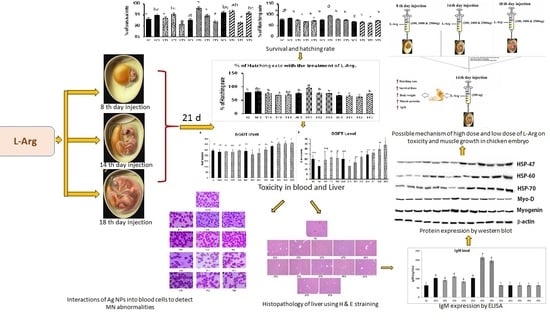
Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals
2.3. Experimental Design and Incubation
2.4. Survival Rate Measurement
2.5. Hatching Rate and Body Weight Measurements
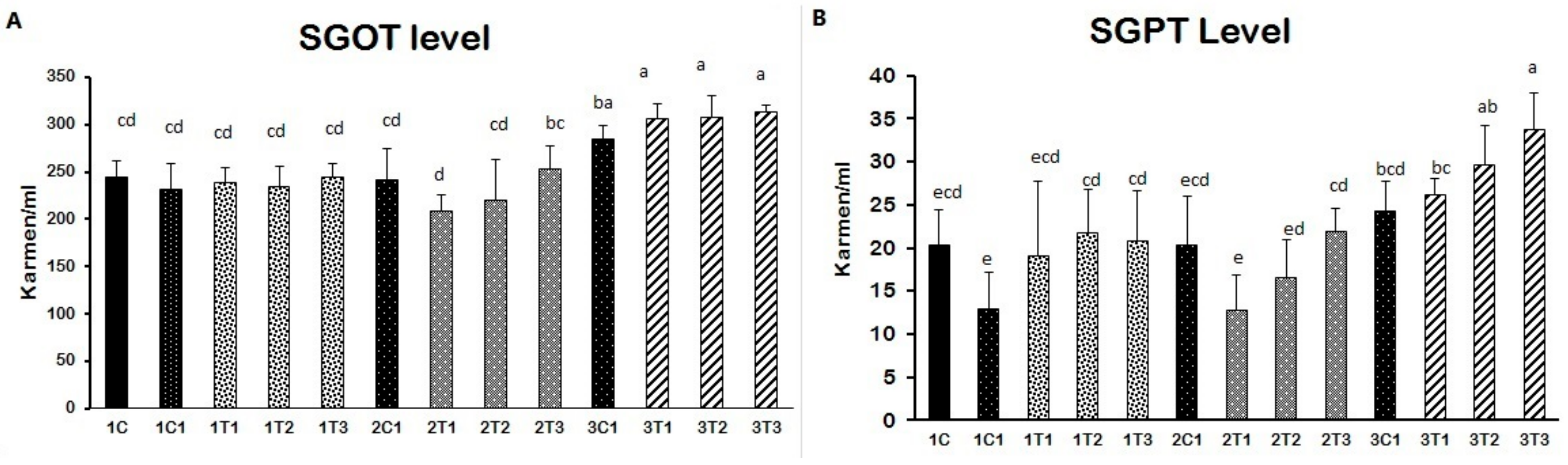
2.6. Biochemical Indices
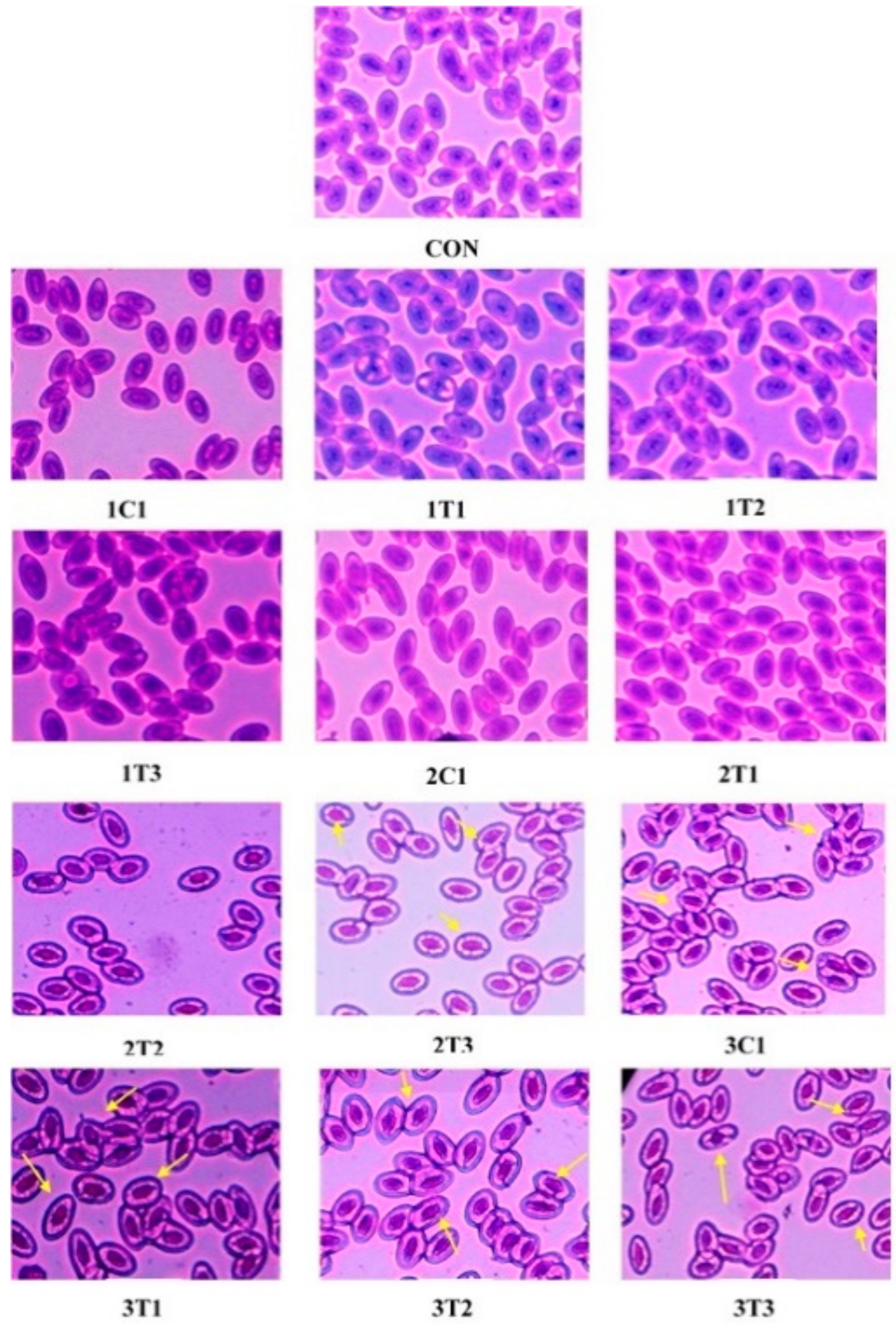
2.7. Micronuclei (MN) and Nuclear Abnormality (NA) Tests Using Periodic Acid Schiff’s (PAS) Staining
2.8. Measurement of IgM Concentration in Serum
2.9. Analysis of Heat-Shock Proteins (HSPs) by Western Blot
2.10. In Silico Molecular Docking Studies
2.11. Histopathological Study of the Liver
2.12. Statistical Analysis
3. Results and Discussion
3.1. Survival Rate and Hatchability
3.2. Biochemical Indices (SGOT and SGPT)
3.3. Micronuclei (MN) and Nuclear Abnormality (NA) Tests Using Periodic Acid-Schiff’s (PAS) Staining
3.4. Protein Analysis by Western Blot
3.5. Measurement of IgM Concentration in Serum
3.6. Histopathology (H&E) Staining
3.7. In Silico Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berri, C.; Wacrenier, N.; Millet, N.; Le Bihan-Duval, E. Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult. Sci. 2001, 80, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Szpinda, M.; Walasik, K.; Bednarczyk, M.; Paruszewska-Achtel, M. Myogenesis–possibilities of its stimulation in chickens. Folia Biol. 2011, 59, 85–90. [Google Scholar] [CrossRef]
- Dos Santos, T.T.; Corzo, A.; Kidd, M.T.; McDaniel, C.D.; Torres Filho, R.A.; Araujo, L.F. Influence of in ovo inoculation with various nutrients and egg size on broiler performance. J. Appl. Poult. Res. 2010, 19, 1–12. [Google Scholar] [CrossRef]
- Dransfield, E.; Sosnicki, A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Ferket, P.R.; Tako, E.; Kedar, O. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 2005, 84, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kidd, M.T.; Ishibashi, T. Embryo growth and amino acid concentration profiles of broiler breeder eggs, embryos, and chicks after in ovo administration of amino acids. Poult. Sci. 2001, 80, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, S.K.; Mandal, A.B. Effect of in ovo injection of critical amino acids on pre- and post-hatch growth, immunocompetence and development of digestive organs in broiler chickens. Asian-Australas J. Anim. Sci. 2005, 18, 524–531. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Kadam, M.M.; Barekatain, M.R.; Bhanja, S.K.; Iji, P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: The science and commercial applications—A review. J. Sci. Food Agric. 2013, 93, 3654–3661. [Google Scholar] [CrossRef]
- Ohta, Y.; Kidd, M.T. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult. Sci. 2001, 80, 1425–1429. [Google Scholar] [CrossRef]
