Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions
Abstract
:1. Introduction and Backgrounds
- First, the cosmogenic noble gases are produced by spallation reactions on specific target elements in the meteorites during their transfer from their parent body to Earth (Table 1). Cosmic rays are energetic particles mainly composed of protons and α-particles, and minor amounts of heavy ions, and can be divided into Galactic Cosmic Rays (GCRs) and Solar Cosmic Rays (SCRs). Galactic Cosmic Rays are omnidirectional in space, most likely produced in supernovae, and are highly energetic. On the other hand, SCRs are produced by the Sun (solar flares and coronal mass ejections); their intensities decrease with increasing distance from the Sun. In meteorites, the signatures of 3He, 21Ne, and 38Ar mostly represent spallation reactions. These cosmogenic noble gases are not only produced in meteorites, but also on airless bodies (Moon, near-Earth objects, asteroids, etc.) and to some extent the surface of Mars, also exposed to GCR spallation reactions in rocks.
- Second, radiogenic and fissiogenic noble gases in rocks are produced by decay and fission of radionuclides, respectively. For example, radiogenic 4Herad is produced mainly from the decay of 235U, 238U, and 232Th. Similarly, radiogenic 40Arrad is produced by the decay of 40K (T1/2 = 1.25 Ga), whereas radiogenic 129Xerad is mainly produced through the decay of short lived but now extinct 129I (T1/2 = 15.7 Ma). On the other hand, heavy isotopes of Kr and Xe are produced by the neutron induced fission of 235U and through spontaneous fission of 238U and the extinct 244Pu [9].
- Mars, where during the recent decades, four space missions have measured atmospheric composition in the Martian atmosphere, including the noble gas abundances as well as their isotopic ratios by mass spectrometry or EUV spectroscopy: the Viking suites of missions (1976–1982), the Curiosity rover on the Mars Science Laboratory (MSL) (2012), the Mars Atmosphere and Volatile Evolution (MAVEN, 2014), or the Mars Orbiter Mission (MOM, 2014). Thus, the Viking mission in 1976 provided the first elemental and isotopic abundances of the volatiles in the Martian atmosphere [18,19,20], etc.
