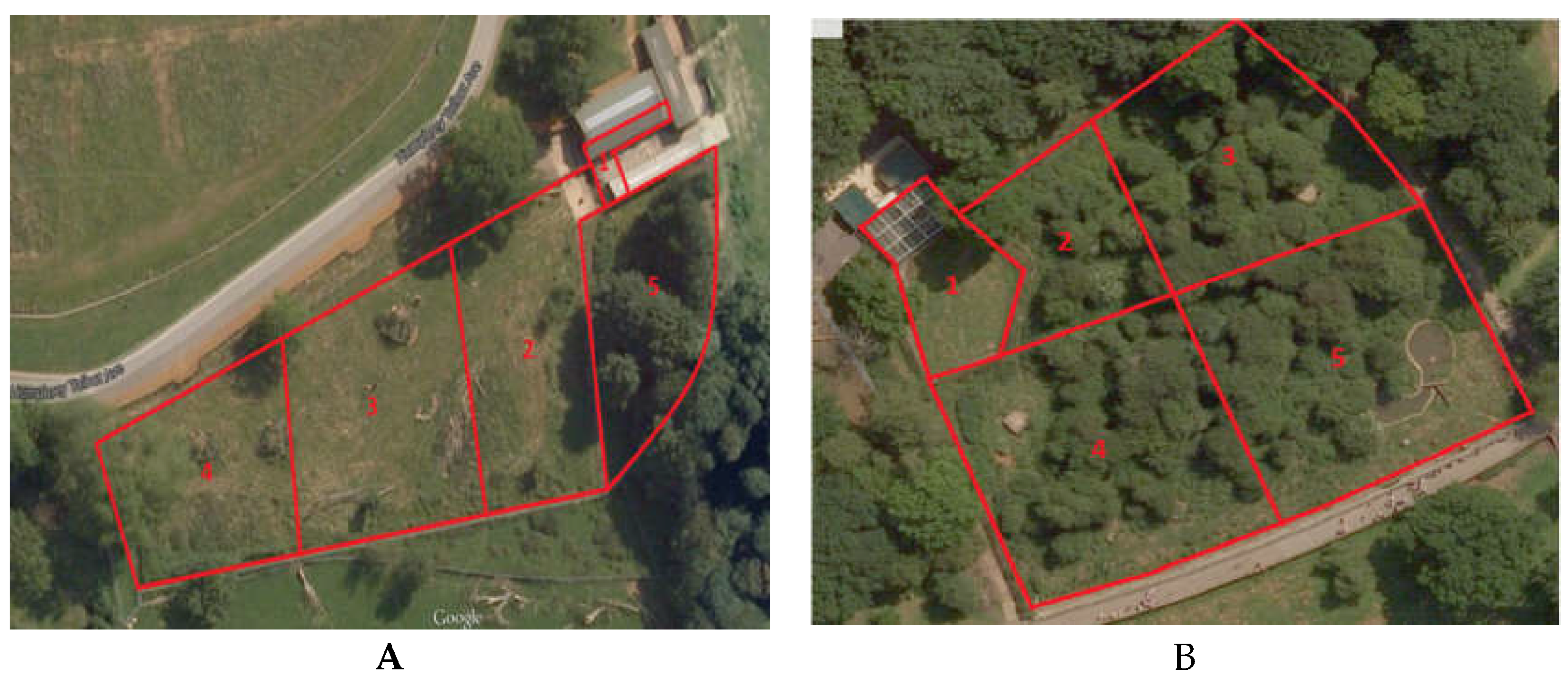
2.1. Study Area and Animals
2.2. Observational Data
2.3. Behavioural Profiles
2.4. Statistical Analysis and Presentation of Data
2.5. Ehtical Statement
3. Results
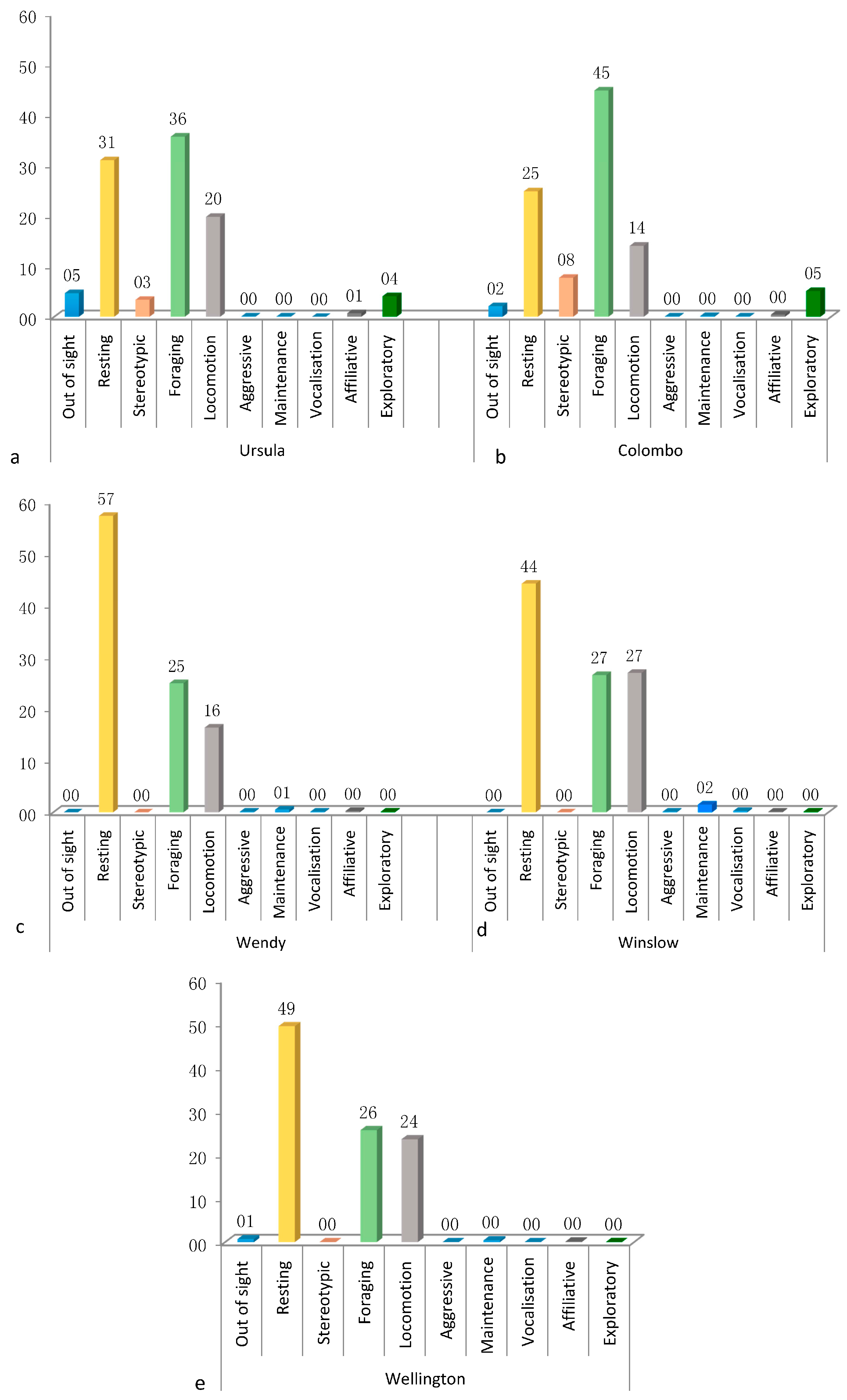
3.1. Activity Budget
3.2. Zone Usage
3.3. Probability of Chance Encounter
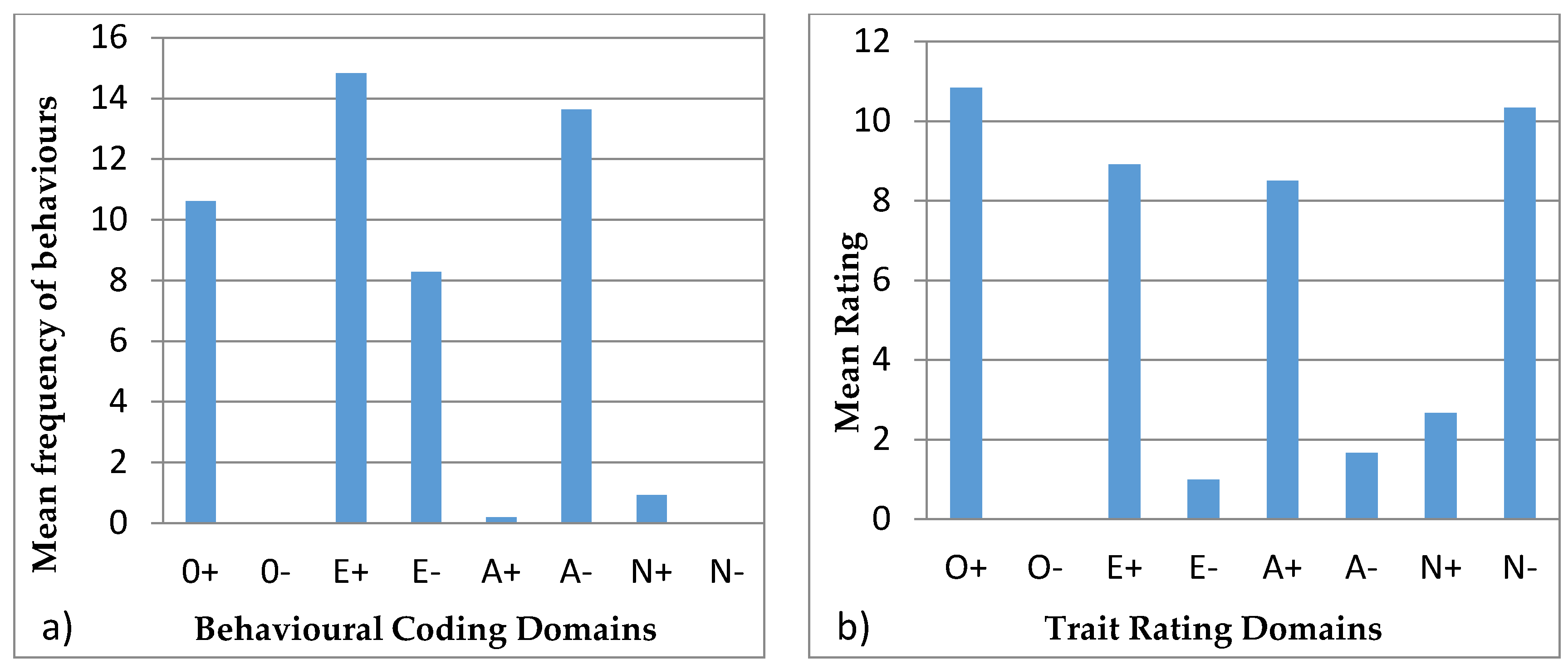
3.4. Behavioural Profiles
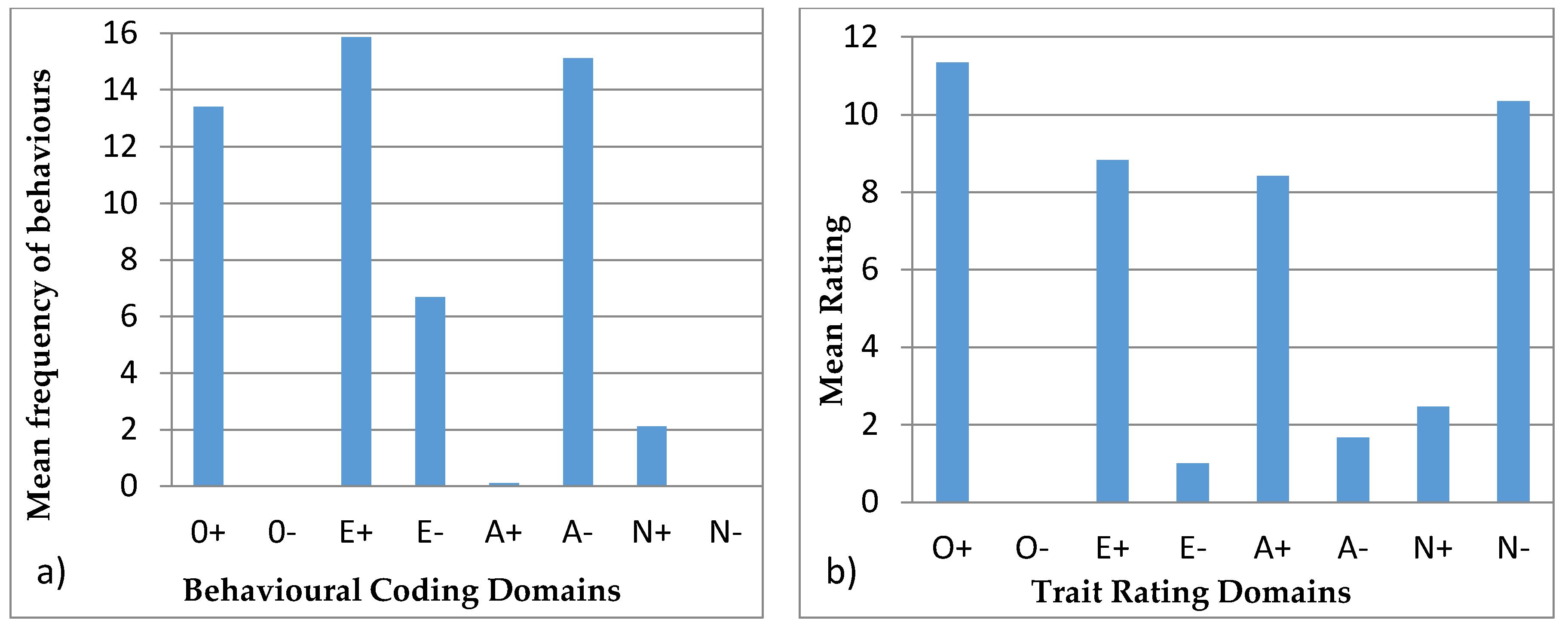
3.4.1. Ursula
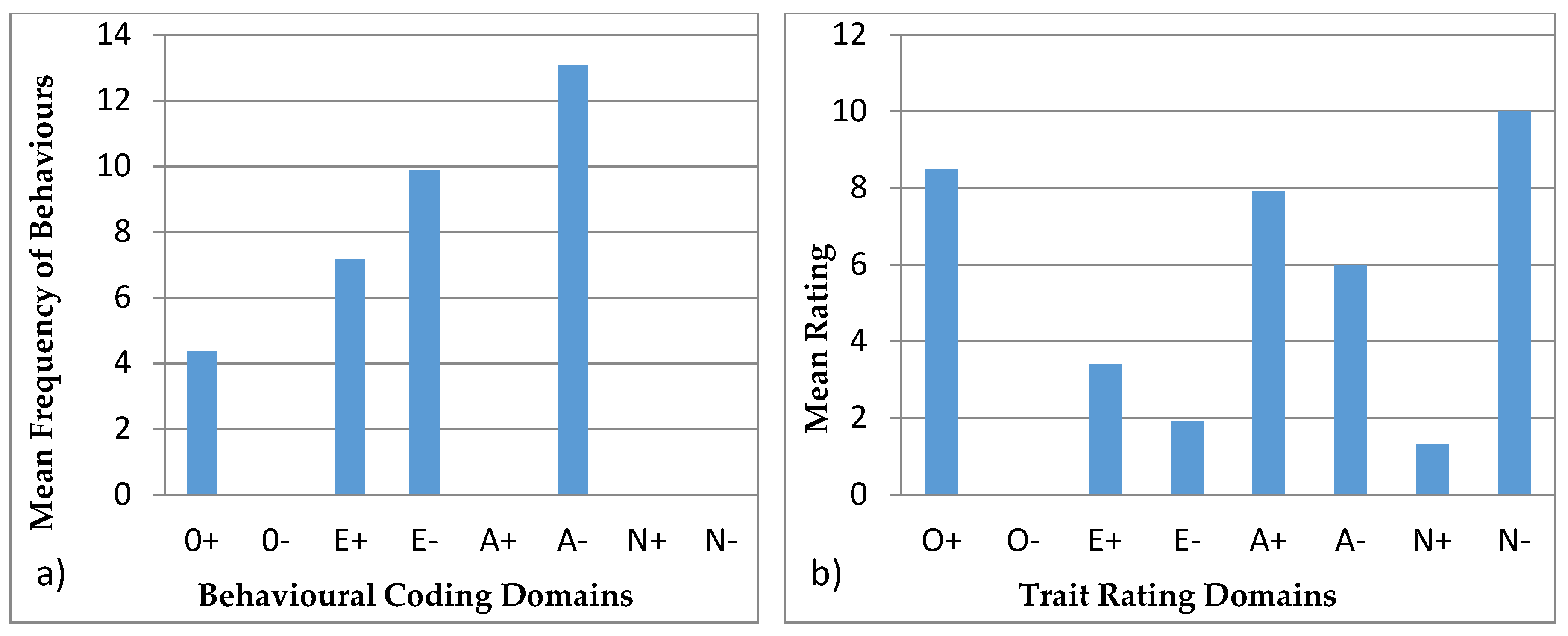
3.4.2. Colombo
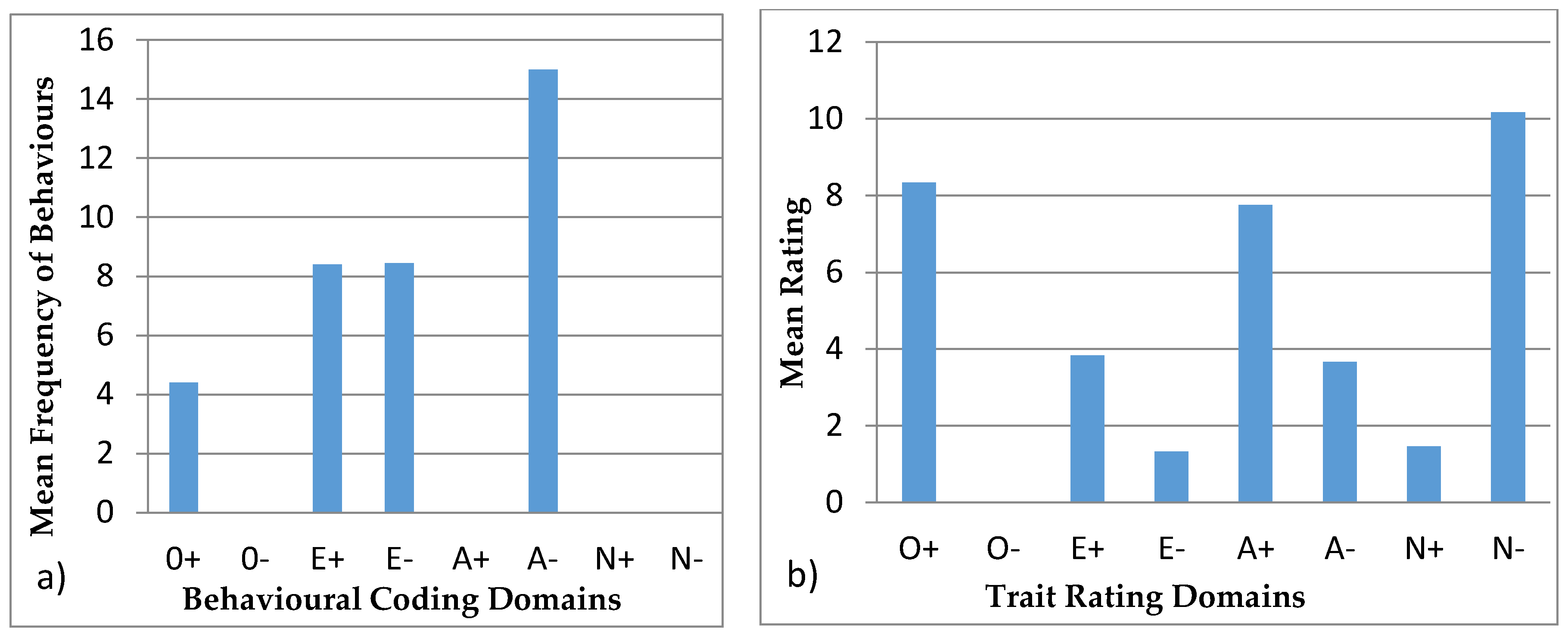
3.4.3. Wendy
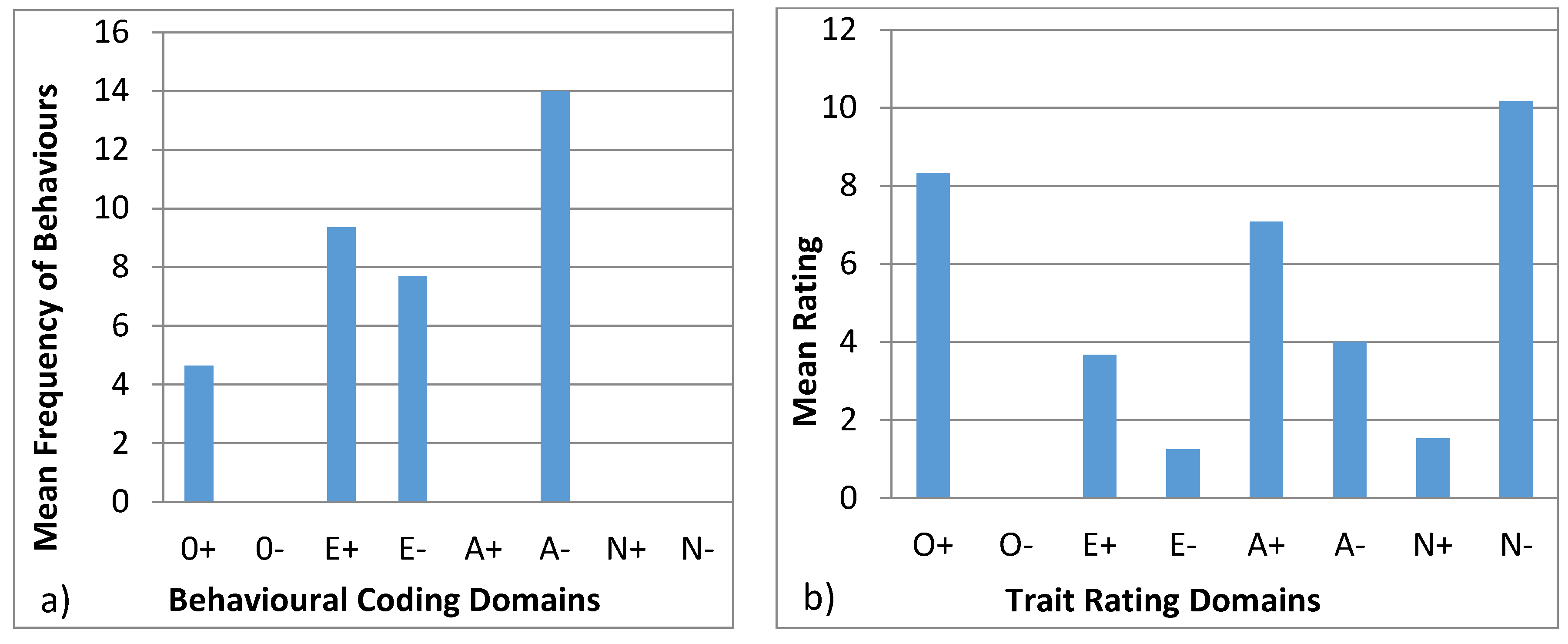
3.4.4. Wellington
3.4.5. Winslow
4. Discussion
4.1. Activity Budget
4.2. Zone Usage
4.3. Probability of Chance Encounter
4.4. Behavioural Profiles
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, R.; Mather, J. Personalities of octopuses. J. Comp. Psychol. 1993, 107, 336–340. [Google Scholar]
- Barber, I.; Dingemanse, N.J. Parasitism and the evolutionary ecology of animal personality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Bergmuller, R.; Taborsky, M. Animal personality due to social niche specialisation. Trends Ecol. Evol. 2010, 25, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, P.T.; Vainikka, A.; Forsman, J.T.; Loukola, O.J.; Kortet, R. How does variation in the environment and individual cognition explain the existence of consistent behavioral differences? Ecol. Evol. 2013, 3, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kortet, R.; Hedrick, A.V.; Vainikka, A. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 2010, 13, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Cote, J.; Evans, M.; Fogarty, S.; Pruitt, J. Ecological implications of behavioural syndromes. Ecol. Lett. 2012, 15, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, C. Social Behaviour and Personality Assessment as a Tool for Improving the Management of Cheetahs (Acinonyx jubatus) in Captivity. Ph.D. Thesis, University of Salford, Manchester, UK, 30 November 2015. [Google Scholar]