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Dankowiakowska, A.; Kułakowska, A.; Szczerba, A.; Bednarczyk, M. The influence of in ovo injection with the prebiotic DiNovo® on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J. Anim. Sci. Biotechnol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Spurlin, J., III; Lwigale, P.A. A Technique to increase accessibility to late-stage chick embryos for in ovo manipulations. Dev Dyn. 2013, 242, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Tsushima, N.; Koide, K.; Kidd, M.T.; Ishibashi, T. Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks. Poult. Sci. 1999, 78, 1493–1498. [Google Scholar] [CrossRef]
- Zielinska, M.; Sawosz, E.; Grodzik, M.; Wierzbicki, M.; Gromadka, M.; Hotowy, A.; Chwalibog, A. Effect of heparan sulfate and gold nanoparticles on muscle development during embryogenesis. Int. J. Nanomed. 2011, 6, 3163. [Google Scholar]
- Noy, Y.; Uni, Z. Early nutritional strategies. Worlds. Poult. Sci. J. 2010, 66, 639–646. [Google Scholar] [CrossRef]
- Foye, O.T.; Uni, Z.; Ferket, P.R. Effect of in ovo feeding egg white protein, β-hydroxy-β-methylbutyrate, and carbohydrates on glycogen status and neonatal growth of turkeys. Poult. Sci. 2006, 85, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. Therapeutic considerations of L-glutamine: A review of the literature. Altern. Med. Rev. Clin. Ther. 1999, 4, 239–248. [Google Scholar]
- Uni, Z.; Ferket, P.R. Enhancement of Development of Oviparous Species by In Ovo Feeding. U.S. Patent 6,592,878, 15 July 2003. [Google Scholar]
- Sawosz, F.; Pineda, L.; Hotowy, A.; Jaworski, S.; Prasek, M.; Sawosz, E.; Chwalibog, A. Nano-nutrition of chicken embryos. The effect of silver nanoparticles and ATP on expression of chosen genes involved in myogenesis. Arch. Anim. Nutr. 2013, 67, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Daraji, H.J.; Al-Mashadani, A.A.; Al-Mashadani, W.K.; Al-Hassani, A.S.; Mirza, H.A. Effect of in ovo injection with L-arginine on productive and physiological traits of Japanese quail. S. Afr. J. Anim. Sci. 2012, 42, 139–145. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Harper, S.L.; Yun, S.I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.S.; Krishnaraj, C.; Sheet, S.; Rampa, D.R.; Belal, S.A.; Kumar, A.; Hwang, I.H.; Yun, S.I.; Shim, K.S. Interaction of silver and gold nanoparticles in mammalian cancer: As real topical bullet for wound healing—A comparative study. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shi, Q.; Bracher, A.; Milicic, G.; Singh, A.K.; Hartl, F.U.; Hayer-Hartl, M. GroEL ring separation and exchange in the chaperonin reaction. Cell 2018, 172, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Hoshikawa, M.; Tochio, N.; Tate, S.I. Substrate binding switches the conformation at the lynchpin site in the substrate-binding domain of human hsp70 to enable allosteric interdomain communication. Molecules 2018, 23, 528. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Shafey, T.M.; Sami, A.S.; Abouheif, M.A. Effects of in ovo feeding of L-glutamine on hatchability performance and hatching time of meat-type breeder eggs. J. Anim. Vet. Adv. 2013, 12, 135–139. [Google Scholar]
