Hydrogen Isotopic Variations in the Shergottites
Abstract
:1. Introduction
2. Water Reservoirs on Mars
3. Alteration Minerals in the Shergottites
4. Evidence of Subsurface Water-Rock Interactions on Mars in the Shergottites
4.1. Melt Inclusions
4.2. Apatite
4.3. Merrillite
4.4. Maskelynite
4.5. Impact Melt Glasses and Groundmass Glasses
4.6. Nominal Anhydrous Minerals/Phases
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bridges, J.C.; Warren, P.H. The SNC meteorites: Basaltic igneous processes on Mars. J. Geol. Soc. 2006, 163, 229–251. [Google Scholar] [CrossRef] [Green Version]
- Mcsween, H.Y. What We Have Learned About Mars from SNC Meteorites. Meteoritics 1994, 29, 757–779. [Google Scholar] [CrossRef]
- Agee, C.B.; Wilson, N.V.; McCubbin, F.M.; Ziegler, K.; Polyak, V.J.; Sharp, Z.D.; Asmerom, Y.; Nunn, M.H.; Shaheen, R.; Thiemens, M.H.; et al. Unique meteorite from early Amazonian Mars: Water-rich basaltic breccia Northwest Africa 7034. Science 2013, 339, 780–785. [Google Scholar] [CrossRef]
- Humayun, M.; Nemchin, A.; Zanda, B.; Hewins, R.H.; Grange, M.; Kennedy, A.; Lorand, J.P.; Gopel, C.; Fieni, C.; Pont, S.; et al. Origin and age of the earliest Martian crust from meteorite NWA 7533. Nature 2013, 503, 513–516. [Google Scholar] [CrossRef]
- Donahue, T.M. Pre-global surveyor evidence for Martian ground water. Proc. Natl. Acad. Sci. USA 2001, 98, 827–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyquist, L.E.; Bogard, D.D.; Shih, C.Y.; Greshake, A.; Stoffler, D.; Eugster, O. Ages and geologic histories of Martian meteorites. Space Sci. Rev. 2001, 96, 105–164. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Barnes, J.J. Origin and abundances of H2O in the terrestrial planets, Moon, and asteroids. Earth Planet. Sci. Lett. 2019, 526, 115771. [Google Scholar] [CrossRef]
- Hallis, L.J. D/H ratios of the inner Solar System. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2017, 375, 20150390. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, C.A. Olivine-phyric martian basalts: A new type of shergottite. Meteorit. Planet. Sci. 2002, 37, B31. [Google Scholar] [CrossRef]
- Howarth, G.H.; Pernet-Fisher, J.F.; Balta, J.B.; Barry, P.H.; Bodnar, R.J.; Taylor, L.A. Two-stage polybaric formation of the new enriched, pyroxene-oikocrystic, lherzolitic shergottite, NWA 7397. Meteorit. Planet. Sci. 2014, 49, 1812. [Google Scholar] [CrossRef]
- Howarth, G.H.; Pernet-Fisher, J.F.; Bodnar, R.J.; Taylor, L.A. Evidence for the exsolution of Cl-rich fluids in martian magmas: Apatite petrogenesis in the enriched lherzolitic shergottite Northwest Africa 7755. Geochim. Cosmochim. Acta 2015, 166, 234. [Google Scholar] [CrossRef]
- Howarth, G.H.; Udry, A. Trace elements in olivine and the petrogenesis of the intermediate, olivine-phyric shergottite NWA 10170. Meteorit. Planet. Sci. 2017, 52, 391–409. [Google Scholar] [CrossRef]
- Howarth, G.H.; Udry, A.; Day, J.M.D. Petrogenesis of basaltic shergottite Northwest Africa 8657: Implications for fO2 correlations and element redistribution during shock melting in shergottites. Meteorit. Planet. Sci. 2018, 53, 249–267. [Google Scholar] [CrossRef]
- Mikouchi, T.; Kurihara, T. Mineralogy and petrology of paired lherzolitic shergottites Yamato 000027, Yamato 000047, and Yamato 000097: Another fragment from a Martian “lherzolite” block. Polar Sci. 2008, 2, 175. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Sanborn, M.; Wadhwa, M.; McSween, H.Y., Jr. Petrology and trace element geochemistry of Robert Massif 04261 and 04262 meteorites, the first examples of geochemically enriched lherzolitic shergottites. Geochim. Cosmochim. Acta 2010, 74, 7283. [Google Scholar] [CrossRef]