- Réale, D.; Dingemanse, N.J.; Kazem, A.J.N.; Wright, J. Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.D.; Gosling, S.D. Personality in nonhuman primates: A review and evaluation of past research. Am. J. Primatol. 2010, 72, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Kurvers, R.H.J.M.; Eijkelenkamp, B.; van Oers, K.; van Lith, B.; van Wieren, S.E.; Ydenberg, R.C.; Prins, H.H.T. Personality differences explain leadership in barnacle geese. Anim. Behav. 2009, 78, 447–453. [Google Scholar] [CrossRef]
- Massen, J.J.; Antonides, A.; Arnold, A.K.; Bionda, T.; Koski, S.E. A behavioral view on chimpanzee personality: Exploration tendency, persistence, boldness, and tool orientation measured with group experiments. Am. J. Primatol. 2013, 75, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Weiss, E.; Brown, J.L. Examination of the interrelationships of behaviour, dominance status, and ovarian activity in captive Asian and African elephants. Zoo Biol. 2004, 23, 431–448. [Google Scholar] [CrossRef]
- Carlstead, K.; Fraser, J.; Bennett, C.; Kleiman, D.G. Black rhinoceros (Diceros bicornis) in U.S. zoos: II. Behaviour, breeding success and mortality in relation to housing facilities. Zoo Biol. 1999, 18, 35–52. [Google Scholar] [CrossRef]
- Wielebnowski, NC. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999, 18, 335–349. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Holland, B.D.; Daniel, J.C. Predator discrimination and “personality” in captive Vancouver Island marmots (Marmota vancouverensis). Anim. Conserv. 2006, 9, 274–282. [Google Scholar] [CrossRef]
- Carlstead, K.; Mellen, J.; Kleiman, D.G. Black rhinoceros (Diceros bicornis) in U.S. zoos: I. Individual behavior profiles and their relationship to breeding success. Zoo Biol. 1999, 18, 17–34. [Google Scholar] [CrossRef]
