Field Propagation Experiments of Male African Savanna Elephant Rumbles: A Focus on the Transmission of Formant Frequencies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sound Recordings of Playback Stimuli
2.2. Study Site and Conditions
2.3. Experimental Design
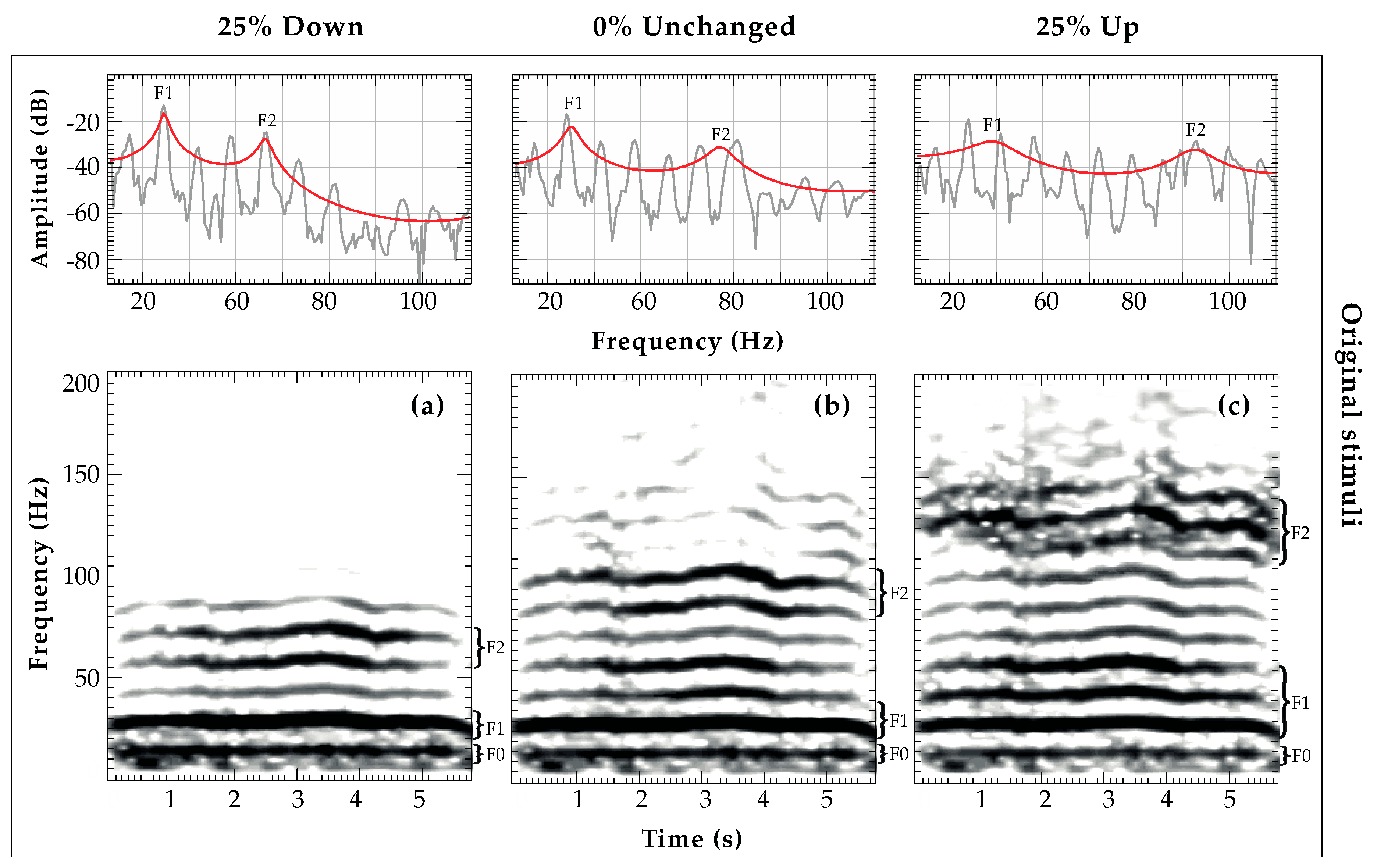
2.3.1. Preparation of Playback Stimuli
2.3.2. Field Recordings
2.4. Acoustic Analyses
2.4.1. Fundamental Frequency (fo) Analysis
2.4.2. Formant Frequency Location (F1, F2) Analysis and Vocal Tract Length
2.4.3. Amplitude Attenuation of Acoustic Features (SNR)
2.5. Statistical Analyses
3. Results
3.1. Transmission Success
3.2. Transmission of Size Information
3.3. Amplitude Attenuation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aureli, F.; Schaffner, C.M.; Boesch, C.; Bearder, S.K.; Call, J.; Chapman, C.A.; Connor, R.; Fiore, A.D.; Dunbar, R.I.M.; Henzi, S.P.; et al. Fission-Fusion Dynamics: New Research Frameworks. Curr. Anthropol. 2008, 49, 627–654. [Google Scholar] [CrossRef]
- Sueur, C.; King, A.J.; Conradt, L.; Kerth, G.; Lusseau, D.; Mettke-Hofmann, C.; Schaffner, C.M.; Williams, L.; Zinner, D.; Aureli, F. Collective decision-making and fission–fusion dynamics: A conceptual framework. Oikos 2011, 120, 1608–1617. [Google Scholar] [CrossRef]
- McComb, K.; Reby, D. Vocal communication networks in large terrestrial mammals. In Animal Communication Networks; McGregor, P.K., Ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 372–389. [Google Scholar]
- Taylor, A.M.; Reby, D. The contribution of source–filter theory to mammal vocal communication research. J. Zool. 2010, 280, 221–236. [Google Scholar] [CrossRef]
- Ryan, M.J.; Kime, N.M. Selection on Long-Distance Acoustic Signals. In Acoustic Communication; Simmons, A.M., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2003; pp. 225–274. [Google Scholar]
- Naguib, M.; Wiley, R.H. Estimating the distance to a source of sound: Mechanisms and adaptations for long-range communication. Anim. Behav. 2001, 62, 825–837. [Google Scholar] [CrossRef]
- Wiley, R.H.; Richards, D.G. Physical Constraints on Acoustic Communication in the Atmosphere: Implications for the Evolution of Animal Vocalizations. Behav. Ecol. Sociobiol. 1978, 3, 69–94. [Google Scholar] [CrossRef]
- Marten, K.; Marler, P. Sound Transmission and Its Significance for Animal Vocalization: I. Temperate Habitats. Behav. Ecol. Sociobiol. 1977, 2, 271–290. [Google Scholar] [CrossRef]
- Forrest, T.G. From sender to receiver: Propagation and environmental effects on acoustic signals. Am. Zool. 1994, 34, 644–654. [Google Scholar] [CrossRef]