- Combs, L.M.; Udry, A.; Howarth, G.H.; Righter, M.; Lapen, T.J.; Gross, J.; Ross, D.K.; Rahib, R.R.; Day, J.M.D. Petrology of the enriched poikilitic shergottite Northwest Africa 10169: Insight into the martian interior. Geochim. Cosmochim. Acta 2019, 266, 435–462. [Google Scholar] [CrossRef]
- Filiberto, J.; Gross, J.; Udry, A.; Trela, J.; Wittmann, A.; Cannon, K.M.; Penniston-Dorland, S.; Ash, R.; Hamilton, V.E.; Meado, A.L.; et al. Shergottite Northwest Africa 6963: A Pyroxene-Cumulate Martian Gabbro. J. Geophys. Res. Planets 2018, 123, 1823–1841. [Google Scholar] [CrossRef]
- Udry, A.; Howarth, G.H.; Lapen, T.J.; Righter, M. Petrogenesis of the NWA 7320 enriched martian gabbroic shergottite: Insight into the martian crust. Geochim. Cosmochim. Acta 2017, 204, 1–18. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Walton, E.L.; Agee, C.B.; Muttik, N.; Ziegler, K.; Shearer, C.K.; Bell, A.S.; Santos, A.R.; Burger, P.V.; Simon, J.I.; et al. The Northwest Africa 8159 martian meteorite: Expanding the martian sample suite to the early Amazonian. Geochim. Cosmochim. Acta 2017, 218, 1–26. [Google Scholar] [CrossRef]
- Lapen, T.J.; Righter, M.; Andreasen, R.; Irving, A.J.; Satkoski, A.M.; Beard, B.L.; Nishiizumi, K.; Jull, A.J.; Caffee, M.W. Two billion years of magmatism recorded from a single Mars meteorite ejection site. Sci. Adv. 2017, 3, e1600922. [Google Scholar] [CrossRef] [Green Version]
- Debaille, V.; Yin, Q.-Z.; Brandon, A.D.; Jacobsen, B. Martian mantle mineralogy investigated by the 176Lu- 176Hf and 147Sm- 143Nd systematics of shergottites. Earth Planet. Sci. Lett. 2008, 269, 186–199. [Google Scholar] [CrossRef]
- McSween, H.Y., Jr. Petrology on Mars. Am. Mineral. 2015, 100, 2380. [Google Scholar] [CrossRef]
- Borg, L.E.; Draper, D.S. A petrogenetic model for the origin and compositional variation of the martian basaltic meteorites. Meteorit. Planet. Sci. 2003, 38, 1713–1731. [Google Scholar] [CrossRef]
- Filiberto, J.; Chin, E.; Day, J.M.D.; Franchi, I.A.; Greenwood, R.C.; Gross, J.; Penniston-Dorland, S.C.; Schwenzer, S.P.; Treiman, A.H. Geochemistry of intermediate olivine-phyric shergottite Northwest Africa 6234, with similarities to basaltic shergottite Northwest Africa 480 and olivine-phyric shergottite Northwest Africa 2990. Meteorit. Planet. Sci. 2012, 47, 1256–1273. [Google Scholar] [CrossRef]
- Jones, J.H. Various aspects of the petrogenesis of the Martian shergottite meteorites. Meteorit. Planet. Sci. 2015, 50, 674–690. [Google Scholar] [CrossRef]
- Longhi, J. Complex magmatic processes on Mars—Inferences from the SNC meteorites. Proc. Lunar Planet. Sci. 1991, 21, 695–709. [Google Scholar]
- Norman, M.D. The composition and thickness of the crust of Mars estimated from rare earth elements and neodymium-isotopic compositions of Martian meteorites. Meteorit. Planet. Sci. 1999, 34, 439–449. [Google Scholar] [CrossRef]
- Borg, L.E.; Nyquist, L.E.; Wiesmann, H.; Reese, Y. Constraints on the petrogenesis of Martian meteorites from the Rb-Sr and Sm-Nd isotopic systematics of the lherzolitic shergottites ALH77005 and LEW88516. Geochim. Cosmochim. Acta 2002, 66, 2037. [Google Scholar] [CrossRef]
- Borg, L.E.; Nyquist, L.E.; Wiesmann, H.; Shih, C.-Y.; Reese, Y. The age of Dar al Gani 476 and the differentiation history of the martian meteorites inferred from their radiogenic isotopic systematics. Geochim. Cosmochim. Acta 2003, 67, 3519. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Borg, L.E.; Jones, J.H.; Papike, J.J. Oxygen fugacity and geochemical variations in the martian basalts: Implications for martian basalt petrogenesis and the oxidation state of the upper mantle of Mars. Geochim. Cosmochim. Acta 2002, 66, 2025–2036. [Google Scholar] [CrossRef]