2. Noble Gases in Martian Atmosphere and In Martian Meteorites Measured In Situ or In the Laboratory: Technical Aspects
2.1. The Atmospheric Noble Gas Composition of Mars: The Latest Significant Missions
2.1.1. The Viking Project (1976–1982)
2.1.2. The Mars Science Laboratory Mission (2012)
2.1.3. MAVEN (2014)
2.1.4. MENCA Onboard MOM (2014)
2.2. Noble Gas Mass Spectrometry Analyses in Laboratory
2.2.1. Sample Preparation
2.2.2. Pyrolysis: Total Fusion or Stepwise Heating
2.2.3. Combustion
2.2.4. Crushing Technique
2.2.5. Separation and Purification
3. The Noble Gas Composition of Martian Atmosphere and Interior
3.1. Helium
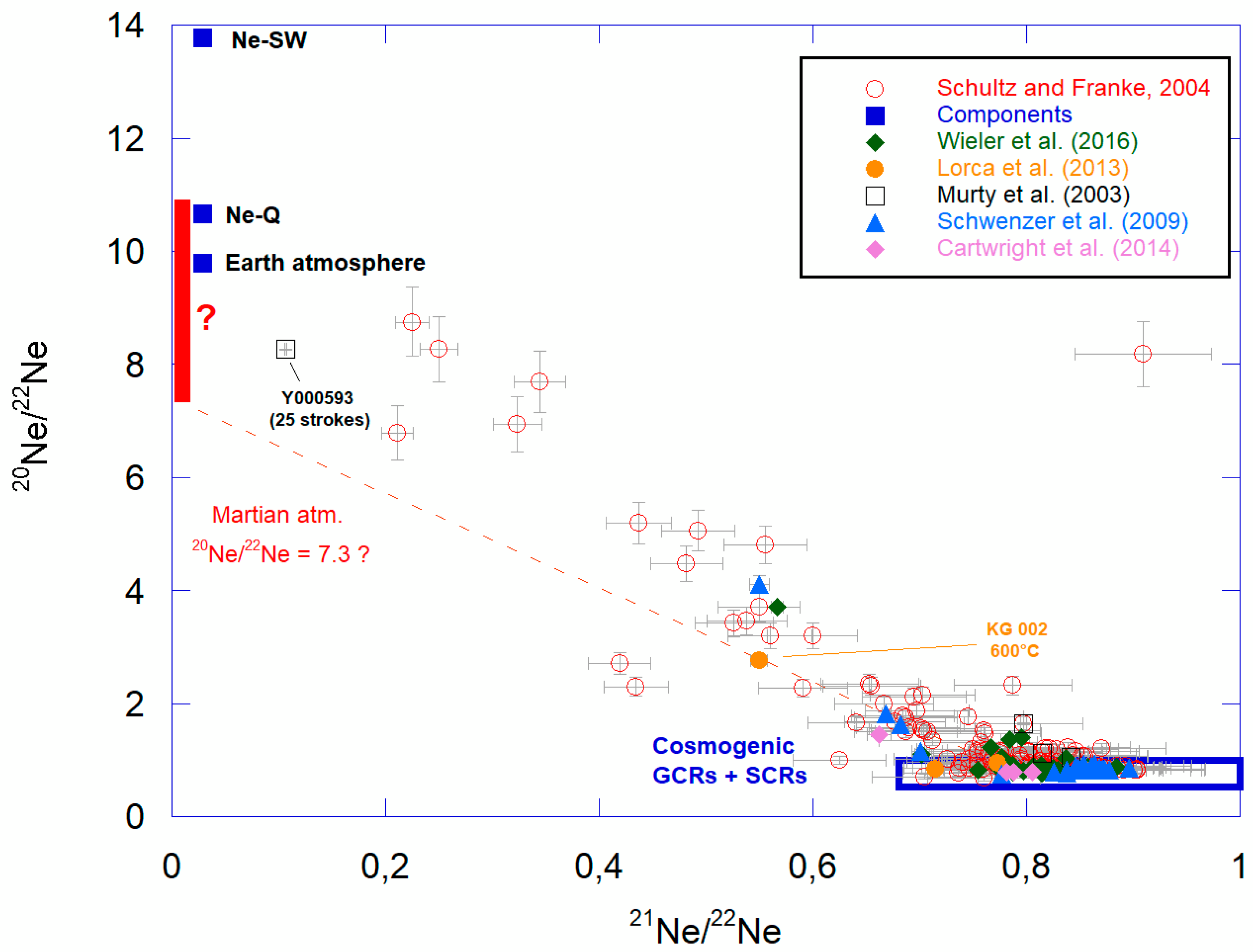
3.2. Neon
3.2.1. In Situ Measurements
3.2.2. Neon Atmospheric Ratio Revealed by Martian Meteorites
3.3. Argon
3.3.1. MOM&MAVEN: Measurements in the Well-Mixed Atmosphere
3.3.2. Loss of Atmospheric Argon to Space Confirmed by Martian Meteorite and Mission Data
Elemental Abundances
Isotopic Ratios
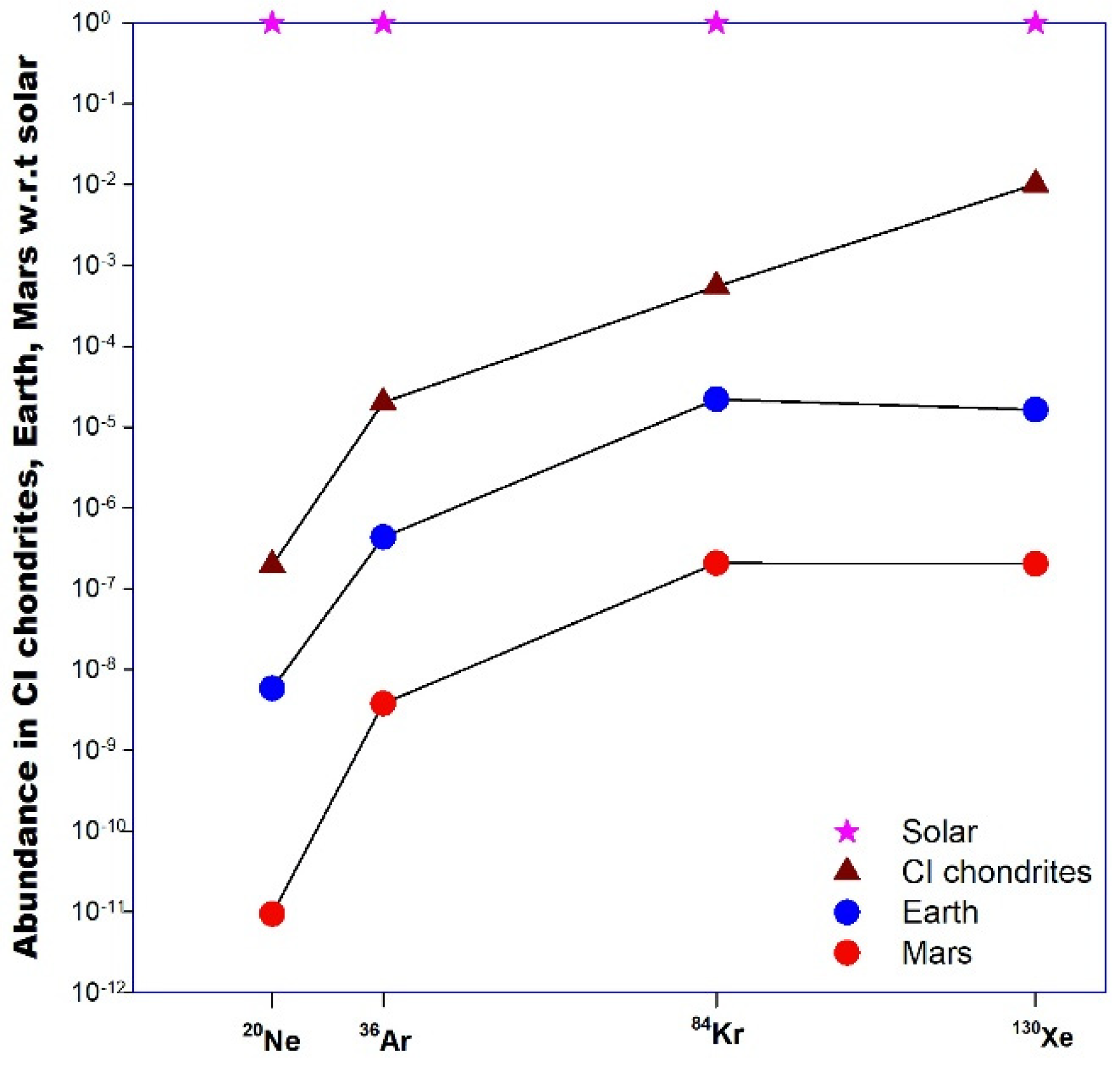
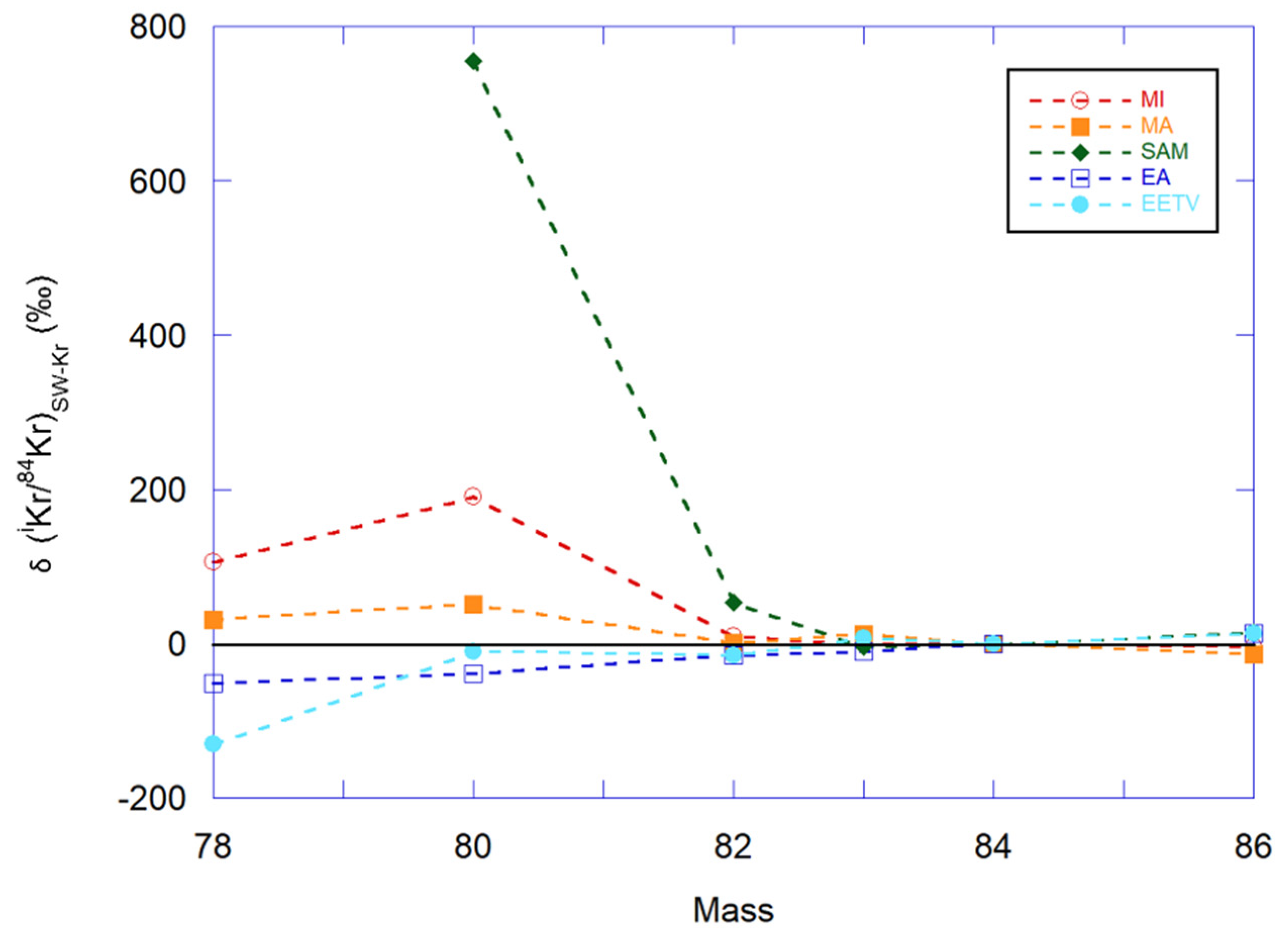
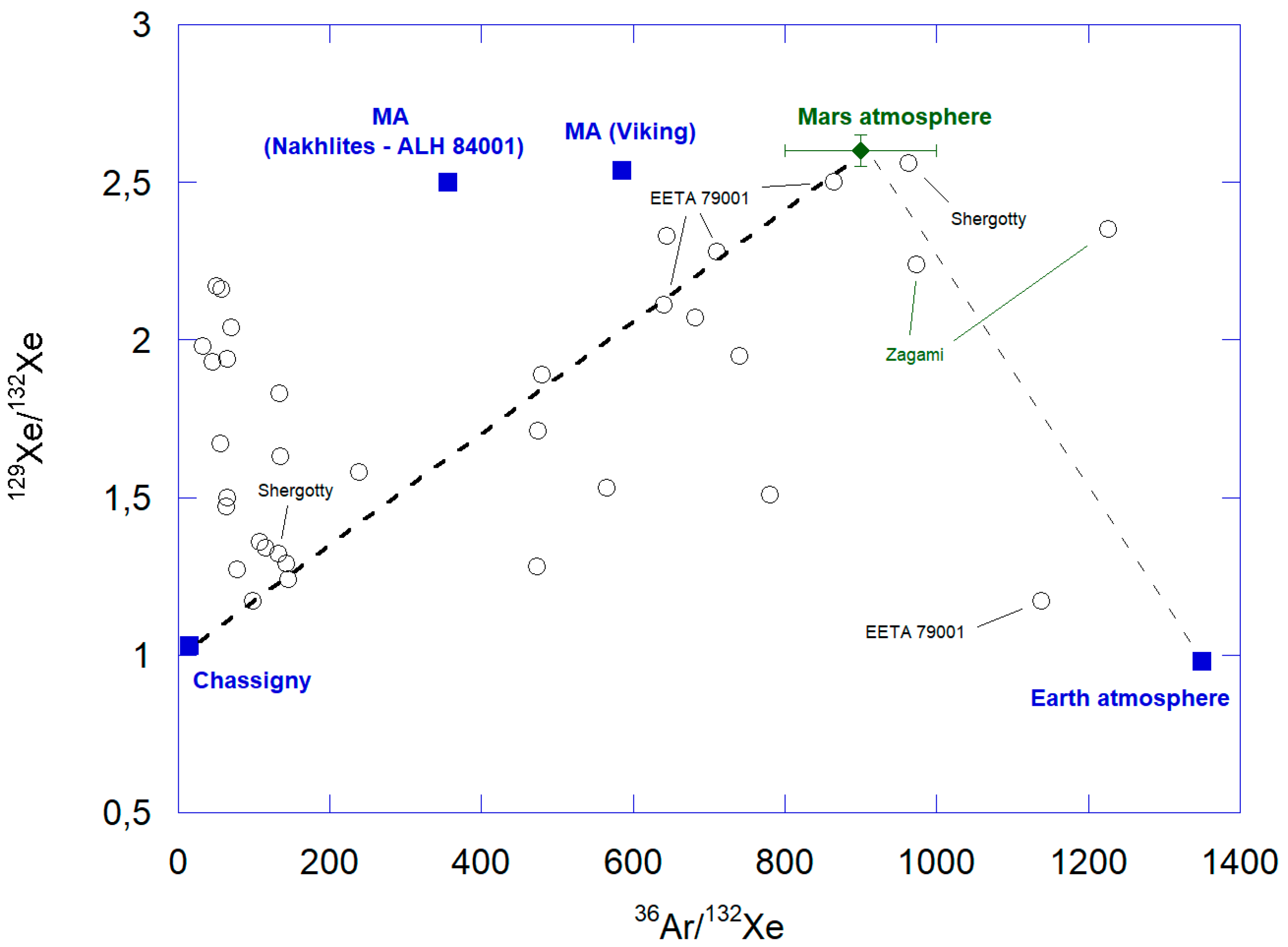
3.4. Krypton and Xenon
3.4.1. Elemental and Isotopic Abundance Patterns
Krypton
Xenon
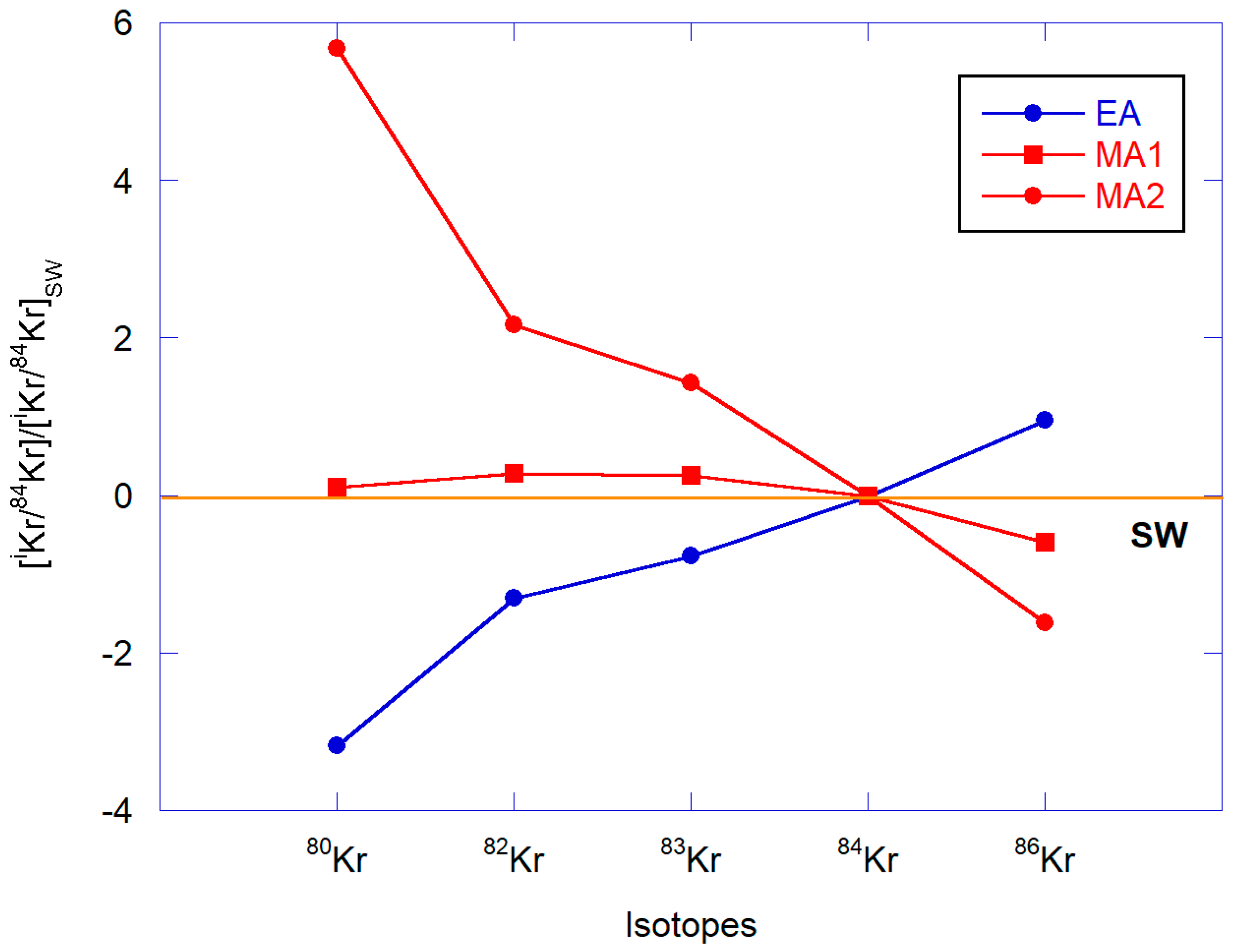
3.4.2. Isotopic Ratios
Krypton
Xenon
3.5. Elemental and Isotopic Heavy Noble Gas Ratios
4. Conclusions
- (i)
- The 36Ar/38Ar ratio is the lowest measured on any planetary material in the solar system, except in some Martian meteorites, with values ranging from 3.5–4.6 (Figure 3). It is generally lower than the first Viking measurements (i.e., 36Ar/38Ar ~ 5.5). A preferential loss of the lighter isotopes and an enrichment in the heavier have been as well identified for the D/H, 14N/15N, 12C/13C, or 16O/18O ratios, all in favor of an atmospheric loss during the last 4 Ga [35]. In addition, the significant enrichments in heavy Xe isotopes observed in the Martian atmosphere could be explained by a preferential loss of light Xe isotopes to space by hydrodynamic escape during the Martian early history [33,34,35];