- Gosling, S.D. Personality in nonhuman animals. Soc. Personal. Psychol. Compass 2008, 2, 985–1001. [Google Scholar] [CrossRef]
- Thurstone, L.L. The vectors of mind. Psychol. Rev. 1934, 41, 1–32. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R. The Neo-PI. NEO-FFI Manual Supplement; Psychological Assessment Resources: Lutz, FL, USA, 1989. [Google Scholar]
- Gosling, S.D.; John, O.P. Personality dimensions in nonhuman animals a cross-species review. Curr. Dir. Psychol. Sci. 1999, 8, 69–75. [Google Scholar] [CrossRef]
- Gosling, S.D. From mice to men: What can we learn about personality from animal research? Psychol. Bull. 2001, 127, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Highfill, L.; Kuczaj, S. Do bottlenose dolphins (Tursiops truncatus) have distinct and stable personalities? Aquat. Mamm. 2007, 33, 380–389. [Google Scholar] [CrossRef]
- Uher, J. Comparative personality research: Methodological approaches. Eur. J. Pers. 2008, 22, 427–455. [Google Scholar] [CrossRef]
- Weiss, A.; Inoue-Murayama, M.; Hong, K.W.; Inoue, E.; Udono, T.; Ochiai, T.; Matsuzawa, T.; Hirata, S.; King, J.E. Assessing chimpanzee personality and subjective well-being in Japan. Am. J. Primatol. 2009, 71, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Birgersson, S. Personality Assessment and Interactions in Eight Captive Bottlenose Dolphins (Tursiops Truncatus). Master’s Thesis, Department of Physics, Chemistry and Biology, Linkopings Universitet, Linkopings, Sweden, 3 June 2011. [Google Scholar]
- Tetley, C.; O’Hara, S. Ratings of animal personality as a tool for improving the breeding, management and welfare of zoo mammals. UFAW J. 2012, 21, 463–476. [Google Scholar] [CrossRef]
- Gartner, M.C.; Weiss, A. Personality in felids: A review. Appl. Anim. Behav. Sci. 2013, 144, 1–13. [Google Scholar] [CrossRef]