- Basu Sarbadhikari, A.; Day, J.M.D.; Liu, Y.; Rumble, D., III; Taylor, L.A. Petrogenesis of olivine-phyric shergottite Larkman Nunatak 06319: Implications for enriched components in martian basalts. Geochim. Cosmochim. Acta 2009, 73, 2190. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Hauri, E.H.; Elardo, S.M.; Vander Kaaden, K.E.; Wang, J.; Shearer, C.K. Hydrous melting of the martian mantle produced both depleted and enriched shergottites. Geology 2012, 40, 683–686. [Google Scholar] [CrossRef]
- Symes, S.J.K.; Borg, L.E.; Shearer, C.K.; Irving, A.J. The age of the martian meteorite Northwest Africa 1195 and the differentiation history of the shergottites. Geochim. Cosmochim. Acta 2008, 72, 1696–1710. [Google Scholar] [CrossRef] [Green Version]
- Warren, P.H.; Greenwood, J.P.; Rubin, A.E. Los Angeles: A tale of two stones. Meteorit. Planet. Sci. 2004, 39, 137–156. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Zhang, J.; Hao, J.; Xing, W.; Zhang, T.; Yang, W.; Changela, H. Ancient geologic events on Mars revealed by zircons and apatites from the Martian regolith breccia NWA 7034. Meteorit. Planet. Sci. 2019, 54, 850–879. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Boyce, J.W.; Novák-Szabó, T.; Santos, A.R.; Tartèse, R.; Muttik, N.; Domokos, G.; Vazquez, J.; Keller, L.P.; Moser, D.E.; et al. Geologic history of Martian regolith breccia Northwest Africa 7034: Evidence for hydrothermal activity and lithologic diversity in the Martian crust. J. Geophys. Res. Planets 2016, 121, 2120–2149. [Google Scholar] [CrossRef]
- Lapen, T.J.; Righter, M.; Brandon, A.D.; Debaille, V.; Beard, B.L.; Shafer, J.T.; Peslier, A.H. A Younger Age for ALH84001 and Its Geochemical Link to Shergottite Sources in Mars. Science 2010, 328, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, J.J.; Nemchin, A.A.; Whitehouse, M.J.; Snape, J.F.; Bland, P.; Benedix, G.K. The Pb isotopic evolution of the Martian mantle constrained by initial Pb in Martian meteorites. J. Geophys. Res. Planets 2015, 120, 2224–2240. [Google Scholar] [CrossRef] [Green Version]
- Moser, D.E.; Chamberlain, K.R.; Tait, K.T.; Schmitt, A.K.; Darling, J.R.; Barker, I.R.; Hyde, B.C. Solving the Martian meteorite age conundrum using micro-baddeleyite and launch-generated zircon. Nature 2013, 499, 454–457. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Takeno, S.; Taylor, L.a.; McSween, H.y. Ion microprobe uranium-thorium-lead dating of Shergotty phosphates. Meteorit. Planet. Sci. 2000, 35, 341–346. [Google Scholar] [CrossRef]
- Zhou, Q.; Herd, C.D.K.; Yin, Q.-Z.; Li, X.-H.; Wu, F.-Y.; Li, Q.-L.; Liu, Y.; Tang, G.-Q.; McCoy, T.J. Geochronology of the Martian meteorite Zagami revealed by U–Pb ion probe dating of accessory minerals. Earth Planet. Sci. Lett. 2013, 374, 156–163. [Google Scholar] [CrossRef]
- Jiang, Y.; Hsu, W. Petrogenesis of Grove Mountains 020090: An enriched “lherzolitic“ shergottite. Meteorit. Planet. Sci. 2012, 47, 1419–1435. [Google Scholar] [CrossRef]
- Bouvier, A.; Blichert-Toft, J.; Albarede, F. Martian meteorite chronology and the evolution of the interior of Mars. Earth Planet. Sci. Lett. 2009, 280, 285–295. [Google Scholar] [CrossRef]
- Bouvier, A.; Blichert-Toft, J.; Vervoort, J.D.; Albarede, F. The age of SNC meteorites and the antiquity of the Martian surface [rapid communication]. Earth Planet. Sci. Lett. 2005, 240, 221–233. [Google Scholar] [CrossRef]
- Carr, M.H. Water on Mars. Nature 1987, 326, 30–35. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; et al. Global Mineralogical and Aqueous Mars History Derived from OMEGA/Mars Express Data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malin, M.C.; Edgett, K.S. Sedimentary Rocks of Early Mars. Science 2000, 290, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53–60. [Google Scholar] [CrossRef]