- (ii)
- SAM onboard Curiosity measured a 40Ar/36Ar value of 1900 ± 300 [40], which is relatively consistent with the ratios measured in Martian meteorites such as EETA 79001 (40Ar/36Ar ~ 2050 ± 70 [63]) or Tissint (40Ar/36Ar = 1714 ± 170 [46]), but not matching the previous measurements by Viking (i.e., 40Ar/36Ar ~ 3000 ± 500, [20]);
- (iii)
- The xenon spectra show a clear enrichment in 129Xe, with, e.g., 129Xe/132Xe ~ 2.38–2.52, both determined by Viking, Curiosity, and in Martian meteorites. Such excesses could be explained by outgassing of radiogenic 129Xerad. Also, excesses in light Xe isotopes such as 124,126Xe have been detected by SAM, possibly reflecting spallation processes produced by GCRs on Ba and some other REEs;
- (iv)
- Similarly, enrichments in 80Kr can be due to neutron capture on 79Br induced by GCRs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozima, M.; Podosek, F.A. Noble Gas Geochemistry; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Wieler, R. Noble gases in the solar system. Rev. Mineral. Geochem. 2002, 47, 21–70. [Google Scholar] [CrossRef]
- Ott, U. Composition of the Martian atmosphere. Space Sci. Rev. 1991, 56, 23–29. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Conrad, P.G.; Coll, P.; Atreya, S.K.; Arvey, R.; Barciniak, M.; Benna, M.; Bleacher, L. The sample analysis at Mars investigation and instrument suite. Space Sci. Rev. 2012, 170, 401–478. [Google Scholar] [CrossRef]
- Caffee, M.; Hudson, G.; Velsko, C.; Huss, G.; Alexander, E.; Chivas, A. Primordial noble gases from Earth’s mantle: Identification of a primitive volatile component. Science 1999, 285, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.; Jenkins, W. Noble gases and mantle reservoirs: Constraints from isotope ratios, degassing fluxes, and noble gas abundances and ratios. AGU Fall Meet. Abstr. 2005, 2005, V22A-01. [Google Scholar]