- Shafey, T.M.; Mahmoud, A.H.; Alsobayel, A.A.; Abouheif, M.A. Effects of in ovo administration of amino acids on hatchability and performance of meat chickens. S. Afr. J. Anim. Sci. 2014, 44, 123–130. [Google Scholar] [CrossRef]
- Azhar, M.; Rahardja, D.P.; Pakiding, W. Embryo Development and Post-Hatch Performances of Kampung Chicken by in Ovo Feeding of L-Arginine. Media Peternak. 2016, 39, 168–172. [Google Scholar] [CrossRef]
- Toghyani, M.; Tahmasebi, S.; Modaresi, M.; Ale Saheb Fosoul, S.S. Effect of arginine and threonine in ovo supplementation on immune responses and some serum biochemical attributes in broiler chickens. Ital. J. Anim. Sci. 2019, 18, 342–349. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Baran Mandal, A.; Agarwal, S.K.; Majumdar, S. Modulation of post hatch-growth and immunocompetence through in ovo injection of limiting amino acids in broiler chickens. Ital. J. Anim. Sci. 2012, 82, 993. [Google Scholar]
- Fouad, A.M.; El-Senousey, H.K.; Yang, X.J.; Yao, J.H. Role of dietary L-arginine in poultry production. Int. J. Poult. Sci. 2012, 11, 718. [Google Scholar]
- Izadinia, M.; Nobakht, M.; Khajali, F.; Faraji, M.; Zamani, F.; Qujeq, D.; Karimi, I. Pulmonary hypertension and ascites as affected by dietary protein source in broiler chickens reared in cool temperature at high altitudes. Anim. Feed Sci. Technol. 2010, 155, 194–200. [Google Scholar] [CrossRef]
- Duan, X.; Li, F.; Mou, S.; Feng, J.; Liu, P.; Xu, L. Effects of dietary L-arginine on laying performance and anti-oxidant capacity of broiler breeder hens, eggs, and offspring during the late laying period. Poult. Sci. 2015, 94, 2938–2943. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sobolewska, A.; Cianciullo, D.; Walasik, K.; Elminowska-Wenda, G.; Sławińska, A.; Tavaniello, S.; Żylińska, J.; Bednarczyk, M. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 2012, 91, 2963–2969. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.; Kolba, N.; Tako, E. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients 2017, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, J.D.; Kida, R.; Singh, A.K.; Kim, Y.S.; Jha, R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017, 96, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Jaja, S.I.; Ogungbemi, S.O.; Kehinde, M.O.; Anigbogu, C.N. Anigbogu. Supplementation with l-arginine stabilizes plasma arginine and nitric oxide metabolites, suppresses elevated liver enzymes and peroxidation in sickle cell anaemia. Pathophysiology 2016, 23, 81–85. [Google Scholar] [CrossRef]