- Wells, A.; Terio, K.A.; Ziccardi, M.H.; Munson, L. The stress response to environmental change in captive cheetahs (Acinonyx jubatus). J. Zoo Wildl. Med. 2004, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Munkwitz, N.M.; Turner, J.M.; Kershner, E.L.; Farabaugh, S.M.; Heath, S.R. Predicting release success of captive reared loggerhead shrikes (Lanius ludovicianus) using pre-release behavior. Zoo Biol. 2005, 24, 447–458. [Google Scholar] [CrossRef]
- Mathews, F.; Orros, M.; McLaren, G.; Gelling, M.; Foster, R. Keeping fit on the ark: Assessing the suitability of captive-bred animals for release. Biol. Conserv. 2005, 121, 569–577. [Google Scholar] [CrossRef]
- Bremner-Harrison, S.; Prodohl, P.; Elwood, R.W. Behavioural trait assessment as a release criterion: Boldness predicts early death in a reintroduction programme of captive-bred swift fox (Vulpes velox). Anim. Conserv. 2004, 7, 313–320. [Google Scholar] [CrossRef]
- Servheen, C.; Herrero, S.; Peyton, B.; Pelletier, K.; Moll, K.; Moll, J. Bears: Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 1999. [Google Scholar]
- Fagen, R.; Fagen, J.M. Individual distinctiveness in brown bears, Ursus arctos L. Ethology 1996, 102, 212–226. [Google Scholar] [CrossRef]
- Kleiman, D.G.; Thompson, K.V.; Baer, C.K. Wild Mammals in Captivity: Principles and Techniques for Zoo Management; Kleiman, D.G., Baer, C.K., Eds.; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- Melfi, V.; Feistner, A. A comparison of the activity budgets of wild and captive Sulawesi Crested Black Macaques (Macaca nigra). Anim. Welf. 2002, 11, 213–222. [Google Scholar]
- Bauer, E.; Babitz, M.; Boedeker, N.; Hellmuth, H. Approaches to understanding and managing pacing in sloth bears in a zoological setting. Int. J. Comp. Psychol. 2013, 26, 53–74. [Google Scholar]
- Montaudouin, S.; Le Pape, G. Comparison between 28 zoological parks: Stereotypic and social behaviours of captive brown bears (Ursus arctos). Appl. Anim. Behav. Sci. 2005, 92, 129–141. [Google Scholar] [CrossRef]