- Webster, C.R.; Mahaffy, P.R.; Flesch, G.J.; Niles, P.B.; Jones, J.H.; Leshin, L.A.; Atreya, S.K.; Stern, J.C.; Christensen, L.E.; Owen, T.; et al. Isotope ratios of H, C, and O in CO2 and H2O of the martian atmosphere. Science 2013, 341, 260–263. [Google Scholar] [CrossRef]
- Filiberto, J.; Baratoux, D.; Beaty, D.; Breuer, D.; Farcy, B.J.; Grott, M.; Jones, J.H.; Kiefer, W.S.; Mane, P.; McCubbin, F.M.; et al. A review of volatiles in the Martian interior. Meteorit. Planet. Sci. 2016, 51, 1935–1958. [Google Scholar] [CrossRef]
- Hallis, L.J.; Taylor, G.J.; Nagashima, K.; Huss, G.R. Magmatic water in the martian meteorite Nakhla. Earth Planet. Sci. Lett. 2012, 359, 84–92. [Google Scholar] [CrossRef]
- Mane, P.; Hervig, R.; Wadhwa, M.; Garvie, L.A.J.; Balta, J.B.; McSween, H.Y. Hydrogen isotopic composition of the Martian mantle inferred from the newest Martian meteorite fall, Tissint. Meteorit. Planet. Sci. 2016, 51, 2073–2091. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Alexander, C.M.D.; Wang, J.; Simon, J.I.; Jones, J.H. Origin of water and mantle–crust interactions on Mars inferred from hydrogen isotopes and volatile element abundances of olivine-hosted melt inclusions of primitive shergottites. Earth Planet. Sci. Lett. 2012, 357–358, 119–129. [Google Scholar] [CrossRef]
- Owen, T.; Maillard, J.P.; de Bergh, C.; Lutz, B.L. Deuterium on Mars: The Abundance of HDO and the Value of D/H. Science 1988, 240, 1767. [Google Scholar] [CrossRef] [PubMed]
- Jakosky, B.M.; Zent, A.P.; Zurek, R.W. The Mars Water Cycle: Determining the Role of Exchange with the Regolith. Icarus 1997, 130, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Leshin, L.A.; Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Coll, P.; Conrad, P.G.; Archer, P.D., Jr.; Atreya, S.K.; Brunner, A.E.; Buch, A.; et al. Volatile, isotope, and organic analysis of martian fines with the Mars Curiosity rover. Science 2013, 341, 1238937. [Google Scholar] [CrossRef]
- Mahaffy, P.; Webster, C.; Stern, J.; Brunner, A.; Atreya, S.; Conrad, P.; Domagal-Goldman, S.; Eigenbrode, J.; Flesch, G.J.; Christensen, L.E. The imprint of atmospheric evolution in the D/H of Hesperian clay minerals on Mars. Science 2015, 347, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.L.; Hutcheon, I.D.; Epstein, S.; Stolper, E.M. Water on Mars: Clues from Deuterium/Hydrogen and Water Contents of Hydrous Phases in SNC Meteorites. Science 1994, 265, 86–90. [Google Scholar] [CrossRef]
- Boctor, N.Z.; Alexander, C.M.O.; Wang, J.; Hauri, E. The sources of water in Martian meteorites: Clues from hydrogen isotopes. Geochim. Cosmochim. Acta 2003, 67, 3971–3989. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Guan, Y.; Eiler, J.M.; Ma, C.; Rossman, G.R.; Taylor, L.A. Evidence in Tissint for recent subsurface water on Mars. Earth Planet. Sci. Lett. 2015, 425, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.P.; Itoh, S.; Sakamoto, N.; Vicenzi, E.P.; Yurimoto, H. Hydrogen isotope evidence for loss of water from Mars through time. Geophys. Res. Lett. 2008, 35, L05203. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.P.; Itoh, S.; Sakamoto, N.; Vicenzi, E.P.; Yurimoto, H. D/H zoning in apatite of Martian meteorites QUE 94201 and Los Angles: Implications for water on Mars. Meteorit. Planet. Sci. 2010, 45, A68. [Google Scholar]
- Guan, Y.; Hsu, W.; Leshin, L.A.; Wang, H.; Wang, R.; Zhang, F.; Lin, C.; Zhang, W. Hydrogen Isotopes of Phosphates in the New Martian Meteorite GRV 99027. In Proceedings of the Lunar and Planetary Institute Science Conference Abstracts, Woodlands, TX, USA, 17–21 March 2003; p. 1830. [Google Scholar]
- Hallis, L.J. Alteration assemblages in the Miller Range and Elephant Moraine regions of Antarctica: Comparisons between terrestrial igneous rocks and Martian meteorites. Meteorit. Planet. Sci. 2013, 48, 165–179. [Google Scholar] [CrossRef]