- Panda, A.K.; Lavanya, G.; Reddy, E.P.K.; Rao, S.R.; Raju, M.V.L.N.; Sunder, G.S. Utilization of quality protein maize in the diet of White Leghorn layers. Anim. Feed Sci. Technol. 2012, 172, 210–216. [Google Scholar] [CrossRef]
- Panda, A.K.; Raju, M.V.L.N.; Rao, S.R.; Lavanya, G.; Reddy, E.P.K.; Sunder, G.S. Nutritional evaluation and utilisation of quality protein maize, Nityashree hybrid maize, and normal maize in broiler chickens. Br. Poult. Sci. 2011, 52, 632–638. [Google Scholar] [CrossRef]
- Nsiah, K.; Dzogbefia, V.P.; Ansong, D.; Akoto, A.O.; Boateng, H.; Ocloo, D. Pattern of AST and ALT changes in relation to hemolysis in sickle cell disease. Clin. Med. Blood Disord. 2011, 4, 1–9. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, A.; Dahia, S.; Shah, V.; Sharma, V.; Mishra, R.M.; Pandey, S.W.; Saxena, R. Biochemical indicator of sickle cell disease: Preliminary report from India. Indian J. Clin. Biochem. 2012, 27, 191–195. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Shukla, G.; Wahi, A.K. Protective effect of L-arginine against necrosis and apoptosis induced by experimental ischemic and reperfusion in rat liver. Saudi J. Gastroenterol. 2009, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Y.; Ozsoy, M.; Coskun, T.; Namlı, K.; Var, A.; Ozyurt, B. The effects of L-arginine on liver damage in experimental acute cholestasis an immunohistochemical study. HPB Surg. 2011, 2011, 306069. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.A.; Belal, S.A.; Choe, H.S.; Shim, K.S. A Comparative Study of Biologically and Chemically Fabricated Synthesized AgNPs’ Supplementation with Respect to Heat-Shock Proteins, Survival, and Hatching Rates of Chicken Embryos: An in ovo Study. J. Clust. Sci. 2018, 29, 129–139. [Google Scholar] [CrossRef]
- Heddle, J.A.; Cimino, M.C.; Hayashi, M.; Romagna, F.; Shelby, M.D.; Tucker, J.D.; MacGregor, J.T. Micronuclei as an index of cytogenetic damage: Past, present, and future. Environ. Mol. Mutagen. 1991, 18, 277–291. [Google Scholar] [CrossRef]
- Hasheimi, S.R.; Zulkifli, I.; Somchit, M.N.; Zunita, Z.; Loh, T.C.; Soleimani, A.F.; Tang, S.C. Dietary supplementation of Zingiber officinale and Zingiber zerumbet to heat-stressed broiler chickens and its effect on heat shock protein 70 expression, blood parameters and body temperature. J. Anim. Physiol. Anim. Nutr. 2013, 97, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.H.; Subramani, V.K.; Moon, Y.S.; Jang, I.S. Telomeric DNA quantity, DNA damage, and heat shock protein gene expression as physiological stress markers in chickens. Poult. Sci. 2012, 91, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Fadda, L.; Mohamed, A.M.; Attia, H.A.; Al-Rasheed, N.M. Regulating effect of carnosine and/or l-arginine on the expression of inflammatory molecules induced nephropathy in the hypoxic rat model. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Mulligan, M.S.; Hevel, J.M.; Marletta, M.A.; Ward, P.A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc. Natl. Acad. Sci. USA 1991, 88, 6338–6342. [Google Scholar] [CrossRef]
