Space Use and Movement of Urban Bobcats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Captures
2.3. GPS Collars and Location Data
2.4. Home Range and Habitat Analyses
2.5. Spatial Proximity and Activity Analysis
3. Results
3.1. Bobcat Captures
3.2. Home Range Analysis
3.3. Habitat Analysis
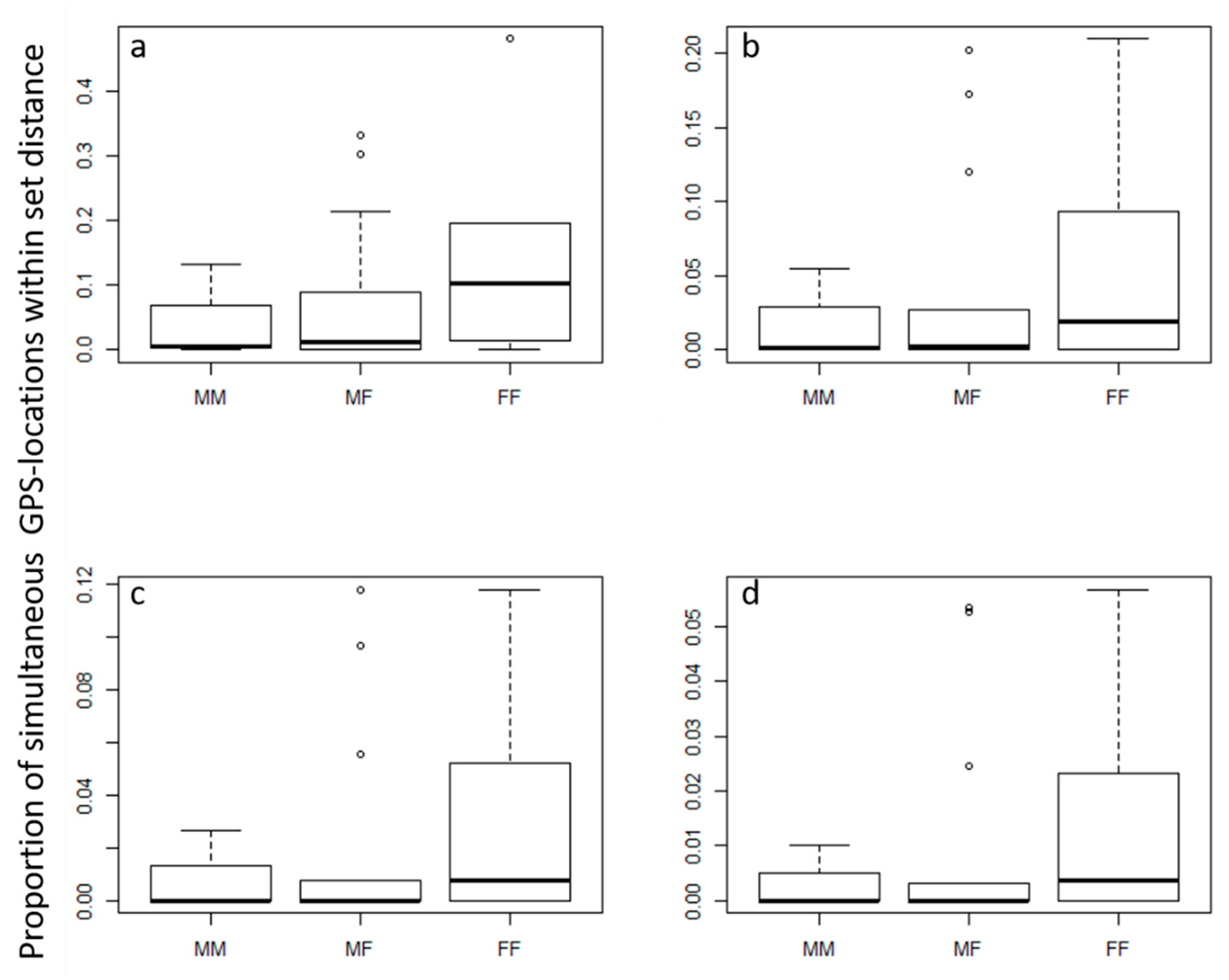
3.4. Spatial Proximity and Activity Analyses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; Rondinini, C.; Boitani, L. Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2642–2651. [Google Scholar] [CrossRef]
- Di Minin, E.; Slotow, R.; Hunter, L.T.B.; Pouzols, F.M.; Toivonen, T.; Verburg, P.H.; Leader-Williams, N.; Petracca, L.; Moilanen, A. Global priorities for national carnivore conservation under land use change. Sci. Rep. 2016, 6, 23814. [Google Scholar] [CrossRef] [Green Version]
- Loveridge, A.J.; Valeix, M.; Chapron, G.; Davidson, Z.; Mtare, G.; Macdonald, D.W. Conservation of large predator populations: Demographic and spatial responses of African lions to the intensity of trophy hunting. Biol. Conserv. 2016, 204, 247–254. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Peres, C.A.; Bogoni, J.A.; Cassano, C.R. Use of agroecosystem matrix habitats by mammalian carnivores (Carnivora): A global-scale analysis. Mamm. Rev. 2018, 48, 312–327. [Google Scholar] [CrossRef]
- Athreya, V.; Odden, M.; Linnell, J.D.; Karanth, K.U. Translocation as a tool for mitigating conflict with leopards in human-dominated landscapes of India. Conserv. Biol. 2011, 25, 133–141. [Google Scholar] [CrossRef]
- Löe, J.; Röskaft, E. Large carnivores and human safety: A review. AMBIO 2004, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Athreya, V.; Odden, M.; Linnell, J.D.; Krishnaswamy, J.; Karanth, U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS ONE 2013, 8, e57872. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Athreya, V.; Grenyer, R.; Macdonald, D.W. Understanding the role of representations of human–leopard conflict in Mumbai through media-content analysis. Conserv. Biol. 2013, 27, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gopalaswamy, A.M.; Karanth, K.U. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J. Mammal. 2010, 91, 1152–1159. [Google Scholar] [CrossRef]
- Abay, G.Y.; Bauer, H.; Gebrihiwot, K.; Deckers, J. Peri-urban spotted hyena (Crocuta crocuta) in northern Ethiopia: Diet, economic impact, and abundance. Eur. J. Wildl. Res. 2011, 57, 759–765. [Google Scholar] [CrossRef]
- Yirga, G.; Ersino, W.; De Iongh, H.H.; Leirs, H.; Gebrehiwot, K.; Deckers, J.; Bauer, H. Spotted hyena (Crocuta crocuta) coexisting at high density with people in Wukro district, northern Ethiopia. Mammal. Biol. 2013, 78, 193–197. [Google Scholar] [CrossRef]
- Boydston, E.E.; Kapheim, K.M.; Watts, H.E.; Szykman, M.; Holekamp, K.E. Altered behaviour in spotted hyenas associated with increased human activity. Anim. Conserv. 2003, 6, 207–219. [Google Scholar] [CrossRef]
