Pleurotus Ostreatus and Volvariella Volvacea Can Enhance the Quality of Purple Field Corn Stover and Modulate Ruminal Fermentation and Feed Utilization in Tropical Beef Cattle
Abstract
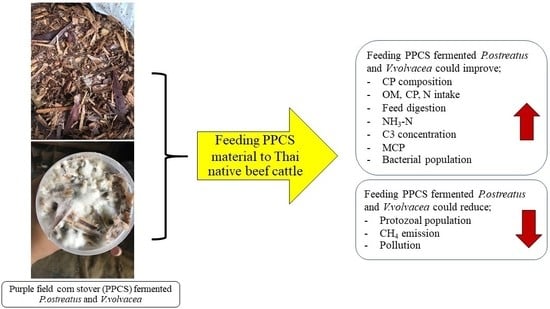
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Care
2.2. Animal and Treatments
2.3. Preparation of Rice Straw, Purple Field Corn Residue, and Fungal-Treated Substrates
2.4. Feed, Feces, Urine, Rumen Fluid, and Blood Sample Collections
2.5. Measurement of Monomeric Anthocyanin Content (MAC)
2.6. Measurement of Lovastatin Content
2.7. Assay of Statistic
3. Results and Discussion
3.1. Nutrient Content of Feeds
3.2. Feed Utilization and Nutrient Digestion
3.3. Ruminal Characteristics, Microbial Count and Blood Metabolite
3.4. Volatile Fatty Acid Profile and CH4 Production
3.5. Nitrogen Balances and Microbial Protein Synthesis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Khampasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic variation in anthocyanins, phenolic compounds, and antioxidant activity in cob and husk of purple field corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Chumpawadee, S.; Ban, C.; Thongpea, S. Short communication: Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J. Dairy. Sci. 2019, 102, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.Z.; Xin, H.; Paengkoum, P.; Paengkoum, S.; Ban, C.; Sorasak, T. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J. Anim. Sci. 2018, 97, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochem 2010, 71, 1198–1222. [Google Scholar] [CrossRef] [PubMed]
- Supapong, C.; Cherdthong, A.; Seankamsorn, A.; Khonkhaeng, B.; Wanapat, M.; Uriyapongson, S.; Gunun, N.; Chanjula, P.; Polyorac, S. Effect of Delonix regia seed meal supplementation in Thai native beef cattle on feed intake, rumen fermentation characteristics and methane production. Anim. Feed. Sci. Technol. 2017, 26, 123–130. [Google Scholar] [CrossRef]
- Chanjula, P.; Petcharat, V.; Cherdthong, A. Effects of fungal (Lentinussajor-caju) treated oil palm frond on performance and carcass characteristics in finishing goats. Asian-Aust. J. Anim. Sci. 2017, 30, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Sarnklong, C.; Cone, J.W.; Pellikaan, W.; Hendriks, W.H. Utilization of rice straw and different treatments to improve its feed value for ruminants: A review. Asian-Aust. J. Anim. Sci. 2010, 23, 680–692. [Google Scholar] [CrossRef]
- Moghazy, M.M.; El-Fadaly, H.M.; Tag, E.; Areda, H.A. Effect of sheep diets containing microbiological treated rice straw on blood parameters and nitrogen balance. J. Microbiol. Res. 2015, 2, 46–56. [Google Scholar]
- Fazaeli, H.; Jelan, Z.A.; Azizi, A.; Liang, J.B.; Mahmodzadeh, H.; Osman, A. Effects of fungal treatment on the nutritive value of wheat straw. Malaysian. J. Anim. Sci. 2002, 7, 61–71. [Google Scholar]