- Hallis, L.J.; Huss, G.R.; Nagashima, K.; Taylor, G.J.; Stöffler, D.; Smith, C.L.; Lee, M.R. Effects of shock and Martian alteration on Tissint hydrogen isotope ratios and water content. Geochim. Cosmochim. Acta 2017, 200, 280–294. [Google Scholar] [CrossRef]
- Hallis, L.J.; Taylor, G.J.; Nagashima, K.; Huss, G.R.; Needham, A.W.; Grady, M.M.; Franchi, I.A. Hydrogen isotope analyses of alteration phases in the nakhlite martian meteorites. Geochim. Cosmochim. Acta 2012, 97, 105–119. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Zhang, J.; Hao, J.; Feng, L.; Xu, L.; Yang, W.; Yang, J. NanoSIMS analyses of apatite and melt inclusions in the GRV 020090 Martian meteorite: Hydrogen isotope evidence for recent past underground hydrothermal activity on Mars. Geochim. Cosmochim. Acta 2014, 140, 321–333. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Zhang, J.; Hao, J.; Yamaguchi, A.; Zhang, T.; Yang, W.; Changela, H. Volatiles in the martian crust and mantle: Clues from the NWA 6162 shergottite. Earth Planet. Sci. Lett. 2020, 530, 115902. [Google Scholar] [CrossRef]
- Koike, M.; Sano, Y.; Takahata, N.; Ishida, A.; Sugiura, N.; Anand, M. Combined investigation of H isotopic compositions and U-Pb chronology of young Martian meteorite Larkman Nunatak 06319. Geochem. J. 2016, 50, 363–377. [Google Scholar] [CrossRef]
- Kuchka, C.R.; Herd, C.D.K.; Walton, E.L.; Guan, Y.; Liu, Y. Martian low-temperature alteration materials in shock-melt pockets in Tissint: Constraints on their preservation in shergottite meteorites. Geochim. Cosmochim. Acta 2017, 210, 228–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, Y.; Guan, Y.; Ma, C.; Rossman, G.R.; Eiler, J.M.; Zhang, Y. Impact-melt hygrometer for Mars: The case of shergottite Elephant Moraine (EETA) 79001. Earth Planet. Sci. Lett. 2018, 490, 206–215. [Google Scholar] [CrossRef]
- Usui, T.; Alexander, C.M.O.D.; Wang, J.; Simon, J.I.; Jones, J.H. Meteoritic evidence for a previously unrecognized hydrogen reservoir on Mars. Earth Planet. Sci. Lett. 2015, 410, 140–151. [Google Scholar] [CrossRef]
- Leshin, L.A. Insights into martian water reservoirs from analyses of martian meteorite QUE94201. Geophys. Res. Lett. 2000, 27, 2017–2020. [Google Scholar] [CrossRef]
- Gillet, P.; Barrat, J.A.; Deloule, E.; Wadhwa, M.; Jambon, A.; Sautter, V.; Devouard, B.; Neuville, D.; Benzerara, K.; Lesourd, M. Aqueous alteration in the Northwest Africa 817 (NWA 817) Martian meteorite. Earth Planet. Sci. Lett. 2002, 203, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, N.; Hoshino, H. Hydrogen-isotopic compositions in Allan Hills 84001 and the evolution of the martian atmosphere. Meteorit. Planet. Sci. 2000, 35, 373–380. [Google Scholar] [CrossRef]
- Sharp, Z.D.; McCubbin, F.M.; Shearer, C.K. A hydrogen-based oxidation mechanism relevant to planetary formation. Earth Planet. Sci. Lett. 2013, 380, 88–97. [Google Scholar] [CrossRef]
- Howarth, G.H.; Liu, Y.; Chen, Y.; Pernet-Fisher, J.F.; Taylor, L.A. Postcrystallization metasomatism in shergottites: Evidence from the paired meteorites LAR 06319 and LAR 12011. Meteorit. Planet. Sci. 2016, 51, 2061–2072. [Google Scholar] [CrossRef]
- Bellucci, J.J.; Whitehouse, M.J.; Nemchin, A.A.; Snape, J.F.; Kenny, G.G.; Merle, R.E.; Bland, P.A.; Benedix, G.K. Tracing martian surface interactions with the triple O isotope compositions of meteoritic phosphates. Earth Planet. Sci. Lett. 2020, 531, 115977. [Google Scholar] [CrossRef]
- Sharp, Z.; Williams, J.; Shearer, C.; Agee, C.; McKeegan, K. The chlorine isotope composition of Martian meteorites 2. Implications for the early solar system and the formation of Mars. Meteorit. Planet. Sci. 2016, 51, 2111–2126. [Google Scholar] [CrossRef] [Green Version]