- Benson, J.F.; Sikich, J.A.; Riley, S.P. Individual and population level resource selection patterns of mountain lions preying on mule deer along an urban-wildland gradient. PLoS ONE 2016, 11, e0158006. [Google Scholar] [CrossRef]
- Gehrt, S.D.; Brown, J.L.; Anchor, C. Is the urban coyote a misanthropic synanthrope? The case from Chicago. Cities Environ. CATE 2011, 4, 3. [Google Scholar]
- Crooks, K.R. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv. Biol. 2002, 16, 488–502. [Google Scholar] [CrossRef]
- Riley, S.P.; Sauvajot, R.M.; Fuller, T.K.; York, E.C.; Kamradt, D.A.; Bromley, C.; Wayne, R.K. Effects of urbanization and habitat fragmentation on bobcats and coyotes in southern California. Conserv. Biol. 2003, 17, 566–576. [Google Scholar] [CrossRef]
- Lombardi, J.V.; Comer, C.E.; Scognamillo, D.G.; Conway, W.C. Coyote, fox, and bobcat response to anthropogenic and natural landscape features in a small urban area. Urban Ecosyst. 2017, 20, 1239–1248. [Google Scholar] [CrossRef]
- Ruell, E.W.; Riley, S.P.; Douglas, M.R.; Pollinger, J.P.; Crooks, K.R. Estimating bobcat population sizes and densities in a fragmented urban landscape using noninvasive capture–recapture sampling. J. Mammal. 2009, 90, 129–135. [Google Scholar] [CrossRef]
- Tigas, L.A.; Van Vuren, D.H.; Sauvajot, R.M. Behavioral responses of bobcats and coyotes to habitat fragmentation and corridors in an urban environment. Biol. Conserv. 2002, 108, 299–306. [Google Scholar] [CrossRef]
- Young, J.K.; Golla, J.M.; Broman, D.; Blankenship, T.; Heilbrun, R. Estimating density of an elusive carnivore in urban areas: Use of spatially explicit capture-recapture models for city-dwelling bobcats. Urban Ecosyst. 2019, 22, 507–512. [Google Scholar] [CrossRef]
- Šálek, M.; Drahníková, L.; Tkadlec, E. Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mammal. Rev. 2015, 45, 1–14. [Google Scholar] [CrossRef]
- Burt, W.H. Territoriality and home range concepts as applied to mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Davison, J.; Huck, M.; Delahay, R.J.; Roper, T.J. Restricted ranging behaviour in a high-density population of urban badgers. J. Zool. 2009, 277, 45–53. [Google Scholar] [CrossRef]
- Harrison, R.L. A comparison of gray fox ecology between residential and undeveloped rural landscapes. J. Wildl. Manag. 1997, 61, 112–122. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2013, 287, 1–23. [Google Scholar] [CrossRef]
- Bailey, T.N. Social organization in a bobcat population. J. Wildl. Manag. 1974, 38, 435–446. [Google Scholar] [CrossRef]
- Larivière, S.; Walton, L.R. Lynx rufus. Mammal. Spec. 1997, 563, 1–8. [Google Scholar] [CrossRef]
- Anderson, E.M. Effects of male removal on spatial distribution of bobcats. J. Mammal. 1988, 69, 637–641. [Google Scholar] [CrossRef]
- Lovallo, M.J.; Anderson, E.M. Range shift by a female bobcat (Lynx rufus) after removal of neighboring female. Am. Midl. Nat. 1995, 134, 409–412. [Google Scholar] [CrossRef]