- Gottlieb, K.; Wacher, V.; Sliman, J.; Pimental, M. Review article: Inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment. Pharmacol. Ther. 2016, 43, 197–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khonkhaeng, B.; Cherdthong, A. Improving nutritive value of purple field corn residue by culturing with white-rot fungi. In Proceedings of the TSRI Congress 2019 Disruptive Technology for World Society, Royal Paragon Hall 3, Bangkok, Thailand, 9 August 2019. [Google Scholar]
- Candyrine, S.C.L.; Mahadzir, M.F.; Garba, S.; Jahromi, M.F.; Ebrahimi, M.; Goh, Y.M.; Samsudin, A.A.; Sazili, A.Q.; Chen, W.L.; Ganesh, S.; et al. Effects of naturally-produced lovastatin on feed digestibility, rumen fermentation, microbiota and methane emissions in goats over a 12-week treatment period. PLoS ONE 2018, 7, e0199840. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.F.; Liang, J.B.; Mohamad, R.; G1oh, Y.M.; Shokryazdan, P.; Ho, Y.W. Lovastatin enriched rice straw enhances biomass quality and suppresses ruminal methanogenesis. BioMed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 19th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 10, 3583–3597. [Google Scholar] [CrossRef]
- Samuel, M.; Sagathewan, S.; Thomas, J.; Mathen, G. An HPLC method for estimation of volatile fatty acids of ruminal fluid. Indian J. Anim. Sci. 1997, 69, 805–807. [Google Scholar]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Galyean, M. Laboratory Procedure in Animal Nutrition Research; Department of Animal and Range Sciences, New Mexico State University: Las Cruces, NM, USA, 1989. [Google Scholar]
- Crocker, C.L. Rapid determination of urea nitrogen in serum or plasma without deproteinization. American. J. Med. Tech. 1967, 33, 361–365. [Google Scholar]
- Chen, X.B.; Gomes, M.J. Estimation of Microbial Protein Supply to Sheep and Cattle Based on Urinary Excretion of Purine Derivative-an Overview of the Technique Details; Occasional Publication 1992; International Feed Resources Unit, Rowett Research Institute: Aberdeen, UK, 1995. [Google Scholar]
- Cherdthong, A.; Wanapat, M.; Wachirapakorn, C. Influence of urea-calcium mixtures as rumen slow-release feed on in vitro fermentation using gas production technique. Ach. Anim. Nutr. 2011, 6, 242–254. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC. Int. 2005, 88, 1269–1278. [Google Scholar]
- Pattanagul, P.; Pinthong, R.; Phianmongkhol, A.; Tharatha, S. Mevalonin, citrinin and pigments of adley angkak fermented by Manascus sp. Int. J. Food Microbiol. 2008, 126, 20–23. [Google Scholar] [CrossRef]
- SAS. User’s guide: Statistics, Version 9.2 Edition; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Min, D.Y.; Chang, H.M.; Jameel, H.; Lucia, L.; Wang, Z.G.; Jin, Y.C. The structure of lignin of corn stover and its changes induced by mild sodium hydroxide treatment. BioResources 2014, 9, 2405–2414. [Google Scholar] [CrossRef] [Green Version]
- Roobha, J.J.; Saravanakumar, M.; Aravindhan, K.M.; Devi, P.S. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res. Plant. Biol. 2011, 1, 5–12. [Google Scholar]