- Shearer, C.K.; Messenger, S.; Sharp, Z.D.; Burger, P.V.; Nguyen, A.N.; McCubbin, F.M. Distinct chlorine isotopic reservoirs on Mars. Implications for character, extent and relative timing of crustal interactions with mantle-derived magmas, evolution of the martian atmosphere, and the building blocks of an early Mars. Geochim. Cosmochim. Acta 2018, 234, 24–36. [Google Scholar] [CrossRef]
- Williams, J.T.; Shearer, C.K.; Sharp, Z.D.; Burger, P.V.; McCubbin, F.M.; Santos, A.R.; Agee, C.B.; McKeegan, K.D. The chlorine isotopic composition of Martian meteorites 1: Chlorine isotope composition of Martian mantle and crustal reservoirs and their interactions. Meteorit. Planet. Sci. 2016, 51, 2092–2110. [Google Scholar] [CrossRef]
- Franz, H.B.; Kim, S.T.; Farquhar, J.; Day, J.M.; Economos, R.C.; McKeegan, K.D.; Schmitt, A.K.; Irving, A.J.; Hoek, J.; Dottin, J., III. Isotopic links between atmospheric chemistry and the deep sulphur cycle on Mars. Nature 2014, 508, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, J.C.; Catling, D.C.; Saxton, J.M.; Swindle, T.D.; Lyon, I.C.; Grady, M.M. Alteration assemblages in martian meteorites: Implications for near-surface processes. Space Sci. Rev. 2001, 96, 365–392. [Google Scholar] [CrossRef]
- Hallis, L.J.; Kemppinen, L.; Lee, M.R.; Taylor, L.A. The origin of alteration “orangettes” in Dhofar 019: Implications for the age and aqueous history of the shergottites. Meteorit. Planet. Sci. 2017, 52, 2695–2706. [Google Scholar] [CrossRef] [Green Version]
- Taylor, L.; Nazarov, M.; Shearer, C.; McSween, H., Jr.; Cahill, J.; Neal, C.; Ivanova, M.; Barsukova, L.; Lentz, R.; Clayton, R. Martian meteorite Dhofar 019: A new shergottite. Meteorit. Planet. Sci. 2002, 37, 1107–1128. [Google Scholar] [CrossRef]
- Gnos, E.; Hofmann, B.; Franchi, I.; Al-Kathiri, A.; Huser, M.; Moser, L. Sayh al Uhaymir 094: A new martian meteorite from the Oman desert. Meteorit. Planet. Sci. 2002, 37, 835–854. [Google Scholar] [CrossRef]
- Kuebler, K.E. A comparison of the iddingsite alteration products in two terrestrial basalts and the Allan Hills 77005 martian meteorite using Raman spectroscopy and electron microprobe analyses. J. Geophys. Res. 2013, 118, 803–830. [Google Scholar] [CrossRef]
- Piercy, J.D.; Bridges, J.C.; Hicks, L.J.; MacArthur, J.L.; Greenwood, R.C.; Franchi, I.A. Olivine Alteration In Shergottite Northwest Africa 10416. In Proceedings of the 82nd Annual Meeting of The Meteoritical Society, Sapporo, Japan, 7–12 July 2019. [Google Scholar]
- Leshin, L.A.; Epstein, S.; Stolper, E.M. Hydrogen isotope geochemistry of SNC meteorites. Geochim. Cosmochim. Acta 1996, 60, 2635–2650. [Google Scholar] [CrossRef]
- Villanueva, G.L.; Mumma, M.J.; Novak, R.E.; Kaufl, H.U.; Hartogh, P.; Encrenaz, T.; Tokunaga, A.; Khayat, A.; Smith, M.D. Strong water isotopic anomalies in the martian atmosphere: Probing current and ancient reservoirs. Science 2015, 348, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Giesting, P.A.; Schwenzer, S.P.; Filiberto, J.; Starkey, N.A.; Franchi, I.A.; Treiman, A.H.; Tindle, A.G.; Grady, M.M. Igneous and shock processes affecting chassignite amphibole evaluated using chlorine/water partitioning and hydrogen isotopes. Meteorit. Planet. Sci. 2015, 50, 433–460. [Google Scholar] [CrossRef]
- Usui, T. Chapter 4—Hydrogen Reservoirs in Mars as Revealed by Martian Meteorites. In Volatiles in the Martian Crust; Filiberto, J., Schwenzer, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 71–88. [Google Scholar] [CrossRef]
- Barnes, J.J.; McCubbin, F.M.; Santos, A.R.; Day, J.M.D.; Boyce, J.W.; Schwenzer, S.P.; Ott, U.; Franchi, I.A.; Messenger, S.; Anand, M.; et al. Multiple early-formed water reservoirs in the interior of Mars. Nat. Geosci. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, H. Diffusion of H, C, and O Components in Silicate Melts. Rev. Mineral. Geochem. 2010, 72, 171–225. [Google Scholar] [CrossRef] [Green Version]