- Mukhopadhyay, S.; Parai, R. Noble gases: A record of Earth’s evolution and mantle dynamics. Annu. Rev. Earth Planet. Sci. 2019, 47, 389–419. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Xiao, Y.; He, H.; Su, B.; Wang, Y.; Zhu, R. He and Ar isotope geochemistry of pyroxene megacrysts and mantle xenoliths in Cenozoic basalt from the Changle–Linqu area in western Shandong. Chin. Sci. Bull. 2014, 59, 396–411. [Google Scholar] [CrossRef]
- Swindle, T.D. Martian noble gases. Rev. Mineral. Geochem. 2002, 47, 171–190. [Google Scholar] [CrossRef]
- Curran, N.; Nottingham, M.; Alexander, L.; Crawford, I.; Füri, E.; Joy, K. A database of noble gases in lunar samples in preparation for mass spectrometry on the Moon. Planet. Space Sci. 2020, 182, 104823. [Google Scholar] [CrossRef]
- Wieler, R.; Heber, V. Noble gas isotopes on the Moon. Space Sci. Rev. 2003, 106, 197–210. [Google Scholar] [CrossRef]
- Niemann, H.; Harpold, D.; Atreya, S.; Carignan, G.; Hunten, D.; Owen, T. Galileo probe mass spectrometer experiment. In The Galileo Mission; Springer: Berlin/Heidelberg, Germany, 1992; pp. 111–142. [Google Scholar]
- Niemann, H.B.; Atreya, S.K.; Carignan, G.R.; Donahue, T.M.; Haberman, J.A.; Harpold, D.N.; Hartle, R.E.; Hunten, D.M.; Kasprzak, W.T.; Mahaffy, P.R. The Galileo probe mass spectrometer: Composition of Jupiter’s atmosphere. Science 1996, 272, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, L.; Altwegg, K.; Balsiger, H.; Berthelier, J.-J.; Bieler, A.; Briois, C.; Calmonte, U.; Combi, M.R.; De Keyser, J.; Dhooghe, F. Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from ROSETTA/ROSINA. Astron. Astrophys. 2015, 583, A1. [Google Scholar] [CrossRef] [Green Version]
- Luspay-Kuti, A.; Hässig, M.; Fuselier, S.; Mandt, K.; Altwegg, K.; Balsiger, H.; Gasc, S.; Jäckel, A.; Le Roy, L.; Rubin, M. Composition-dependent outgassing of comet 67P/Churyumov-Gerasimenko from ROSINA/DFMS-implications for nucleus heterogeneity? Astron. Astrophys. 2015, 583, A4. [Google Scholar] [CrossRef] [Green Version]