- Piercy, J.E.; Embleton, T.F.W. Review of noise propagation in the atmosphere. J. Acoust. Soc. Am. 1977, 61, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Wiley, R.D.; Richards, D.C. Adaptation for acoustic communication in birds: Sound transmission and signal detection. In Acoustic Communication in Birds; Kroodsma, D.E., Miller, E.H., Quellet, H., Eds.; Academic Press: Cambridge, UK, 1982; pp. 131–181. [Google Scholar]
- Marten, K.; Quine, D.; Marler, P. Sound Transmission and Its Significance for Animal Vocalization: II. Tropical Forest Habitats. Behav. Ecol. Sociobiol. 1977, 2, 291–302. [Google Scholar] [CrossRef]
- Ey, E.; Rahn, C.; Hammerschmidt, K.; Fischer, J. Wild Female Olive Baboons Adapt their Grunt Vocalizations to Environmental Conditions. Ethology 2009, 115, 493–503. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Ellers, J.; Smith, T.B. Birdsong and Sound Transmission: The Benefits of Reverberations. Condor 2002, 104, 564–573. [Google Scholar] [CrossRef]
- Nemeth, E.; Dabelsteen, T.; Pedersen, S.B.; Winkler, H. Rainforests as concert halls for birds: Are reverberations improving sound transmission of long song elements? J. Acoust. Soc. Am. 2006, 119, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Attenborough, K. Sound propagation close to the ground. Annu. Rev. Fluid Mech. 2002, 34, 51–82. [Google Scholar] [CrossRef]
- Brumm, H.; Naguib, M. Chapter 1 Environmental Acoustics and the Evolution of Bird Song. In Advances in the Study of Behavior; Academic Press: Cambridge, UK, 2009; Volume 40, pp. 1–33. [Google Scholar]
- Richards, D.G. Estimation of Distance of Singing Conspecifics by the Carolina Wren. Auk 1981, 98, 127–133. [Google Scholar]
- Morton, E.S. Grading, Discreteness, Redundancy, and Motivation-Structural Rules. In Acoustic Communication in Birds; Kroodsma, D.E., Miller, E.H., Eds.; Academic Press: San Diego, CA, USA, 1982; pp. 183–212. [Google Scholar]
- Ringler, M.; Szipl, G.; Hödl, W.; Khil, L.; Kofler, B.; Lonauer, M.; Provin, C.; Ringler, E. Acoustic ranging in poison frogs—it is not about signal amplitude alone. Behav. Ecol. Sociobiol. 2017, 71, 114. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S.; Galimberti, F. Source level of male vocalizations in the genus Mirounga: Repeatability and correlates. Bioacoustics 2003, 14, 47–59. [Google Scholar] [CrossRef]
- Wyman, M.T.; Mooring, M.S.; McCowan, B.; Penedo, M.C.T.; Hart, L.A. Amplitude of bison bellows reflects male quality, physical condition and motivation. Anim. Behav. 2008, 76, 1625–1639. [Google Scholar] [CrossRef]
- Waser, P.M.; Brown, C.H. Habitat acoustics and primate communication. Am. J. Primatol. 1986, 10, 135–154. [Google Scholar] [CrossRef]