- Francis, F. Food colourants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Kavitha, V.; Janani, B.; Angayarkanni, J. Optimization of process parameters for lovastatin production from red gram bran by solid state fermentation. Int. J. Sci. Res. 2012, 3, 1413–1418. [Google Scholar]
- Lai, L.S.T.; Tsai, T.H.; Wang, T.C.; Cheng, T.Y. The influence of culturing environments on lovastatin production by Aspergillus terreus in submerged cultures. Enzym. Microb. Technol. 2005, 36, 737–748. [Google Scholar] [CrossRef]
- Osman, M.E.; Khattab, O.H.; Zaghlol, G.M.; El-Hameed, R.M.A. Optimization of some physical and chemical factors for lovastatin productivity by local strain of Aspergillus terreus. Aust. J. Basic Appl. Sci. 2011, 5, 718–732. [Google Scholar]
- Li, S.W.; Li, M.; Song, H.P.; Feng, J.L.; Tai, X.S. Induction of a high-yield lovastatin mutant of Aspergillus terreus by 12c6+ heavy-ion beam irradiation and the influence of culture conditions on lovastatin production under submerged fermentation. Appl. Biochem. Biotechnol. 2011, 165, 913–925. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; SernaSaldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2011, 152, 46–55. [Google Scholar] [CrossRef]
- Hosoda, K.; Miyaji, M.; Matsuyama, H.; Haga, S.; Ishizaki, H.; Nonaka, K. Effect of supplementation of purple pigment from anthocyanin-rich corn (Zea mays L.) on blood antioxidant activity and oxidation resistance in sheep. Livest. Sci. 2012, 145, 266–270. [Google Scholar] [CrossRef]
- Cherdthong, A.; Prachumchai, R.; Wanapat, M.; Foiklang, S.; Chanjula, P. Effects of supplementation with royal poinciana seed meal (Delonix regia) on ruminal fermentation pattern, microbial protein synthesis, blood metabolites and mitigation of methane emissions in native Thai beef cattle. Animals 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Safe 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Südekum, K.H.; Brusemeister, F.; Schroder, A.; Stangassinger, M. Effects of amount of intake and stage of forage maturity on urinary allantoin excretion and estimated microbial crude protein synthesis in the rumen of steers. J. Anim. Physiol. Anim. Nutr. 2006, 90, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Khonkhaeng, B.; Seankamsorn, A.; Supapong, C.; Wanapat, M.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. Effects of feeding fresh cassava root with high-sulfur feed block on feed utilization, rumen fermentation, and blood metabolites in Thai native cattle. Trop. Anim. Health Prod. 2018, 50, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Wanapat, M. In vitro gas production in rumen fluid of buffalo as affected by urea-calcium mixture in highquality feed block. Anim. Sci. J. 2014, 85, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Etter, R.P.; Ropp, J.K.; Grandeen, K.L. Effect of dietary crude protein level and degradability on ruminal fermentation and nitrogen utilization in lactating dairy cows. J. Anim. Sci. 2004, 82, 3219–3229. [Google Scholar] [CrossRef] [Green Version]