- Mall, U.; Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.-J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.R. High-time resolution in situ investigation of major cometary volatiles around 67P/C–G at 3.1–2.3 AU measured with Rosina-RTOF. Astrophys. J. 2016, 819, 126. [Google Scholar] [CrossRef]
- Marty, B.; Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Bekaert, D.; Berthelier, J.-J.; Bieler, A.; Briois, C.; Calmonte, U.; Combi, M. Xenon isotopes in 67P/Churyumov-Gerasimenko show that comets contributed to earth’s atmosphere. Science 2017, 356, 1069–1072. [Google Scholar] [CrossRef] [Green Version]
- Owen, T.; Biemann, K. Composition of the atmosphere at the surface of Mars: Detection of argon-36 and preliminary analysis. Science 1976, 193, 801–803. [Google Scholar] [CrossRef]
- Owen, T.; Biemann, K.; Rushneck, D.; Biller, J.; Howarth, D.; LaFleur, A. The atmosphere of Mars: Detection of krypton and xenon. Science 1976, 194, 1293–1295. [Google Scholar] [CrossRef]
- Owen, T.; Biemann, K.; Rushneck, D.; Biller, J.; Howarth, D.; Lafleur, A. The composition of the atmosphere at the surface of Mars. J. Geophys. Res. 1977, 82, 4635–4639. [Google Scholar] [CrossRef]
- Mousis, O.; Atkinson, D.H.; Cavalié, T.; Fletcher, L.; Amato, M.; Aslam, S.; Ferri, F.; Renard, J.B.; Spilker, T.; Venkatapathy, E. Scientific rationale for Uranus and Neptune in situ explorations. Planet. Space Sci. 2018, 155, 12–40. [Google Scholar] [CrossRef] [Green Version]
- Pepin, R. Meteorites: Evidence of Martian origins. Nature 1985, 317, 473–474. [Google Scholar] [CrossRef]
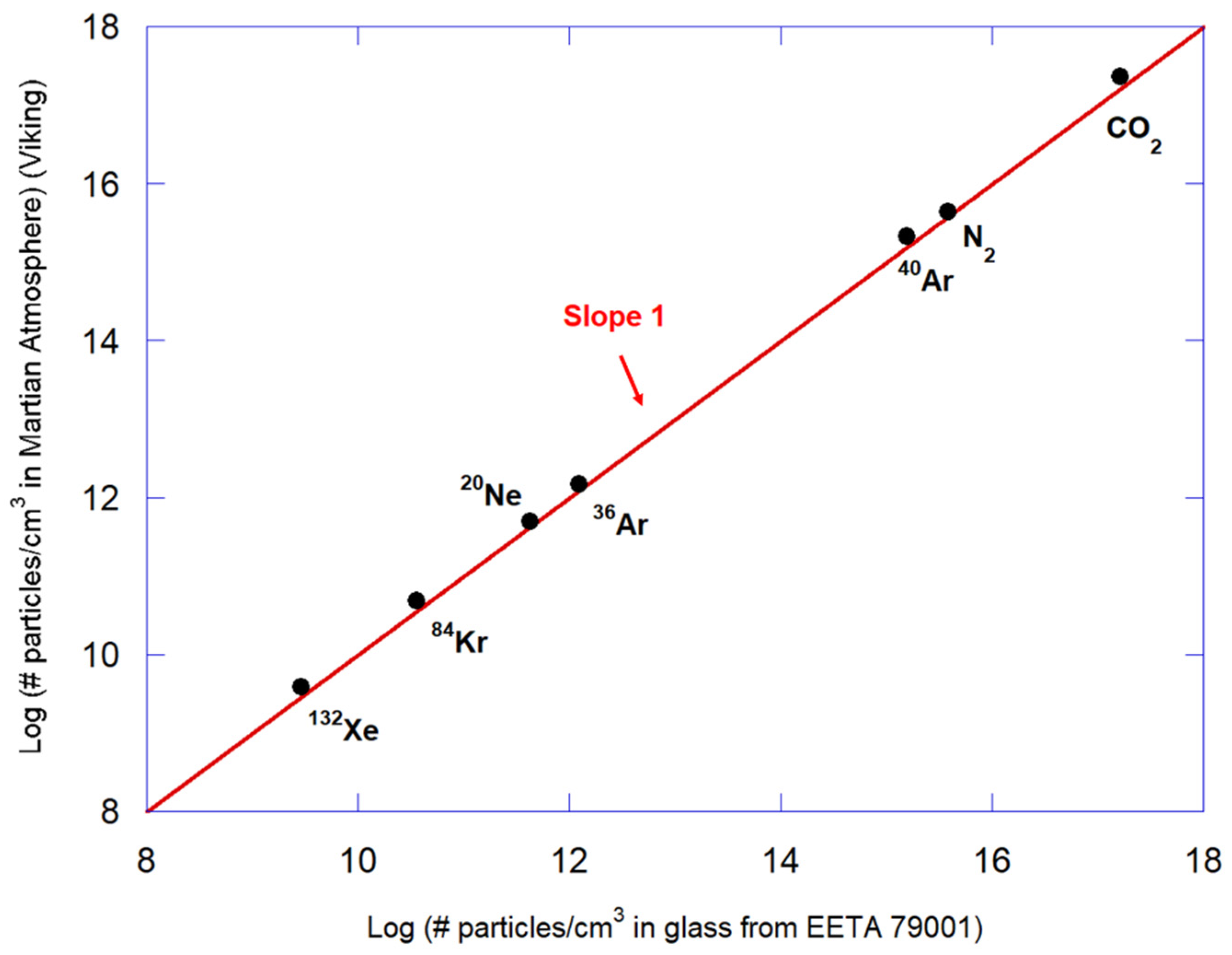
- Becker, R.; Pepin, R. The case for a martian origin of the shergottites: Nitrogen and noble gases in EETA 79001. Earth Planet. Sci. Lett. 1984, 69, 225–242. [Google Scholar] [CrossRef]
- Bogard, D.D.; Johnson, P. Martian gases in an Antarctic meteorite? Science 1983, 221, 651–654. [Google Scholar] [CrossRef]
- Irving, A.J. Martian Meteorites. Available online: https://imca.cc/mars/martian-meteorites.htm (accessed on 10 September 2020).