- Murray, J.; Auwerx, J.; Huss, J.M. Impaired myogenesis in estrogen-related receptor γ (ERRγ)-deficient skeletal myocytes due to oxidative stress. FASEB J. 2013, 27, 135–150. [Google Scholar] [CrossRef]
- Wust, R.C.; Degens, H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 289–300. [Google Scholar]
- Mok, G.F.; Mohammed, R.H.; Sweetman, D. Expression of myogenic regulatory factors in chicken embryos during somite and limb development. J. Anat. 2015, 227, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Ruan, Z.; Gao, Y.; Yin, Y.; Zhou, X.; Wang, L.; Wu, G. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn-and soybean meal-based diet. Amino Acid. 2010, 39, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Wong, C.W.; Nolan, J.V. Long-term effects of early life L-arginine supplementation on growth performance, lymphoid organs and immune responses in Leghorn-type chickens. Br. Poult. Sci. 2005, 46, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Cha, J.; Ivanova, S.; Kalinovsky, T.; Roomi, M.W.; Rath, M.; Niedzwiecki, A. Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int. J. Mol. Med. 2008, 22, 731–741. [Google Scholar] [PubMed]
- Wu, G.H.; Zhang, Y.W.; Wu, Z.H. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J. Gastroenterol. 2001, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Can L-arginine manipulation reduce renal disease? Semin. Nephrol. 1999, 19, 304–309. [Google Scholar] [PubMed]
- Kline, J.A.; Watts, J.; Courtney, D.; Lee, Y.Y.; Hwang, S. Severe pulmonary embolism decreases plasma L-arginine. Eur. Respir. J. 2014, 43, 906–909. [Google Scholar] [CrossRef]
- Kang, K.; Shu, X.L.; Zhong, J.X.; Yu, T.T.; Lei, T. Effect of L-arginine on immune function: A meta-analysis. Asia Pac. J. Clin. Nutr. 2014, 23, 351–359. [Google Scholar]
- Alabi, O.O.; Shoyombo, A.J.; Animashahun, R.A.; Olawoye, S.O.; Abdulazeez, J.O.; Faduhunsi, O.O.; Oladehinbo, D.O. Effects of L-Arginine supplementation of drinking water on the kidney and liver of Sasso chickens. Int. J. Livest. Prod. 2018, 9, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Atakisi, O.; Atakisi, E.; Kart, A. Effects of dietary zinc and l-arginine supplementation on total antioxidants capacity, lipid peroxidation, nitric oxide, egg weight, and blood biochemical values in Japanase quails. Biol. Trace Elem. Res. 2009, 132, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Oh, S.K.; Lee, J.S.; Wu, C.; Lee, S.J. Effects of l-arginine on growth hormone and insulin-like growth factor 1. Food Sci. Biotechnol. 2017, 26, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, Q.; Liu, J.; Jia, Y.; Cai, D.; Idriss, A.A.; Omer, N.A.; Zhao, R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Rep. 2017, 7, 40251. [Google Scholar] [CrossRef] [PubMed]