- Morton, E.S. Ecological Sources of Selection on Avian Sounds. Am. Naturalist 1975, 109, 17–34. [Google Scholar] [CrossRef]
- Fischer, J.; Hammerschmidt, K.; Cheney, D.L.; Seyfarth, R.M. Acoustic features of male baboon loud calls: Influences of context, age, and individuality. J. Acoust. Soc. Am. 2002, 111, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Mitani, J.C.; Nishida, T. Contexts and social correlates of long-distance calling by male chimpanzees. Anim. Behav. 1993, 45, 735–746. [Google Scholar] [CrossRef]
- Gersick, A.S.; Cheney, D.L.; Schneider, J.M.; Seyfarth, R.M.; Holekamp, K.E. Long-distance communication facilitates cooperation among wild spotted hyaenas, Crocuta crocuta. Anim. Behav. 2015, 103, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.H.; Payne, K.; Langbauer, W.R.; Moss, C.J. The social contexts of some very low frequency calls of African elephants. Behav. Ecol. Sociobiol. 1988, 22, 385–392. [Google Scholar] [CrossRef]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W.M. The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef]
- Moss, C.J.; Poole, J.H. Relationships and social structure of African elephants. In Primate Social Relations: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific Publication: Oxford, UK, 1983; pp. 315–325. [Google Scholar]
- O’Connell-Rodwell, C.E.; Wood, J.D.; Wyman, M.; Redfield, S.; Puria, S.; Hart, L.A. Antiphonal vocal bouts associated with departures in free-ranging African elephant family groups (Loxodonta africana). Bioacoustics 2012, 21, 215–224. [Google Scholar] [CrossRef]
- Soltis, J. Vocal communication in African Elephants (Loxodonta africana). Zoo Biol. 2010, 29, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Langbauer, W.R.; Payne, K.B.; Charif, R.A.; Rapaport, L.; Osborn, F. African Elephants Respond to Distant Playbacks of Low-Frequency Conspecific Calls. J. Exp. Biol. 1991, 157, 35–46. [Google Scholar]
- Poole, J.H. Behavioral contexts of elephant acoustic communication. In The Amboseli Elephants: A Long-Term Perspective on A Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; The University of Chicago: Chicago, IL, USA, 2011; pp. 125–161. [Google Scholar]
- Stoeger, A.S.; Zeppelzauer, M.; Baotic, A. Age-group estimation in free-ranging African elephants based on acoustic cues of low-frequency rumbles. Bioacoustics 2014, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Soltis, J.; Blowers, T.E.; Savage, A. Measuring positive and negative affect in the voiced sounds of African elephants (Loxodonta africana). J. Acoust. Soc. Am. 2011, 129, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, A.S.; Baotic, A. Information content and acoustic structure of male African elephant social rumbles. Sci. Rep. 2016, 6, 27585. [Google Scholar] [CrossRef] [PubMed]