- McSween, H.Y., Jr. What we have learned about Mars from SNC meteorites. Meteoritics 1994, 29, 757–779. [Google Scholar] [CrossRef]
- Ott, U. Noble gases in SNC meteorites: Shergotty, Nakhla, Chassigny. Geochim. Cosmochim. Acta 1988, 52, 1937–1948. [Google Scholar] [CrossRef]
- McSween, H.Y., Jr. Petrology on Mars. Am. Mineral. 2015, 100, 2380–2395. [Google Scholar] [CrossRef]
- Marti, K.; Kim, J.; Thakur, A.; McCoy, T.J.; Keil, K. Signatures of the Martian atmosphere in glass of the Zagami meteorite. Science 1995, 267, 1981–1984. [Google Scholar] [CrossRef] [Green Version]
- Odert, P.; Lammer, H.; Erkaev, N.V.; Nikolaou, A.; Lichtenegger, H.I.; Johnstone, C.P.; Kislyakova, K.G.; Leitzinger, M.; Tosi, N. Escape and fractionation of volatiles and noble gases from Mars-sized planetary embryos and growing protoplanets. Icarus 2018, 307, 327–346. [Google Scholar] [CrossRef] [Green Version]
- Atreya, S.K.; Trainer, M.G.; Franz, H.B.; Wong, M.H.; Manning, H.L.; Malespin, C.A.; Mahaffy, P.R.; Conrad, P.G.; Brunner, A.E.; Leshin, L.A. Primordial argon isotope fractionation in the atmosphere of Mars measured by the SAM instrument on Curiosity and implications for atmospheric loss. Geophys. Res. Lett. 2013, 40, 5605–5609. [Google Scholar] [CrossRef] [Green Version]
- Jakosky, B.M.; Slipski, M.; Benna, M.; Mahaffy, P.; Elrod, M.; Yelle, R.; Stone, S.; Alsaeed, N. Mars’ atmospheric history derived from upper-atmosphere measurements of 38Ar/36Ar. Science 2017, 355, 1408–1410. [Google Scholar] [CrossRef] [Green Version]
- Cassata, W.S. Meteorite constraints on Martian atmospheric loss and paleoclimate. Earth Planet. Sci. Lett. 2017, 479, 322–329. [Google Scholar] [CrossRef]
- Luhmann, J.; Johnson, R.; Zhang, M. Evolutionary impact of sputtering of the Martian atmosphere by O+ pickup ions. Geophys. Res. Lett. 1992, 19, 2151–2154. [Google Scholar] [CrossRef]
- Pepin, R.O. On the origin and early evolution of terrestrial planet atmospheres and meteoritic volatiles. Icarus 1991, 92, 2–79. [Google Scholar] [CrossRef]
- Koike, M.; Sumino, H.; Sano, Y.; Ozima, M. Combined stepwise heating and vacuum crushing analyses of noble gases in shergottites. In Proceedings of the Lunar and Planetary Science Conference, Woodlands, TX, USA, 20–24 March 2017. [Google Scholar]
- Park, J.; Nagao, K. New insights on Martian atmospheric neon from Martian meteorite, Dhofar 378. In Proceedings of the 37th Lunar and Planetary Science Conference, Woodlands, TX, USA, 14–18 March 2006. [Google Scholar]
- Park, J.; Nyquist, L.; Herzog, G.; Nagao, K.; Mikouchi, T.; Kusakabe, M. 20Ne/22Ne in the Martian atmosphere: New evidence from Martian meteorites. In Proceedings of the Lunar and Planetary Science Conference, Woodlands, TX, USA, 20–24 March 2017. [Google Scholar]
- Wiens, R.; Becker, R.; Pepin, R. The case for a Martian origin of the Shergottites, ii. Trapped and indigenous gas components in EETA 79001 glass. Earth Planet. Sci. Lett. 1986, 77, 149–158. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Webster, C.R.; Atreya, S.K.; Franz, H.; Wong, M.; Conrad, P.G.; Harpold, D.; Jones, J.J.; Leshin, L.A.; Manning, H. Abundance and isotopic composition of gases in the Martian atmosphere from the Curiosity rover. Science 2013, 341, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Conrad, P.G.; Malespin, C.A.; Franz, H.B.; Pepin, R.O.; Trainer, M.G.; Schwenzer, S.P.; Atreya, S.; Freissinet, C.; Jones, J.; Manning, H. In situ measurement of atmospheric krypton and xenon on Mars with Mars Science Laboratory. Earth Planet. Sci. Lett. 2016, 454, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mahaffy, P.R.; Benna, M.; King, T.; Harpold, D.N.; Arvey, R.; Barciniak, M.; Bendt, M.; Carrigan, D.; Errigo, T.; Holmes, V. The neutral gas and ion mass spectrometer on the Mars atmosphere and volatile evolution mission. Space Sci. Rev. 2015, 195, 49–73. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Mohankumar, S.; Das, T.P.; Pradeepkumar, P.; Sreelatha, P.; Sundar, B.; Nandi, A.; Vajja, D.P.; Dhanya, M.; Naik, N. MENCA experiment aboard India’s Mars orbiter mission. Curr. Sci. 2015, 109, 1106–1113. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Thampi, S.V.; Das, T.P.; Dhanya, M.; Naik, N.; Vajja, D.P.; Pradeepkumar, P.; Sreelatha, P.; Thampi, R.S.; Yadav, V.K. Observation of suprathermal argon in the exosphere of Mars. Geophys. Res. Lett. 2017, 44, 2088–2095. [Google Scholar] [CrossRef]
- Wieler, R. Noble gas mass spectrometry. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 15, pp. 355–373. [Google Scholar]
- Avice, G.; Bekaert, D.; Chennaoui Aoudjehane, H.; Marty, B. Noble gases and nitrogen in Tissint reveal the composition of the Mars atmosphere. Geochem. Perspect. Lett. 2018, 6, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Wieler, R.; Huber, L.; Busemann, H.; Seiler, S.; Leya, I.; Maden, C.; Masarik, J.; Meier, M.; Nagao, K.; Trappitsch, R. Noble gases in 18 Martian meteorites and Angrite Northwest Africa 7812—Exposure ages, trapped gases, and a re-evaluation of the evidence for solar cosmic ray-produced neon in shergottites and other achondrites. Meteorit. Planet. Sci. 2016, 51, 407–428. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhu, R.; Saxton, J. Noble gas isotopes in corundum and peridotite xenoliths from the eastern north China craton: Implication for comprehensive refertilization of lithospheric mantle. Phys. Earth Planet. Inter. 2011, 189, 185–191. [Google Scholar] [CrossRef]
- Ranjith, P.; He, H.; Miao, B.; Su, F.; Zhang, C.; Xia, Z.; Xie, L.; Zhu, R. Petrographic shock indicators and noble gas signatures in a H and an L chondrite from Antarctica. Planet. Space Sci. 2017, 146, 20–29. [Google Scholar] [CrossRef]
- Frick, U.; Pepin, R. On the distribution of noble gases in Allende: A differential oxidation study. Earth Planet. Sci. Lett. 1981, 56, 45–63. [Google Scholar] [CrossRef]
- Wiens, R. Noble gases released by vacuum crushing of EETA 79001 glass. Earth Planet. Sci. Lett. 1988, 91, 55–65. [Google Scholar] [CrossRef]
- Blard, P.H.; Puchol, N.; Farley, K. Constraints on the loss of matrix-sited helium during vacuum crushing of mafic phenocrysts. Geochim. Cosmochim. Acta 2008, 72, 3788–3803. [Google Scholar] [CrossRef]
- Ott, U.; Swindle, T.D.; Schwenzer, S.P. Noble gases in Martian meteorites: Budget, sources, sinks, and processes. In Volatiles in the Martian Crust; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–70. [Google Scholar]