- Allen, M.L.; Wallace, C.F.; Wilmers, C.C. Patterns in bobcat (Lynx rufus) scent marking and communication behaviors. J. Ethol. 2015, 33, 9–14. [Google Scholar] [CrossRef]
- Benson, J.F.; Chamberlain, M.J.; Leopold, B.D. Land tenure and occupation of vacant home ranges by bobcats (Lynx rufus). J. Mammal. 2004, 85, 983–988. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Woolf, A. Spatial organization of bobcats (Lynx rufus) in southern Illinois. Am. Midl. Nat. 2001, 146, 43–52. [Google Scholar] [CrossRef]
- López-Vidal, J.C.; Elizalde-Arellano, C.; Hernández, L.; Laundré, J.W.; González-Romero, A.; Cervantes, F.A. Foraging of the bobcat (Lynx rufus) in the Chihuahuan Desert: Generalist or specialist? Southwest. Nat. 2014, 59, 157–167. [Google Scholar] [CrossRef]
- McCleery, R.A. Urban mammals. In Urban Ecosystem Ecology; American Society of Agronomy: Madison, WI, USA; Crop Science Society of America: Fitchburg, WI, USA; Soil Science Society of America: Madison, WI, USA, 2010; pp. 87–102. [Google Scholar]
- Kertson, B.N.; Spencer, R.D.; Marzluff, J.M.; Hepinstall-Cymerman, J.; Grue, C.E. Cougar space use and movements in the wildland–urban landscape of western Washington. Ecol. Appl. 2011, 21, 2866–2881. [Google Scholar] [CrossRef]
- Gould, F.W. The Grasses of Texas; Texas A&M University, Texas Agricultural Experiment Station: College Station, TX, USA, 1975. [Google Scholar]
- Beltrán, J.F.; Tewes, M.E. Immobilization of ocelots and bobcats with ketamine hydrochloride and xylazine hydrochloride. J. Wildl. Dis. 1995, 31, 43–48. [Google Scholar] [CrossRef]
- Heilbrun, R.D.; Silvy, N.J.; Peterson, M.J.; Tewes, M.E. Estimating bobcat abundance using automatically triggered cameras. J. Wildl. Manag. 2006, 34, 69–73. [Google Scholar] [CrossRef]
- Elizalde-Arellano, C.; López-Vidal, J.C.; Hernández, L.; Laundré, J.W.; Cervantes, F.A.; Alonso-Spilsbury, M. Home range size and activity patterns of bobcats (Lynx rufus) in the southern part of their range in the Chihuahuan Desert, Mexico. Am. Midl. Nat. 2012, 168, 247–264. [Google Scholar] [CrossRef]
- D’Eon, R.G.; Delparte, D. Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. J. Appl. Ecol. 2005, 42, 383–388. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10.1; Environmental Systems Research Institute: Redlands, CA, USA, 2013. [Google Scholar]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; MacDonald, T.L.; Erickson, W.P. Resource Selection by Animals. Statistical Design and Analysis for Field Studies; Kluwer Academic Publisher: London, UK, 2002. [Google Scholar]
- Benhamou, S.; Cornélis, D. Incorporating movement behavior and barriers to improve kernel home range space use estimates. J. Wildl. Manag. 2010, 74, 1353–1360. [Google Scholar] [CrossRef]
- Calenge, C.; Fortmann-Roe, S. adehabitatHR: Home Range Estimation. R Package Version 0.4.7. 2013. Available online: http://cran.r-project.org/web/packages/adehabitatHR/ (accessed on 21 March 2019).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org (accessed on 21 March 2019).