- Cieślak, A.; Zmora, P.; Matkowski, A.; Nawrot-Hadzik, I.; Pers-Kamczyc, E.; El-Sherbiny, M.; Bryszak, M.; Szumacher-Strabe, M. Tannins from Sanguisorba officinalis affect in vitro rumen methane production and fermentation. J. Anim. Plant. Sci. 2016, 26, 54–62. [Google Scholar]
- Ndjonka, D.; Abladam, E.; Djafsia, B.; Ajonina-Ekoti, I.; Achukwi, M.; Liebau, E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J. Helminthol. 2014, 88, 481–488. [Google Scholar] [CrossRef]
- Newbold, C.J.; Lopez, S.; Nelson, N.; Ouda, J.O.; Wallace, R.J.; Moss, A.R. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br. J. Nutr. 2005, 94, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Pino, F.; Mitchell, L.K.; Jones, C.M.; Heinrichs, A.J. Comparison of diet digestibility, rumen fermentation, rumen rate of passage, and feed efficiency in dairy heifers fed ad-libitum versus precision diets with low and high quality forages. J. Appl. Anim. Res. 2018, 46, 1296–1306. [Google Scholar] [CrossRef]
- Moran, J. Feeding management for small holder dairy farmers in the humid tropics. In Tropical Dairy Farming; Landlinks Press: Collingwood, VIC, Australia, 2005; 312p. [Google Scholar]
- Varga, M. Chapter 1—Rabbit Basic Science. In Textbook of Rabbit Medicine, 2nd ed.; Butterworth Heinemann Publisher: Oxford, UK, 2014; pp. 3–108. [Google Scholar]
- Patra, A.K.; Yu, Z. Effects of vanillin, quillaja saponin, and essential oils on in vitro fermentation and protein-degrading microorganisms of the rumen. Appl. Microbiol. Biotechnol. 2014, 98, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.; Mushegian, A. Biosynthesis of isoprenoids via mevalonate in Archaea: The lost pathway. Genom. Res. 2016, 10, 1468–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, B.S.; Hansen, E.E.; Manchester, J.K.; Coutinho, P.M.; Henrissat, B.; Fulton, R.; Latreille, P.; Kim, K.; Wilson, R.K.; Gordon, J.I. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. USA 2007, 104, 10643–10648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belanche, A.; de la Fuente, G.; Newbold, C.J. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014, 90, 663–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenchel, T.; Finley, B.J. The diversity of microbes: Resurgence of the phenotype. Philos. Trans. R. Soc. B 2006, 361, 1965–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushida, K.; Newbold, C.J.; Jouany, J.P. Interspecies hydrogen transfer between the rumen ciliate Polyplastron multivesiculatum and Methanosarcina barkeri. J. Gen. Appl. Microbiol. 1997, 43, 129–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutrignelli, M.I.; Piccolo, G.; D’Urso, S.; Calabrò, S.; Bovera, F.; Tudisco, R.; Infascelli, F. Urinary excreti on of purine derivatives in dry buffalo and Fresian cows. Ital. J. Anim. Sci. 2007, 6 (Suppl. 2), 563–566. [Google Scholar] [CrossRef] [Green Version]
- Jardstedt, M.; Hessle, A.; Nørgaard, P.; Richardt, W.; Nadeau, E. Feed intake and urinary excretion of nitrogen and purine derivatives in pregnant suckler cows fed alternative roughage-based diets. Livest. Sci. 2017, 202, 82–88. [Google Scholar] [CrossRef]