- Guo, W.; He, H.; Hilton, D.R.; Zheng, Y.; Su, F.; Liu, Y.; Zhu, R. Recycled noble gases preserved in podiform chromitites from Luobusa, Tibet. Chem. Geol. 2017, 469, 97–109. [Google Scholar] [CrossRef]
- Riebe, M.E.; Welten, K.C.; Meier, M.M.; Wieler, R.; Barth, M.; Ward, D.; Laubenstein, M.; Bischoff, A.; Caffee, M.W.; Nishiizumi, K. Cosmic-ray exposure ages of six chondritic Almahata Sitta fragments. Meteorit. Planet. Sci. 2017, 52, 2353–2374. [Google Scholar] [CrossRef]
- Lott, D.E., III. Improvements in noble gas separation methodology: A nude cryogenic trap. Geochem. Geophys. Geosyst. 2001, 2. [Google Scholar] [CrossRef]
- Mahajan, R.R. Exposure ages, noble gases and nitrogen in the ordinary chondrite Karimati (L5). Earth Moon Planets 2020, 124, 3–13. [Google Scholar] [CrossRef]
- Mahajan, R.R. Noble gases and nitrogen in metal from the ordinary chondrites Katol (L6), Itawa Bhopji (L3-5) and Portales Valley (H6). Astrophys. Space Sci. 2020, 365, 130. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Schwenzer, S.P.; Herrmann, S.; Murty, S.; Ott, U.; Gilmour, J.D. Noble gases and nitrogen in Martian meteorites Dar Al Gani 476, Sayh Al Uhaymir 005 and Lewis Cliff 88516: EFA and extra neon. Geochim. Cosmochim. Acta 2009, 73, 1505–1522. [Google Scholar] [CrossRef]
- Heber, V.S.; Wieler, R.; Baur, H.; Olinger, C.; Friedmann, T.A.; Burnett, D.S. Noble gas composition of the solar wind as collected by the Genesis mission. Geochim. Cosmochim. Acta 2009, 73, 7414–7432. [Google Scholar] [CrossRef]
- Garrison, D.H.; Bogard, D.D. Isotopic composition of trapped and cosmogenic noble gases in several Martian meteorites. Meteorit. Planet. Sci. 1998, 33, 721–736. [Google Scholar] [CrossRef] [Green Version]
- Bogard, D.D. A reappraisal of the Martian 36Ar/38Ar ratio. J. Geophys. Res. Planets 1997, 102, 1653–1661. [Google Scholar] [CrossRef]
- Bogard, D.; Clayton, R.; Marti, K.; Owen, T.; Turner, G. Martian volatiles: Isotopic composition, origin, and evolution. In Chronology and Evolution of Mars; Springer: Berlin/Heidelberg, Germany, 2001; pp. 425–458. [Google Scholar]
- Mathew, K.; Marti, K. Early evolution of Martian volatiles: Nitrogen and noble gas components in ALH 84001 and Chassigny. J. Geophys. Res. Planets 2001, 106, 1401–1422. [Google Scholar] [CrossRef]
- Owen, T. The composition and early history of the atmosphere of Mars. In Mars; University of Arizona Press: Tucson, AZ, USA, 1992; pp. 818–834. [Google Scholar]
- Nakamura, M.; Yamashita, K.; Yoshikawa, I.; Shiomi, K.; Yamazaki, A.; Sasaki, S.; Takizawa, Y.; Hirahara, M.; Miyake, W.; Saito, Y. Helium observation in the Martian ionosphere by an X-ray ultraviolet scanner on Mars orbiter Nozomi. Earth Planets Space 1999, 51, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Krasnopolsky, V.; Bowyer, S.; Chakrabarti, S.; Gladstone, G.; McDonald, J. First measurement of helium on Mars: Implications for the problem of radiogenic gases on the terrestrial planets. Icarus 1994, 109, 337–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnopolsky, V.A.; Gladstone, G.R. Helium on Mars and Venus: EUVE observations and modeling. Icarus 2005, 176, 395–407. [Google Scholar] [CrossRef]
- Brennecka, G.; Borg, L.; Wadhwa, M. Insights into the Martian mantle: The age and isotopics of the meteorite fall Tissint. Meteorit. Planet. Sci. 2014, 49, 412–418. [Google Scholar] [CrossRef]
- Nishiizumi, K.; Nagao, K.; Caffee, M.; Jull, A.; Irving, A. Cosmic-ray exposure chronologies of depleted olivine-phyric shergottites. In Proceedings of the 42nd Lunar and Planetary Science Conference, Woodlands, TX, USA, 7–11 March 2011; p. 2371. [Google Scholar]
- Povinec, P.; Koeberl, C.; Jull, A.; Sýkora, I.; Ferrière, L.; Kovácik, A. Radionuclides in the Tissint meteorite: Implications for its martian origin. In Proceedings of the Lunar and Planetary Science Conference, Woodlands, TX, USA, 18–22 March 2019. [Google Scholar]
- Busemann, H.; Baur, H.; Wieler, R. Primordial noble gases in “phase Q” in carbonaceous and ordinary chondrites studied by closed-system stepped etching. Meteorit. Planet. Sci. 2000, 35, 949–973. [Google Scholar] [CrossRef]
- Schultz, L.; Franke, L. Helium, neon, and argon in meteorites: A data collection. Meteorit. Planet. Sci. 2004, 39, 1889–1890. [Google Scholar] [CrossRef]
- Llorca, J.; Roszjar, J.; Cartwright, J.A.; Bischoff, A.; Ott, U.; Pack, A.; Merchel, S.; Rugel, G.; Fimiani, L.; Ludwig, P.; et al. The Ksar Ghilane 002 shergottite-the 100th registered Martian fragment. Meteorit. Planet. Sci. 2013, 48, 493–513. [Google Scholar] [CrossRef]
- Murty, S.; Mahajan, R.; Das, J.; Sinha, N.; Goswami, J.J.P.R. Trapped and cosmogenic gas components and nuclear tracks in the Nakhlite Y000593. In Proceedings of the International Symposium, Evolution of Solar System Materials: A New Perspective from Antarctic Meteorites. National Institute of Polar Research, Tokyo, Japan, 3–5 September 2003; pp. 90–91. [Google Scholar]
- Schwenzer, S.P.; Herrmann, S.; Ott, U. Noble gases in two shergottites and one nakhlite from Antarctica: Y00027, Y00097, and Y000593. Polar Sci. 2009, 3, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, J.; Ott, U.; Herrmann, S.; Agee, C. Modern atmospheric signatures in 4.4 Ga Martian meteorite NWA 7034. Earth Planet. Sci. Lett. 2014, 400, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, K.; Matsuda, J. Anomalous Ne enrichments in tektites. Meteoritics 1991, 26, 217–220. [Google Scholar] [CrossRef]
- Jakosky, B.M.; Pepin, R.O.; Johnson, R.E.; Fox, J.L. Mars atmospheric loss and isotopic fractionation by solar-wind-induced sputtering and photochemical escape. Icarus 1994, 111, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Marti, K.; Severinghaus, J.P.; Kawamura, K.; Yoo, H.-S.; Lee, J.B.; Kim, J.S. A redetermination of the isotopic abundances of atmospheric Ar. Geochim. Cosmochim. Acta 2006, 70, 4507–4512. [Google Scholar] [CrossRef]
- Mahaffy, P.; Niemann, H.; Alpert, A.; Atreya, S.; Demick, J.; Donahue, T.; Harpold, D.; Owen, T. Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo probe mass spectrometer. J. Geophys. Res. Planets 2000, 105, 15061–15071. [Google Scholar] [CrossRef]