- Baotic, A.; Stoeger, A.S. Sexual dimorphism in African elephant social rumbles. PLoS ONE 2017, 12, e0177411. [Google Scholar] [CrossRef] [PubMed]
- Leighty, K.A.; Soltis, J.; Wesolek, C.M.; Savage, A. Rumble vocalizations mediate interpartner distance in African elephants, Loxodonta africana. Anim. Behav. 2008, 76, 1601–1608. [Google Scholar] [CrossRef]
- Titze, I.R. Principles of Voice Production; Prentice Hall: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- Titze, I.R. Vocal Fold Mass Is Not A Useful Quantity for Describing F0 in Vocalization. J. Speech Lang. Hear. Res. 2011, 54, 520–522. [Google Scholar] [CrossRef]
- Reby, D.; McComb, K.; Cargnelutti, B.; Darwin, C.; Fitch, W.T.; Clutton-Brock, T. Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 272, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.; Ellis, W.H.; Larkin, R.; Tecumseh Fitch, W. Perception of size-related formant information in male koalas (Phascolarctos cinereus). Anim. Cogn. 2012, 15, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Reby, D.; McComb, K. Size communication in domestic dog, Canis familiaris, growls. Anim. Behav. 2010, 79, 205–210. [Google Scholar] [CrossRef]
- Fitch, W.T. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 1997, 102, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.; Hauser, M. Unpacking “Honesty”: Vertebrate Vocal Production and the Evolution of Acoustic Signals. In Acoustic Communication; Simmons, A., Fay, R., Popper, A., Eds.; Springer: New York, NY, USA, 2003; Volume 16, pp. 65–137. [Google Scholar]
- Charlton, B.D.; Reby, D. The evolution of acoustic size exaggeration in terrestrial mammals. Nat. Commun. 2016, 7, 12739. [Google Scholar] [CrossRef] [PubMed]
- Herbst, C.T.; Švec, J.G.; Lohscheller, J.; Frey, R.; Gumpenberger, M.; Stoeger, A.S.; Fitch, W.T. Complex vibratory patterns in an elephant larynx. J. Exp. Biol. 2013, 216, 4054–4064. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, A.S.; Heilmann, G.; Zeppelzauer, M.; Ganswindt, A.; Hensman, S.; Charlton, B.D. Visualizing Sound Emission of Elephant Vocalizations: Evidence for Two Rumble Production Types. PLoS ONE 2012, 7, e48907. [Google Scholar] [CrossRef] [PubMed]
- Low, A.B.; Rebelo, A.G. Vegetation of South Africa, Lesotho and Swaziland: A Companion to the Vegetation Map of South Africa, Lesotho and Swaziland; Department of Environmental Affairs and Tourism: Pretoria, South Africa, 1998.