- Anantasook, N.; Wanapat, M.; Gunun, P.; Cherdthong, A. Reducing methane production by supplementation of Terminalia chebula RETZ. containing tannins and saponins. Anim. Sci. J. 2016, 87, 783–790. [Google Scholar] [CrossRef]
- Williams, A.G.; Coleman, G.S. The Rumen Protozoa; Springer-Verlag New York Inc.: New York, NY, USA, 1992. [Google Scholar]
- Belanche, A.; de la Fuente, G.; Newbold, C.J. Effect of progressive inoculation of fauna-free sheep with holotrich protozoa and total-fauna on rumen fermentation, microbial diversity and methane emissions. FEMS. Microbiol. Ecol. 2015, 362, 1–10. [Google Scholar] [CrossRef]
| Parameters | Concentrate | Before fermented | After fermented | ||||
|---|---|---|---|---|---|---|---|
| RS | PPCS | RS | PPCS | ||||
| P. ostreatus | V. volvacea | P. ostreatus | V. volvacea | ||||
| Ingredients, (%DM) | |||||||
| Cassava chip | 55.00 | ||||||
| Rice bran | 11.00 | ||||||
| Palm kernel meal, solvent | 13.50 | ||||||
| Coconut kernel meal, solvent | 12.90 | ||||||
| Molasses, liquid | 2.00 | ||||||
| Urea | 2.60 | ||||||
| Pure sulfur | 1.00 | ||||||
| Mineral premix * | 1.00 | ||||||
| Salt | 1.00 | ||||||
| Chemical composition | |||||||
| Dry matter, % | 87.40 | 90.10 | 94.40 | 55.80 | 56.23 | 55.65 | 56.14 |
| Organic matter, %DM | 94.70 | 91.80 | 96.50 | 91.80 | 91.50 | 95.39 | 94.74 |
| Neutral detergent fiber, %DM | 12.20 | 65.40 | 70.60 | 61.97 | 61.32 | 67.60 | 64.45 |
| Acid detergent fiber, %DM | 8.40 | 40.20 | 53.20 | 37.57 | 37.22 | 42.37 | 43.87 |
| Crude protein, %DM | 14.20 | 2.60 | 4.90 | 6.95 | 7.38 | 9.47 | 9.56 |
| Plant secondary metabolites | |||||||
| Anthocyanin, %DM | - | - | 11.8 | 0.53 | 0.57 | 6.25 | 5.76 |
| Lovastatin, g/kg | - | - | - | 33.92 | 33.96 | 32.49 | 34.90 |
| Parameters | RS | PPCS | SEM | Contrast | ||||
|---|---|---|---|---|---|---|---|---|
| P. ostreatus | V. volvacea | P. ostreatus | V. volvacea | R | WRF | R × WRF | ||
| DM intake | ||||||||
| Roughage | ||||||||
| kg/d | 3.54 | 3.39 | 3.23 | 3.80 | 0.18 | 0.80 | 0.30 | 0.08 |
| g/kg BW0.75 | 87.69 | 83.52 | 79.27 | 92.12 | 5.44 | 0.98 | 0.44 | 0.14 |
| Concentrate | ||||||||
| kg/d | 1.39 | 1.40 | 1.41 | 1.43 | 0.04 | 0.56 | 0.79 | 0.95 |
| g/kg BW0.75 | 34.32 | 34.38 | 34.48 | 34.57 | 0.27 | 0.55 | 0.79 | 0.95 |
| Total | ||||||||
| kg/d | 4.93 | 4.79 | 4.65 | 5.23 | 0.18 | 0.69 | 0.27 | 0.07 |
| g/kg BW0.75 | 122.01 | 117.91 | 113.75 | 126.69 | 5.31 | 0.96 | 0.42 | 0.13 |
| Nutrients intake, kg/d | ||||||||
| Dry matter | 4.93 | 4.79 | 4.65 | 5.23 | 0.18 | 0.69 | 0.27 | 0.07 |
| Organic matter | 4.55 a | 4.21 a | 4.73 b | 4.90 b | 0.17 | 0.02 | 0.64 | 0.17 |
| Crude protein | 0.39 a | 0.38 a | 0.49 b | 0.51 b | 0.02 | <0.01 | 0.75 | 0.52 |
| Neutral detergent fiber | 2.91 | 2.65 | 3.14 | 3.13 | 0.20 | 0.11 | 0.53 | 0.56 |
| Acid detergent fiber | 1.82 a | 1.66 a | 2.01 b | 2.13 b | 0.14 | 0.03 | 0.91 | 0.33 |