- Veeraselvam, M.; Sridhar, R.; Jayathangaraj, M.; Perumal, P. Behavioural study of captive sloth bears using environmental enrichment tools. Int. J. Zool. 2013, 2013, 526905. [Google Scholar] [CrossRef]
- Anderson, C.; Arun, A.S.; Jensen, P. Habituation to environmental enrichment in captive sloth bears—Effect on stereotypies. Zoo Biol. 2010, 29, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Plowman, A. A note on a modification of the spread of participation index allowing for unequal zones. Appl. Anim. Behav. Sci. 2003, 83, 331–336. [Google Scholar] [CrossRef]
- Rose, P.; Robert, R. Evaluating the activity patterns and enclosure usage of a little-studied zoo species, the sitatunga (Tragelaphus spekii). JZAR 2013, 1, 14–19. [Google Scholar]
- Shepherdson, D.J.; Mellen, J.D.; Hutchins, M. Second Nature: Environmental Enrichment for Captive Animals; Smithsonian Institution: Washington, DC, USA, 2012. [Google Scholar]
- Yoerg, S.I. Solitary is not asocial: Effects of social contact in kangaroo rats (Heteromyidae: Dipodomys heermanni). Ethology 1999, 105, 317–333. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P.P.G. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- MacHutchon, A.G. Grizzly bear activity budget and pattern in the Firth River Valley, Yukon. Ursus 2001, 12, 189–198. [Google Scholar]
- Ramesh, T.; Kalle, R.; Sankar, K.; Qureshi, Q. Activity pattern of sloth bear Melursus ursinus (Mammalia: Ursidae) in mudumalai tiger reserve, Western Ghats, India. J. Threat. Taxa 2013, 5, 3989–3992. [Google Scholar] [CrossRef]
- Seryodkin, I.; Kostyria, A.; Goodrich, J.; Miquelle, D. Daily activity patterns of brown bear (Ursus arctos) of the sikhote-alin mountain range (Primorskiy Krai, Russia). Russ. J. Ecol. 2013, 44, 50–55. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies: A critical review. Anim. Behav. 1991, 41, 1015–1037. [Google Scholar] [CrossRef]