- Whitehouse, A.M.; Irwin, P.R. A field Guide to the Addo Elephants; Rhodes University, Environmental Education Unit: Port Elizabeth, South Africa, 2002. [Google Scholar]
- Whitehouse, A.M.; Schoeman, D.S. Ranging behaviour of elephants within a small, fenced area in Addo Elephant National Park, South Africa. Afr. Zool. 2003, 38, 95–108. [Google Scholar] [CrossRef]
- Garstang, M. Long-distance, low-frequency elephant communication. J. Comp. Physiol. A 2004, 190, 791–805. [Google Scholar] [CrossRef] [PubMed]
- McComb, K.; Reby, D.; Baker, L.; Moss, C.; Sayialel, S. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 2003, 65, 317–329. [Google Scholar] [CrossRef]
- Soltis, J.; Leighty, K.A.; Wesolek, C.M.; Savage, A. The Expression of Affect in African Elephant (Loxodonta africana) Rumble Vocalizations. J. Comp. Psychol. 2009, 123, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Soltis, J.; King, L.E.; Douglas-Hamilton, I.; Vollrath, F.; Savage, A. African Elephant Alarm Calls Distinguish between Threats from Humans and Bees. PLoS ONE 2014, 9, e89403. [Google Scholar] [CrossRef] [PubMed]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 6.0.15. 2014. Available online: http://www.praat.org/ (accessed on 25 March 2016).
- AppicDesign. GPS Tour [Mobile Application Software]. Version 2.0. 2016. Available online: http://itunes.apple.com (accessed on 24 March 2016).
- UNAVCO. Geoid Height Calculator. 2018. Available online: https://www.unavco.org/software/geodetic-utilities/geoid-height-calculator/geoid-height-calculator.html (accessed on 26 March 2016).
- Noll, A.; White, J. S_Tools-STx Online Manual. Available online: https://www.kfs.oeaw.ac.at/stx/docs/wiki/index.php (accessed on 21 March 2017).
- Michelsen, A. Sound Reception in Different Environments. In Sensory Ecology: Review and Perspectives; Ali, M.A., Ed.; Springer: Boston, MA, USA, 1978; pp. 345–373. [Google Scholar]
- Cohen, J. Multiple Regression and Correlation Analysis. In Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA; Routledge: Abingdon, UK, 1988; pp. 407–466. [Google Scholar]
- SPSS-Inc. IBM SPSS Statistics v.23; SPSS Inc.: Chicago, IL, USA, 2015. [Google Scholar]
- Brumm, H.; Slabbekoorn, H. Acoustic Communication in Noise. In Advances in the Study of Behavior; Academic Press: Cambridge, UK, 2005; Volume 35, pp. 151–209. [Google Scholar]
- Schopf, C.; Zimmermann, E.; Tünsmeyer, J.; Kästner, S.B.R.; Hubka, P.; Kral, A. Hearing and Age-Related Changes in the Gray Mouse Lemur. J. Assoc. Res. Otolaryngol. 2014, 15, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, F.A.; Mills, J.H.; Norton, B.L. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hear. Res. 1993, 71, 137–145. [Google Scholar] [CrossRef]
- Boettcher, F.A.; Mills, J.H.; Norton, B.L.; Schmiedt, R.A. Age-related changes in auditory evoked potentials of gerbils. II. Response latencies. Hear. Res. 1993, 71, 146–156. [Google Scholar] [CrossRef]