- Filiberto, J.; Gross, J.; McCubbin, F.M. Constraints on the water, chlorine, and fluorine content of the Martian mantle. Meteorit. Planet. Sci. 2016, 51, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- McCubbin, F.M.; Boyce, J.W.; Srinivasan, P.; Santos, A.R.; Elardo, S.M.; Filiberto, J.; Steele, A.; Shearer, C.K. Heterogeneous distribution of H2O in the Martian interior: Implications for the abundance of H2O in depleted and enriched mantle sources. Meteorit. Planet. Sci. 2016, 51, 2036–2060. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, G.A.; O’Leary, J.A.; Shimizu, N.; Bucholz, C.E.; Newville, M. Rapid reequilibration of H2O and oxygen fugacity in olivine-hosted melt inclusions. Geology 2012, 40, 915–918. [Google Scholar] [CrossRef]
- Minitti, M.E.; Leshin, L.A.; Dyar, M.D.; Ahrens, T.J.; Guan, Y.; Luo, S.N. Assessment of shock effects on amphibole water contents and hydrogen isotope compositions: 2. Kaersutitic amphibole experiments. Earth Planet. Sci. Lett. 2008, 266, 288–302. [Google Scholar] [CrossRef]
- Stephant, A.; Garvie, L.A.J.; Mane, P.; Hervig, R.; Wadhwa, M. Terrestrial exposure of a fresh Martian meteorite causes rapid changes in hydrogen isotopes and water concentrations. Sci. Rep. 2018, 8, 12385. [Google Scholar] [CrossRef] [Green Version]
- McCubbin, F.M.; Smirnov, A.; Nekvasil, H.; Wang, J.; Hauri, E.; Lindsley, D.H. Hydrous magmatism on Mars: A source of water for the surface and subsurface during the Amazonian. Earth Planet. Sci. Lett. 2010, 292, 132–138. [Google Scholar] [CrossRef]
- Dasgupta, R.; Nelson, J.D.; Chi, H.; Ding, S.; Li, Y.; Duncan, M.S.; Tsuno, K. Carbon in the Martian Interior: Core-Mantle Fractionation and Extraction by Mantle Melting at Oxidized Conditions. In Proceedings of the Workshop on Volatiles in the Martian Interior, Houston, TX, USA, 3–5 November 2014; p. 1012. [Google Scholar]
- Bellucci, J.J.; Whitehouse, M.J.; John, T.; Nemchin, A.A.; Snape, J.F.; Bland, P.A.; Benedix, G.K. Halogen and Cl isotopic systematics in Martian phosphates: Implications for the Cl cycle and surface halogen reservoirs on Mars. Earth Planet. Sci. Lett. 2017, 458, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.J. The bulk composition of Mars. Chem. Der Erde Geochem. 2013, 73, 401–420. [Google Scholar] [CrossRef]
- Orosei, R.; Lauro, S.E.; Pettinelli, E.; Cicchetti, A.; Coradini, M.; Cosciotti, B.; Di Paolo, F.; Flamini, E.; Mattei, E.; Pajola, M.; et al. Radar evidence of subglacial liquid water on Mars. Science 2018, 361, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Kounaves, S.P.; Carrier, B.L.; O’Neil, G.D.; Stroble, S.T.; Claire, M.W. Evidence of martian perchlorate, chlorate, and nitrate in Mars meteorite EETA79001: Implications for oxidants and organics. Icarus 2014, 229, 206–213. [Google Scholar] [CrossRef]
- Rao, M.N.; Nyquist, L.E.; Wentworth, S.J.; Sutton, S.R.; Garrison, D.H. The nature of Martian fluids based on mobile element studies in salt-assemblages from Martian meteorites. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.; Ito, M.; Rao, M.; Hervig, R.; Williams, L.; Nyquist, L.E.; Peslier, A. Jarosite in the Shergottite Que 94201. In Proceedings of the Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2019. [Google Scholar]
- Changela, H.G.; Bridges, J.C. Alteration assemblages in the nakhlites: Variation with depth on Mars. Meteorit. Planet. Sci. 2010, 45, 1847–1867. [Google Scholar] [CrossRef]