- Getz, W.M.; Wilmers, C.C. A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography 2004, 27, 489–505. [Google Scholar] [CrossRef] [Green Version]
- Lichti, N.I.; Swihart, R.K. Estimating utilization distributions with kernel versus local convex hull methods. J. Wildl. Manag. 2011, 75, 413–422. [Google Scholar] [CrossRef]
- Signer, J.; Balkenhol, N. Reproducible home ranges (rhr): A new, user-friendly R package for analyses of wildlife telemetry data. Wildl. Soc. Bull. 2015, 39, 358–363. [Google Scholar] [CrossRef]
- Homer, C.; Dewitz, J.; Yang, L.M.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.; Wickham, J.; Megown, K. Completion of the 2011 National Land Cover Database for the Conterminous United States—Representing a Decade of Land Cover Change Information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Texas Water Development Board. Available online: https://www.twdb.texas.gov/mapping/gisdata.asp (accessed on 20 March 2016).
- Gillies, C.S.; Hebblewhite, M.; Nielsen, S.E.; Krawchuk, M.A.; Aldridge, C.L.; Frair, J.L.; Saher, D.J.; Stevens, C.E.; Jerde, C.L. Application of random effects to the study of resource selection by animals. J. Anim. Ecol. 2006, 75, 887–898. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin, Germany, 2002. [Google Scholar]
- Avgar, T.; Lele, S.R.; Keim, J.L.; Boyce, M.S. Relative selection strength: Quantifying effect size in selection inference. Ecol. Evol. 2017, 7, 5322–5330. [Google Scholar] [CrossRef]
- Bertrand, M.R.; DeNicola, A.J.; Beissinger, S.R.; Swihart, R.K. Effects of parturition on home ranges and social affiliations of female white-tailed deer. J. Wildl. Manag. 1996, 60, 899–909. [Google Scholar] [CrossRef]
- Long, J.A. wildlifeDI—A Suite of R Tools for Exploring Dynamic Interaction Patterns in Wildlife Telemetry Data Version 0.1 (2014). Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.565.460 (accessed on 21 May 2019).