- Jensen, A.M.; Delfour, F.; Carter, T. Anticipatory behavior in captive bottlenose dolphins (Tursiops truncatus): A preliminary study. Zoo Biol. 2013, 32, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.W.; Jeppesen, L.L. Temperament, stereotypies and anticipatory behaviour as measures of welfare in mink. Appl. Anim. Behav. Sci. 2006, 99, 172–182. [Google Scholar] [CrossRef]
- Perret, M.; Predine, J. Effects of long-term grouping on serum cortisol levels in Microcebus murinus (Prosimii). Horm. Behav. 1984, 18, 346–358. [Google Scholar] [CrossRef]
- Lodé, T. Kin recognition versus familiarity in a solitary mustelid, the european polecat mustela putorius. C. R. Biol. 2008, 331, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 2007, 82, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Peck, D. The effects of personality of keepers and tigers (Panthera tigris tigris) on their behaviour in an interactive zoo exhibit. Appl. Anim. Behav. Sci. 2007, 106, 244–258. [Google Scholar] [CrossRef]
- Kiley-Worthington, M. The tail movements of ungulates, canids and felids with particular reference to their causation and function as displays. Behaviour 1976, 56, 69–114. [Google Scholar] [CrossRef]
- Highfill, L.; Hanbury, D.; Kristiansen, R.; Kuczaj, S.; Watson, S. Rating vs. coding in animal personality research. Zoo Biol. 2010, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Vazire, S.; Gosling, S.D.; Dickey, A.S.; Schapiro, S.J. Measuring personality in nonhuman animals. In Handbook of Research Methods in Personality Psychology; Guilford Press: New York, NY, USA, 2007; pp. 190–206. [Google Scholar]
| Name | Species | Sex | Born | Born in | Relationship to Other Study Animals |
|---|---|---|---|---|---|
| Wendy | Brown Bear | Female | 26/03/1991 | Captivity | None |