- Boettcher, F.A.; White, D.R.; Mills, J.H.; Schmiedt, B.N. Age-related changes in auditory evoked potentials of gerbils. III. Low-frequency responses and repetition rate effects. Hear. Res. 1995, 87, 208–219. [Google Scholar] [CrossRef]
- Torre, P.; Fowler, C.G. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta). Hear. Res. 2000, 142, 131–140. [Google Scholar] [CrossRef]
- Poole, J.H. Signals and assessment in African elephants: Evidence from playback experiments. Anim. Behav. 1999, 58, 185–193. [Google Scholar] [CrossRef] [PubMed]
- McComb, K.; Moss, C.; Sayialel, S.; Baker, L. Unusually extensive networks of vocal recognition in African elephants. Anim. Behav. 2000, 59, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, A.S.; Baotic, A. Male African elephants discriminate and prefer vocalizations of unfamiliar females. Sci. Rep. 2017, 7, 46414. [Google Scholar] [CrossRef] [PubMed]
- Soltis, J.; Leong, K.; Savage, A. African elephant vocal communication II: Rumble variation reflects the individual identity and emotional state of callers. Anim. Behav. 2005, 70, 589–599. [Google Scholar] [CrossRef]
- Hedwig, D.; DeBellis, M.; Wrege, P.H. Not so far: Attenuation of low-frequency vocalizations in a rainforest environment suggests limited acoustic mediation of social interaction in African forest elephants. Behav. Ecol. Sociobiol. 2018, 72, 33. [Google Scholar] [CrossRef]
- Charlton, B.D.; Whisson, D.A.; Reby, D. Free-Ranging Male Koalas Use Size-Related Variation in Formant Frequencies to Assess Rival Males. PLoS ONE 2013, 8, e70279. [Google Scholar] [CrossRef] [PubMed]
- Riede, T.; Fitch, T. Vocal tract length and acoustics of vocalization in the domestic dog (Canis familiaris). J. Exp. Biol. 1999, 202, 2859–2867. [Google Scholar] [PubMed]
- Charlton, B.D.; Zhihe, Z.; Snyder, R.J. Giant pandas perceive and attend to formant frequency variation in male bleats. Anim. Behav. 2010, 79, 1221–1227. [Google Scholar] [CrossRef]
- Reby, D.; McComb, K. Anatomical constraints generate honesty: Acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 2003, 65, 519–530. [Google Scholar] [CrossRef]
- Sinnott, J.M.; Brown, C.H.; Malik, W.T.; Kressley, R.A. A multidimensional scaling analysis of vowel discrimination in humans and monkeys. Percept. Psychophys. 1997, 59, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Garstang, M.; Larom, D.; Raspet, R.; Lindeque, M. Atmospheric controls on elephant communication. J. Exp. Biol. 1995, 198, 939–951. [Google Scholar] [PubMed]






| Location, Year of Data Collection | Individual | Age (Years) | Approx. Shoulder Height (m) |
|---|---|---|---|
| Pilanesberg, 2014 | Mike | ∼29 | 3.20 m |
| Bela Bela, 2014 | Chishuru | ∼18 | 2.40 m |
| Hazyview, 2014 | Medwa | ∼19 | 2.60 m |
| Addo Elephant Back Safaries, 2016 | Thaba | ∼31 | 3.25 m 1 |
| Habitat | Distance (m) | Acoustic Features | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formant 1 (Hz) | Formant 2 (Hz) | f0 mean (Hz) | Duration (s) | |||||||||||||||||