| Digestibility coefficients, % | ||||||||
| Dry matter | 60.54 a | 60.20 a | 64.85 b | 65.16 b | 0.36 | <0.01 | 0.96 | 0.39 |
| Organic matter | 64.96 a | 63.43 a | 67.93 b | 67.78 b | 0.99 | <0.01 | 0.41 | 0.50 |
| Crude protein | 62.55 a | 61.23 a | 65.21 b | 65.36 b | 0.88 | <0.01 | 0.52 | 0.42 |
| Neutral detergent fiber | 41.43 a | 42.42 a | 47.81 b | 46.56 b | 0.80 | <0.01 | 0.73 | 0.25 |
| Acid detergent fiber | 37.24 a | 35.94 a | 41.73 b | 41.72 b | 0.91 | <0.01 | 0.74 | 0.29 |
| Parameters | RS | PPCS | SEM | Contrast | ||||
|---|---|---|---|---|---|---|---|---|
| P. osteratus | V. volvacea | P. osteratus | V. volvacea | R | WRF | R × WRF | ||
| Rumen ecology | ||||||||
| Ruminal temperature, °C | ||||||||
| 0 h post feeding | 38.30 | 38.47 | 38.55 | 38.12 | 0.26 | 0.85 | 0.63 | 0.28 |
| 4 h post feeding | 39.33 | 39.36 | 39.26 | 38.86 | 0.28 | 0.34 | 0.52 | 0.46 |
| Mean | 38.82 | 38.91 | 38.91 | 38.49 | 0.24 | 0.52 | 0.53 | 0.32 |
| Ruminal pH | ||||||||
| 0 h post feeding | 7.08 | 7.19 | 7.12 | 6.97 | 0.18 | 0.63 | 0.91 | 0.51 |
| 4 h post feeding | 7.06 | 7.06 | 6.95 | 6.52 | 0.18 | 0.11 | 0.26 | 0.26 |
| Mean | 6.95 | 7.00 | 7.04 | 6.75 | 0.28 | 0.58 | 0.43 | 0.26 |
| Ammonia nitrogen (NH3-N) concentration, mg/dL | ||||||||
| 0 h post feeding | 12.95 | 12.60 | 14.36 | 14.36 | 1.51 | 0.31 | 0.91 | 0.91 |
| 4 h post feeding | 14.71 a | 14.51 a | 16.75 b | 17.60 b | 0.28 | <0.01 | 0.27 | 0.08 |
| Mean | 14.53 | 14.43 | 14.85 | 15.11 | 0.80 | 0.54 | 0.92 | 0.83 |
| Volatile fatty acid profile, mol/100 mol | ||||||||
| Acetic acid | ||||||||
| 0 h post feeding | 53.52 | 53.51 | 55.14 | 56.63 | 2.70 | 0.39 | 0.78 | 0.78 |
| 4 h post feeding | 55.44 a | 55.17 a | 58.70 b | 62.19 b | 2.14 | 0.03 | 0.46 | 0.39 |
| Mean | 54.48 | 54.59 | 56.85 | 54.59 | 2.04 | 0.10 | 0.57 | 0.57 |
| Propionic acid | ||||||||
| 0 h post feeding | 28.51 a | 28.99 a | 33.66 b | 32.09 b | 1.51 | 0.03 | 0.72 | 0.51 |
| 4 h post feeding | 32.59 a | 32.26 a | 36.37 b | 35.47 b | 1.48 | 0.01 | 0.68 | 0.85 |
| Mean | 31.12 a | 30.62 a | 35.01 b | 33.78 b | 1.29 | 0.01 | 0.51 | 0.78 |
| Butyric acid | ||||||||
| 0 h post feeding | 12.26 | 11.10 | 10.14 | 11.00 | 2.00 | 0.59 | 0.94 | 0.62 |
| 4 h post feeding | 12.78 | 12.12 | 10.88 | 12.72 | 2.20 | 0.77 | 0.79 | 0.58 |
| Mean | 12.52 | 11.06 | 10.51 | 10.72 | 1.52 | 0.45 | 0.69 | 0.59 |
| Total VFA | ||||||||
| 0 h post feeding | 98.38 | 96.54 | 101.66 | 103.02 | 3.16 | 0.14 | 0.94 | 0.62 |
| 4 h post feeding | 96.73 a | 96.29 a | 103.99 b | 107.00 b | 1.98 | <0.01 | 0.53 | 0.40 |
| Mean | 98.57 a | 95.69 a | 101.60 b | 102.90 b | 1.81 | 0.01 | 0.66 | 0.27 |
| Blood urea nitrogen, mg/dL | ||||||||
| 0 h post feeding | 16.75 | 15.00 | 13.25 | 14.25 | 1.63 | 0.21 | 0.82 | 0.41 |
| 4 h post feeding | 18.25 | 14.00 | 16.25 | 16.50 | 1.44 | 0.86 | 0.19 | 0.14 |
| Mean | 17.50 | 14.50 | 14.75 | 15.37 | 1.18 | 0.44 | 0.33 | 0.15 |
| Methane estimation *, mM/L | ||||||||