| Wellington | Brown Bear | Female | 15/01/1993 | Captivity | Sister of Winslow |
| Winslow | Brown Bear | Female | 15/01/1993 | Captivity | Sister of Wellington |
| Ursula | Sloth Bear | Female | 04/02/2001 | Captivity | Sister of Colombo |
| Colombo | Sloth Bear | Male | 05/01/1998 | Captivity | Brother of Ursula |
| Behaviour | Description |
|---|---|
| Inactive | Bear is lying, sitting, standing on four paws or upright on two paws whether on land or water. Lying can be on the side, stomach, or back. |
| Foraging and Eating | Bear is actively searching and consuming food. This includes digging to get to food. Distinction between foraging and eating is not made because handling time is minimal or absent in some cases. |
| Locomotion | Movement of the bear like walking on land and water, running or climbing trees or other structures. |
| Stereotypic | Behaviour not exhibited in the wild. In this case it is pacing, which is walking repeatedly, for more than 30’, along the same path. |
| Aggression | Aggressive displays towards conspecifics or people. |
| Maintenance | Natural somatic behaviours like drinking water, urinating, defecating, grooming, or scratching. |
| Affiliation | Positive/friendly behaviours towards conspecifics like playing, sniffing, and rubbing. |
| Exploration | Interacting with the environment whether handling, sniffing, or rubbing against objects or parts of the enclosure. |
| Vocal | Sounds emitted by the bears to show danger, alarm, anger, or intimidation like barking and growling. |
| Openness to Experience | Extroversion | Agreeableness | Neuroticism |
|---|---|---|---|
| + | + | + | + |
| Exploration | Vocal | Affiliation | Aggression |
| Foraging and eating | Locomotion | Stereotypic | |
| Foraging and eating | |||
| − | − | − | − |
| Inactive | Solitary |
| Openness to Experience | Extroversion | Agreeableness | Neuroticism |
|---|---|---|---|