- Pepin, R.O.; Schlutter, D.J.; Becker, R.H.; Reisenfeld, D.B. Helium, neon, and argon composition of the solar wind as recorded in gold and other Genesis collector materials. Geochim. Cosmochim. Acta 2012, 89, 62–80. [Google Scholar] [CrossRef]
- Garrison, D.; Hamlin, S.; Bogard, D. Chlorine abundances in meteorites. Meteorit. Planet. Sci. 2000, 35, 419–429. [Google Scholar] [CrossRef]
- Wong, M.H.; Atreya, S.K.; Mahaffy, P.N.; Franz, H.B.; Malespin, C.; Trainer, M.G.; Stern, J.C.; Conrad, P.G.; Manning, H.L.; Pepin, R.O. Isotopes of nitrogen on Mars: Atmospheric measurements by Curiosity’s mass spectrometer. Geophys. Res. Lett. 2013, 40, 6033–6037. [Google Scholar] [CrossRef] [Green Version]
- Murty, S.; Mohapatra, R. Nitrogen and heavy noble gases in ALH 84001: Signatures of ancient Martian atmosphere. Geochim. Cosmochim. Acta 1997, 61, 5417–5428. [Google Scholar] [CrossRef]
- Cassata, W.S.; Shuster, D.L.; Renne, P.R.; Weiss, B.P. Trapped Ar isotopes in meteorite ALH 84001 indicate Mars did not have a thick ancient atmosphere. Icarus 2012, 221, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.N.; Bogard, D.D.; Nyquist, L.E.; McKay, S.D.; Masarik, J. Neutron capture isotopes in the Martian regolith and implications for Martian atmospheric noble gases. Icarus 2002, 156, 353–372. [Google Scholar] [CrossRef]
- Mazor, E.; Heymann, D.; Anders, E. Noble gases in carbonaceous chondrites. Geochim. Cosmochim. Acta 1970, 34, 781–824. [Google Scholar] [CrossRef]
- Cartwright, J. Noble Gas Components in Martian Meteorites. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2010. [Google Scholar]
- Swindle, T.; Caffee, M.; Hohenberg, C.J.G. Xenon and other noble gases in Shergottites. Geochim. Cosmochim. Acta 1986, 50, 1001–1015. [Google Scholar] [CrossRef]
- Avice, G.; Marty, B. Perspectives on atmospheric evolution from noble gas and nitrogen isotopes on Earth, Mars & Venus. Space Sci. Rev. 2020, 216, 36. [Google Scholar] [CrossRef] [Green Version]
- Bekaert, D.V.; Broadley, M.W.; Marty, B. The origin and fate of volatile elements on Earth revisited in light of noble gas data obtained from comet 67P/Churyumov-Gerasimenko. Sci. Rep. 2020, 10, 5796. [Google Scholar] [CrossRef]
- Yokochi, R.; Marty, B. A determination of the neon isotopic composition of the deep mantle. Earth Planet. Sci. Lett. 2004, 225, 77–88. [Google Scholar] [CrossRef]
- Broadley, M.W.; Barry, P.H.; Bekaert, D.V.; Byrne, D.J.; Caracausi, A.; Ballentine, C.J.; Marty, B. Indentification of chondritic krypton and xenon in Yellowstone gases and the timing of terrestrial volatile accretion. Proc. Natl. Acad. Sci. USA 2020, 117, 13997–14004. [Google Scholar] [CrossRef]
- Holland, G.; Cassidy, M.; Ballentine, C.J. Meteorite Kr in Earth’s mantle suggests a late accretionary source for the atmosphere. Science 2009, 326, 1522–1525. [Google Scholar] [CrossRef]
- Pepin, R.O. On the Isotopic Composition of Primordial Xenon in Terrestrial Planet Atmospheres. Space Sci. Rev. 2000, 92, 371–395. [Google Scholar] [CrossRef]
- Gilmour, J.; Whitby, J.; Turner, G. Xenon isotopes in irradiated ALH84001: Evidence for shock-induced trapping of ancient Martian atmosphere. Geochim. Cosmochim. Acta 1998, 62, 2555–2571. [Google Scholar] [CrossRef]
- Bogard, D.D.; Garrison, D.H. Relative abundances of argon, krypton, and xenon in the Martian atmosphere as measured in Martian meteorites. Geochim. Cosmochim. Acta 1998, 62, 1829–1835. [Google Scholar] [CrossRef]
- Grady, M.M. Exploring Mars with returned samples. Space Sci. Rev. 2020, 216, 51. [Google Scholar] [CrossRef]





| Nuclide | Production Path | Main Target Elements |
|---|---|---|
| 3He | Spallation | O, Mg, Al, Si, Ca, Fe, Ni |
| 4He | Radiogenic Daughter of 235,238U and 232Th decay chains | O, Mg, Al, Si, Ca, Fe, Ni |
| 20,21,22Ne | Spallation | Mg, Al, Si, Ca, Fe, Ni |
| 36Ar | Decay product of 36Cl | Cl, Ca, Ti, Fe, Ni |
| 38Ar | Spallation | Cl, Ca, Ti, Fe, Ni |
| 40Ar | Radiogenic | Decay product of 40K |
| 81Kr | Radioactive, T1/2 = 2.29 × 105 years | Rb, Sr, Y, Zr |
| 78,83,84,86Kr | Spallation | Rb, Sr, Y, Zr |
| 80,82Kr | Spallation Neutron capture of 79,81Br | Br, Rb, Sr, Y, Zr |
| 124,126,128,130Xe | Spallation | Te, Ba, La, Ce |
| 129,131,132,134,126Xe | Spontaneous, and induced fission on U and extinct 244Pu | Te, Ba, La, Ce, U |
| 129Xe | Radiogenic 129Xe is the decay product of extinct 129I | Te, Ba, La, Ce, U |
| Gas | Abundance |
|---|---|
| CO2 | 95.32% |
| N2 | 2.7% |
| Ar | 1.6% |
| O2 | 0.13% |
| CO | 0.07% |
| H2O | 0.03% |
| Ne | 2.5 ppm |
| Kr | 0.3 ppm |
| Xe | 0.08 ppm |
| O3 | 0.03 ppm |
| Mission | Goals | Payloads | Achievements |
|---|---|---|---|
| Viking | −Detect and identify organic compounds −Determine the composition of the lower atmosphere | Mass spectrometer coupled with gas chromatograph | −Atmospheric elemental abundances, including noble gases |
| Curiosity | −Understand the potential of the present or past Martian environments to support life −Chemical and isotopic composition of the atmosphere | Sample Analysis at Mars (SAM): quadrupole mass spectrometer, tunable laser spectrometer, gas chromatograph | −Measurements of all of the stable isotopes of the heavy noble gases in the Martian atmosphere |
| MAVEN | −Study the Martian atmosphere, and the geological evolution and potential habitability of the planet −Evaluate the amount of atmospheric gas which has been lost to space over geological time | Neutral Gas and Ion Mass Spectrometer (NGIMS), a quadrupole mass spectrometer | −Characterization of the Martian upper atmospheric structure −Fractionation of argon |
| MOM | Understand the escape of the Martian atmosphere over geological time | Mars Exospheric Neutral Composition Analyser (MENCA), a quadrupole mass spectrometer | −Exospheric composition of Mars −Altitude profiles of 40Ar in the Martian exosphere |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, T.; Ranjith, P.M.; He, H.; Zhu, R. Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions. Geosciences 2020, 10, 439. https://doi.org/10.3390/geosciences10110439
Smith T, Ranjith PM, He H, Zhu R. Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions. Geosciences. 2020; 10(11):439. https://doi.org/10.3390/geosciences10110439
Chicago/Turabian StyleSmith, Thomas, P. M. Ranjith, Huaiyu He, and Rixiang Zhu. 2020. "Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions" Geosciences 10, no. 11: 439. https://doi.org/10.3390/geosciences10110439