- Kelley, P.M.; Schlesioger, M.J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol. Cell. Biol. 1982, 2, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, T.; Slater, M.; Craig, E. Saccharomycescerevislae contains a complex multigene family related to the major heat shock inducible gene of Drosophila. Mol. Cell. Biol. 1982, 2, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P.; Fakan, S.; Tissieres, A. Localization of the heat shock induced proteins in Drosophila melanogaster tissue culture cells. Dev. Biol. 1980, 78, 86–103. [Google Scholar] [CrossRef]
- Morimoto, R.; Fodor, E. Cell-specific expression of heat shock proteins in chicken reticulocytes and lymphocytes. J. Cell Biol. 1984, 99, 1316–1323. [Google Scholar] [CrossRef]








| Group | Dosage | No. of Eggs & No. of Replication | Total No. of Eggs |
|---|---|---|---|
| 1C | Control | 20 eggs × 4 replicates | 80 |
| 1C1 (8th day) | PBS/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 1T1 (8th day) | 100 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 1T2 (8th day) | 1000 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 1T3 (8th day) | 2500 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 2C1 (14th day) | PBS/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 2T1 (14th day) | 100 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 2T2 (14th day) | 1000 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 2T3 (14th day) | 2500 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 3C1 (18th day) | PBS/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 3T1 (18th day) | 100 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 3T2 (18th day) | 1000 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
| 3T2 (18th day) | 2500 µg/100 µL/egg | 20 eggs × 4 replicates | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniyan, S.A.; Kang, D.R.; Park, J.R.; Siddiqui, S.H.; Ravichandiran, P.; Yoo, D.J.; Na, C.S.; Shim, K.S. Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins. Animals 2019, 9, 357. https://doi.org/10.3390/ani9060357
Subramaniyan SA, Kang DR, Park JR, Siddiqui SH, Ravichandiran P, Yoo DJ, Na CS, Shim KS. Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins. Animals. 2019; 9(6):357. https://doi.org/10.3390/ani9060357
Chicago/Turabian StyleSubramaniyan, Sivakumar Allur, Da Rae Kang, Jin Ryong Park, Sharif Hasan Siddiqui, Palanisamy Ravichandiran, Dong Jin Yoo, Chong Sam Na, and Kwan Seob Shim. 2019. "Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins" Animals 9, no. 6: 357. https://doi.org/10.3390/ani9060357