| 0 h post feeding | 20.75 a | 21.05 a | 18.12 b | 18.72 b | 1.09 | 0.04 | 0.68 | 0.89 |
| 4 h post feeding | 23.68 a | 25.76 a | 20.04 b | 19.44 b | 1.17 | <0.01 | 0.54 | 0.27 |
| Mean | 22.21 a | 23.40 a | 19.08 b | 18.87 b | 1.09 | <0.01 | 0.66 | 0.53 |
| Microbial populations, cell/mL | ||||||||
| Bacteria, ×1010 | ||||||||
| 0 h post feeding | 4.27 a | 5.00 a | 8.25 b | 6.53 b | 1.11 | 0.04 | 0.67 | 0.31 |
| 4 h post feeding | 4.87 a | 5.15 a | 9.65 b | 8.84 b | 2.02 | 0.05 | 0.89 | 0.79 |
| Mean | 5.51 a | 5.07 a | 8.65 b | 7.82 b | 1.06 | 0.01 | 0.55 | 0.85 |
| Protozoa, ×106 | ||||||||
| 0 h post feeding | 7.20 | 5.50 | 3.00 | 4.83 | 1.58 | 0.18 | 0.91 | 0.32 |
| 4 h post feeding | 6.62 a | 4.50 a | 3.50 b | 3.96 b | 0.66 | 0.01 | 0.25 | 0.09 |
| Mean | 6.91 a | 5.00 a | 3.25 b | 3.73 b | 0.78 | 0.02 | 0.34 | 0.16 |
| Parameters | Rice Straw | Purple corn stove | SEM | Contrast | ||||
|---|---|---|---|---|---|---|---|---|
| P. osteratus | V. volvacea | P. osteratus | V. volvacea | R | WRF | R × WRF | ||
| N intake (NI), g/d | 62.91 a | 62.77 a | 77.93 b | 81.90 b | 3.84 | <0.01 | 0.62 | 0.60 |
| Total N excretion, g/d | 50.23 a | 51.02 a | 55.56 b | 58.23 b | 0.58 | 0.02 | 0.88 | 0.65 |
| Fecal N excretion, g/d | 15.07 | 15.31 | 16.67 | 17.47 | 1.89 | 0.18 | 0.68 | 0.44 |
| Urinary N excretion, g/d | 35.16 a | 35.71 a | 38.89 b | 40.76 b | 1.35 | 0.01 | 0.58 | 0.71 |
| N absorption, g/d | 47.84 a | 47.46 a | 61.26 b | 64.43 b | 2.36 | <0.01 | 0.83 | 0.36 |
| % of N absorption | 30.10 a | 29.79 a | 47.74 b | 52.77 b | 1.55 | 0.02 | 0.65 | 0.89 |
| N retention, g/d | 12.68 a | 11.75 a | 22.37 b | 23.67 b | 1.23 | 0.03 | 0.33 | 0.47 |
| % of N retention to N intake | 20.16 a | 18.72 a | 28.71 b | 28.90 b | 2.19 | 0.05 | 0.53 | 0.77 |
| PD, mmol/d | ||||||||
| Allantoin excretion | 80.55 | 80.27 | 88.17 | 88.15 | 0.51 | <0.01 | 0.81 | 0.84 |
| Allantoin absorption | 74.62 a | 74.27 a | 82.92 b | 82.77 b | 0.58 | <0.01 | 0.67 | 0.86 |
| Microbial crude protein, g/d | 275.11 a | 274.16 a | 301.14 b | 301.07 b | 3.97 | <0.01 | 0.73 | 0.19 |
| EMPS * | 64.16 a | 64.50 a | 68.20 b | 68.34 b | 0.92 | 0.03 | 0.34 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khonkhaeng, B.; Cherdthong, A. Pleurotus Ostreatus and Volvariella Volvacea Can Enhance the Quality of Purple Field Corn Stover and Modulate Ruminal Fermentation and Feed Utilization in Tropical Beef Cattle. Animals 2019, 9, 1084. https://doi.org/10.3390/ani9121084
Khonkhaeng B, Cherdthong A. Pleurotus Ostreatus and Volvariella Volvacea Can Enhance the Quality of Purple Field Corn Stover and Modulate Ruminal Fermentation and Feed Utilization in Tropical Beef Cattle. Animals. 2019; 9(12):1084. https://doi.org/10.3390/ani9121084
Chicago/Turabian StyleKhonkhaeng, Benjamad, and Anusorn Cherdthong. 2019. "Pleurotus Ostreatus and Volvariella Volvacea Can Enhance the Quality of Purple Field Corn Stover and Modulate Ruminal Fermentation and Feed Utilization in Tropical Beef Cattle" Animals 9, no. 12: 1084. https://doi.org/10.3390/ani9121084