| N | TS | U | Z | P | N | TS | U | Z | P | N | TS | U | Z | P | N | U | Z | P | ||
| Dense | 50 | 36 | 100 | - | - | - | 36 | 100 | - | - | - | 36 | 100 | - | - | - | 36 | - | - | - |
| 50–100 | 36 | 100 | 636 | −0.135 | 0.892 | 36 | 100 | 646 | −0.028 | 0.978 | 36 | 100 | 645 | −0.039 | 0.969 | 36 | 622 | −0.298 | 0.765 | |
| 50–200 | 36 | 100 | 629 | −0.220 | 0.826 | 28 | 78 | 454 | −0.683 | 0.494 | 31 | 86 | 542 | −0.201 | 0.840 | 36 | 604 | −0.501 | 0.616 | |
| 50–400 | 34 | 94 | 587 | −0.300 | 0.764 | 21 | 58 | 356 | −0.364 | 0.716 | 24 | 67 | 364 | −1.027 | 0.305 | 36 | 557 | −1.031 | 0.303 | |
| 50–800 | 29 | 81 | 501 | −0.277 | 0.782 | 8 | 22 | 120 | −0.730 | 0.465 | 7 | 19 | 72 | −1.778 | 0.075 | 32 | 376 | −2.464 | 0.014 | |
| 50–1000 | 32 | 89 | 556 | −0.252 | 0.801 | 6 | 17 | 72 | −1.294 | 0.196 | 8 | 22 | 129 | −0.442 | 0.659 | 36 | 442 | −2.320 | 0.020 | |
| 50–1500 | 32 | 89 | 561 | −0.184 | 0.854 | 14 | 39 | 200 | −1.124 | 0.261 | 7 | 19 | 117 | −0.313 | 0.754 | 36 | 432 | −2.433 | 0.015 | |
| Open | 50 | 36 | 100 | - | - | - | 36 | 100 | - | - | - | 36 | 100 | - | - | - | 36 | - | - | - |
| 50–100 | 36 | 100 | 642 | −0.068 | 0.946 | 36 | 100 | 637 | −0.130 | 0.897 | 36 | 100 | 623 | −0.282 | 0.778 | 36 | 621 | −0.304 | 0.761 | |
| 50–200 | 36 | 100 | 608 | −0.451 | 0.652 | 36 | 100 | 646 | −0.023 | 0.982 | 36 | 100 | 645 | −0.039 | 0.969 | 36 | 644 | −0.051 | 0.960 | |
| 50–400 | 36 | 100 | 610 | −0.434 | 0.665 | 34 | 94 | 588 | −0.282 | 0.778 | 27 | 75 | 411 | −1.042 | 0.297 | 36 | 613 | −0.400 | 0.689 | |
| 50–800 | 27 | 75 | 450 | −0.500 | 0.617 | 10 | 28 | 118 | −1.665 | 0.096 | 5 | 14 | 62 | −1.117 | 0.264 | 34 | 435 | −2.086 | 0.037 | |
| 50–1000 | 28 | 78 | 453 | −0.697 | 0.486 | 4 | 11 | 26 | −2.074 | 0.038 | 2 | 6 | 36 | 0.000 | 1.000 | 31 | 350 | −2.616 | 0.009 | |
| 50–1500 | 17 | 47 | 298 | −0.162 | 0.871 | 3 | 8 | 30 | −1.265 | 0.206 | 3 | 8. | 45 | −0.475 | 0.635 | 21 | 138 | −3.979 | 0.000 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baotic, A.; Garcia, M.; Boeckle, M.; Stoeger, A. Field Propagation Experiments of Male African Savanna Elephant Rumbles: A Focus on the Transmission of Formant Frequencies. Animals 2018, 8, 167. https://doi.org/10.3390/ani8100167
Baotic A, Garcia M, Boeckle M, Stoeger A. Field Propagation Experiments of Male African Savanna Elephant Rumbles: A Focus on the Transmission of Formant Frequencies. Animals. 2018; 8(10):167. https://doi.org/10.3390/ani8100167
Chicago/Turabian StyleBaotic, Anton, Maxime Garcia, Markus Boeckle, and Angela Stoeger. 2018. "Field Propagation Experiments of Male African Savanna Elephant Rumbles: A Focus on the Transmission of Formant Frequencies" Animals 8, no. 10: 167. https://doi.org/10.3390/ani8100167
APA StyleBaotic, A., Garcia, M., Boeckle, M., & Stoeger, A. (2018). Field Propagation Experiments of Male African Savanna Elephant Rumbles: A Focus on the Transmission of Formant Frequencies. Animals, 8(10), 167. https://doi.org/10.3390/ani8100167