- Riley, S.P. Spatial ecology of bobcats and gray foxes in urban and rural zones of a national park. J. Wildl. Manag. 2006, 70, 1425–1435. [Google Scholar] [CrossRef]
- Ordeñana, M.A.; Crooks, K.R.; Boydston, E.E.; Fisher, R.N.; Lyren, L.M.; Siudyla, S.; Haas, C.D.; Harris, S.; Hathaway, S.A.; Turschak, G.M.; et al. Effects of urbanization on carnivore species distribution and richness. J. Mammal. 2010, 91, 1322–1331. [Google Scholar] [CrossRef] [Green Version]
- Lawhead, D.N. Bobcat Lynx rufus home range, density and habitat preference in south-central Arizona. Southwest. Nat. 1984, 29, 105–113. [Google Scholar] [CrossRef]
- Hamilton, D.A. Ecology of the Bobcat in Missouri. Master’s Thesis, University of Missouri, Columbia, OH, USA, 1982. [Google Scholar]
- Zezulak, D.S.; Schwab, R.G. A comparison of density, home range, and habitat utilization of bobcat populations at Lava Beds and Joshua Tree National Monuments, California. Proc. 1979 Bobcat Res. Conf. National Wildl. Fed. Sci. Tech. Series 1979, 6, 74–79. [Google Scholar]
- Conner, L.M.; Chamberlain, M.J.; Leopold, B.D. Bobcat home range size relative to habitat quality. Proc. Annu. Conf. SEAFWA 2001, 55, 418–426. [Google Scholar]
- Ruell, E.W.; Riley, S.P.D.; Douglas, M.R.; Antolin, M.F.; Pollinger, J.R.; Tracey, J.A.; Lyren, L.M.; Boydston, E.E.; Fisher, R.N.; Crooks, K.R. Urban habitat fragmentation and genetic population structure of bobcats in coastal southern California. Am. Midl. Nat. 2012, 168, 265–281. [Google Scholar] [CrossRef]
- Blankenship, T.L. Ecological Response of Bobcats to Fluctuating Prey Populations on the Welder Wildlife Foundation Refuge. Ph.D. Dissertation, Texas A&M University-Kingsville and Texas A&M University-College Station, Kingsville, TX, USA, 2000. [Google Scholar]
- Fedriani, J.M.; Fuller, T.K.; Sauvajot, R. Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 2001, 24, 325–331. [Google Scholar] [CrossRef]
- Poessel, S.A.; Burdett, C.L.; Boydston, E.E.; Lyren, L.M.; Alonso, R.S.; Fisher, R.N.; Crooks, K.R. Roads influence movement and home ranges of a fragmentation-sensitive carnivore, the bobcat, in an urban landscape. Biol. Conserv. 2014, 180, 224–232. [Google Scholar] [CrossRef]
- Cuo, L.; Lettenmaier, D.P.; Alberti, M.; Richey, J.E. Effects of a century of land cover and climate change on the hydrology of the Puget Sound basin. Hydrol. Process. 2009, 23, 907–933. [Google Scholar] [CrossRef]