| + | + | + | + |
| Curious | Active | Friendly to conspecifics | Aggression to conspecifics |
| Smart | Playful | Friendly to keepers | Aggression to familiars |
| Vocal | Friendly to familiars | Aggression to unfamiliars | |
| Excitable | Friendly to unfamiliars | Tense | |
| Eccentric | |||
| − | − | − | − |
| Fear of conspecifics | Solitary | Calm | |
| Fear of familiars | Self-Assured | ||
| Fear of unfamiliars | |||
| Insecure |
| Species | Animal | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | SPI | Mean SPI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | D | N | D | N | D | N | D | N | D | ||||
| Sloth | Ursula | 327 | 309 | 366 | 72 | 176 | −120 | 122 | −97 | 59 | −163 | 0.369 | 0.365 |
| Colombo | 286 | 268 | 398 | 104 | 213 | −83 | 133 | −86 | 20 | −201 | 0.360 | ||
| Brown | Wendy | 97 | 37 | 64 | −20 | 50 | −107 | 347 | 150 | 156 | −60 | 0.286 | 0.310 |
| Wellington | 132 | 72 | 62 | −22 | 45 | −112 | 365 | 168 | 110 | −106 | 0.367 | ||
| Winslow | 87 | 27 | 29 | −55 | 30 | −127 | 349 | 152 | 219 | −3 | 0.278 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorino, G.Q.; Christodoulides, Y.; Curone, G.; Pearce-Kelly, P.; Faustini, M.; Albertini, M.; Preziosi, R.; Mazzola, S.M. Behavioural Profiles of Brown and Sloth Bears in Captivity. Animals 2017, 7, 39. https://doi.org/10.3390/ani7050039
Pastorino GQ, Christodoulides Y, Curone G, Pearce-Kelly P, Faustini M, Albertini M, Preziosi R, Mazzola SM. Behavioural Profiles of Brown and Sloth Bears in Captivity. Animals. 2017; 7(5):39. https://doi.org/10.3390/ani7050039
Chicago/Turabian StylePastorino, Giovanni Quintavalle, Yiannis Christodoulides, Giulio Curone, Paul Pearce-Kelly, Massimo Faustini, Mariangela Albertini, Richard Preziosi, and Silvia Michela Mazzola. 2017. "Behavioural Profiles of Brown and Sloth Bears in Captivity" Animals 7, no. 5: 39. https://doi.org/10.3390/ani7050039