- Cresswell, W.J.; Harris, S. Foraging behaviour and home-range utilization in a suburban badger (Meles meles) population. Mammal. Rev. 1988, 18, 37–49. [Google Scholar] [CrossRef]
- Abrahms, B.; Jordan, N.R.; Golabek, K.A.; McNutt, J.W.; Wilson, A.M.; Brashares, J.S. Lessons from integrating behaviour and resource selection: Activity-specific responses of African wild dogs to roads. Anim. Conserv. 2016, 19, 247–255. [Google Scholar] [CrossRef]
- Kitchen, A.M.; Gese, E.M.; Schauster, E.R. Changes in coyote activity patterns due to reduced exposure to human persecution. Can. J. Zool. 2000, 78, 853–857. [Google Scholar] [CrossRef]
- Smith, J.A.; Suraci, J.P.; Clinchy, M.; Crawford, A.; Roberts, D.; Zanette, L.Y.; Wilmers, C.C. Fear of the human ‘super predator’ reduces feeding time in large carnivores. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170433. [Google Scholar] [CrossRef]
- Breck, S.W.; Poessel, S.A.; Mahoney, P.; Young, J.K. The intrepid urban coyote: A comparison of bold and exploratory behavior in coyotes from urban and rural environments. Sci. Rep. 2019, 9, 2104. [Google Scholar] [CrossRef] [PubMed]

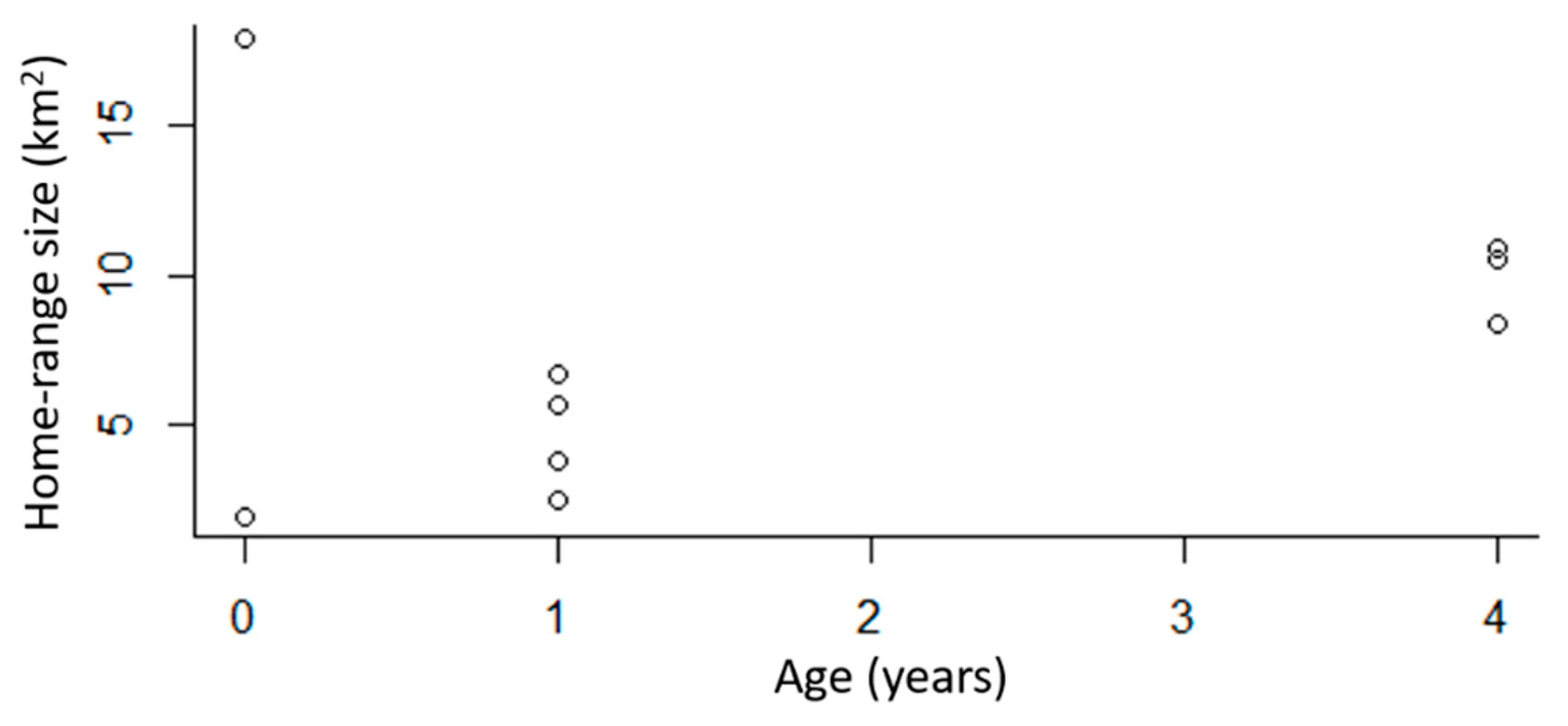
| Distance Threshold (m) | All Bobcats (n = 9) | Without Disperser (n = 8) | ||||
|---|---|---|---|---|---|---|
| Age | Sex | Random | Age | Sex | Random | |
| 200 | 0.80 | 0.53 | 0.67 | 0.79 | 0.65 | 0.64 |
| 300 | 0.70 | 0.55 | 0.45 | 0.68 | 0.66 | 0.41 |
| 500 | 0.69 | 0.56 | 0.82 | 0.68 | 0.64 | 0.79 |
| 700 | 0.71 | 0.58 | 0.82 | 0.73 | 0.64 | 0.83 |
| 1000 | 0.85 | 0.36 | 0.83 | 0.86 | 0.45 | 0.82 |
| 1500 | 0.91 | 0.21 | 0.68 | 0.92 | 0.32 | 0.69 |
| 2000 | 0.73 | 0.14 | 0.74 | 0.73 | 0.28 | 0.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, J.K.; Golla, J.; Draper, J.P.; Broman, D.; Blankenship, T.; Heilbrun, R. Space Use and Movement of Urban Bobcats. Animals 2019, 9, 275. https://doi.org/10.3390/ani9050275
Young JK, Golla J, Draper JP, Broman D, Blankenship T, Heilbrun R. Space Use and Movement of Urban Bobcats. Animals. 2019; 9(5):275. https://doi.org/10.3390/ani9050275
Chicago/Turabian StyleYoung, Julie K., Julie Golla, John P. Draper, Derek Broman, Terry Blankenship, and Richard Heilbrun. 2019. "Space Use and Movement of Urban Bobcats" Animals 9, no. 5: 275. https://doi.org/10.3390/ani9050275