- Muttik, N.; McCubbin, F.M.; Keller, L.P.; Santos, A.R.; McCutcheon, W.A.; Provencio, P.P.; Rahman, Z.; Shearer, C.K.; Boyce, J.W.; Agee, C.B. Inventory of H2O in the ancient Martian regolith from Northwest Africa 7034: The important role of Fe oxides. Geophys. Res. Lett. 2014, 41, 8235–8244. [Google Scholar] [CrossRef]
- Boctor, N.Z.; Alexander, C.M.O.D.; Wang, J.; Hauri, E. The Source of Extraterrestrial Water in Martian Meteorites: Clues from Hydrogen Isotope Composition of Impact-melted Glasses and Magmatic Melt-Inclusion Glasses. In Proceedings of the Eleventh Annual V. M. Goldschmidt Conference, Baltimore, MA, USA, 20–24 May 2001. [Google Scholar]
- Boctor, N.Z.; Wang, J.; Alexander, C.M.O.D.; Hauri, E.; Bertka, C.M.; Fei, Y. Hydrogen Isotope Studies of Feldspathic and Mafic Glasses in Martian Meteorites ALH 84001 and EETA 79001. In Proceedings of the Lunar and Planetary Institute Science Conference Abstracts, Houston, TX, USA, 15–19 March 1999; p. 1397. [Google Scholar]
- Boctor, N.Z.; Alexander, C.M.O.D.; Steele, A.; Armstrong, J. Hydrogen Isotope Signatures and Water Abundances in Nominally Anhydrous Minerals from the Shergottite Y-984028. Meteorit. Planet. Sci. 2010, 73, 5176. [Google Scholar]
- Sonzogni, Y.; Treiman, A. Small melt inclusions can record bulk magma compositions: A planetary example from the Martian basalt (shergottite) Tissint. Meteorit. Planet. Sci. 2015. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Nekvasil, H. Maskelynite-hosted apatite in the Chassigny meteorite: Insights into late-stage magmatic volatile evolution in martian magmas. Am. Mineral. 2008, 93, 676–684. [Google Scholar] [CrossRef]
- Hauri, E.H.; Weinreich, T.; Saal, A.E.; Rutherford, M.C.; Van Orman, J.A. High pre-eruptive water contents preserved in lunar melt inclusions. Science 2011, 333, 213. [Google Scholar] [CrossRef] [Green Version]
- Stephant, A.; Anand, M.; Zhao, X.; Chan, Q.H.S.; Bonifacie, M.; Franchi, I.A. The chlorine isotopic composition of the Moon: Insights from melt inclusions. Earth Planet. Sci. Lett. 2019, 523, 115715. [Google Scholar] [CrossRef]
- Wallace, P.J.; Edmonds, M. The Sulfur Budget in Magmas: Evidence from Melt Inclusions, Submarine Glasses, and Volcanic Gas Emissions. Rev. Mineral. Geochem. 2011, 73, 215–246. [Google Scholar] [CrossRef]
- Filiberto, J.; Treiman, A.H. Martian magmas contained abundant chlorine, but little water. Geology 2009, 37, 1087–1090. [Google Scholar] [CrossRef]
- Boyce, J.W.; Tomlinson, S.M.; McCubbin, F.M.; Greenwood, J.P.; Treiman, A.H. The lunar apatite paradox. Science 2014, 344, 400–402. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, F.M.; Vander Kaaden, K.E.; Tartèse, R.; Boyce, J.W.; Mikhail, S.; Whitson, E.S.; Bell, A.S.; Anand, M.; Franchi, I.A.; Wang, J.; et al. Experimental investigation of F, Cl, and OH partitioning between apatite and Fe-rich basaltic melt at 1.0–1.2 GPa and 950–1000 °C. Am. Mineral. 2015, 4779, 83–89. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, S.; Miao, B.; Xu, L.; Liu, Y.; Xie, L.; Feng, L.; Yang, J. Grove Mountains 020090 enriched lherzolitic shergottite: A two-stage formation model. Meteorit. Planet. Sci. 2013, 48, 1572–1589. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Shearer, C.K.; Burger, P.V.; Hauri, E.H.; Wang, J.; Elardo, S.M.; Papike, J.J. Volatile abundances of coexisting merrillite and apatite in the martian meteorite Shergotty: Implications for merrillite in hydrous magmas. Am. Mineral. 2014, 99, 1347–1354. [Google Scholar] [CrossRef]
- Boctor, N.Z.; Alexander, C.M.O.D.; Wang, J.; Hauri, E. Hydrogen Isotope Studies of Mafic, Feldspathic, and Melt Inclusion Glasses in Martian Meteorite Alan Hills 77005. In Proceedings of the Lunar and Planetary Institute Science Conference Abstracts, Houston, TX, USA, 13–17 March 2000; p. 1759. [Google Scholar]
- Boctor, N.Z.; Alexander, C.M.O.D.; Wang, J.; Hauri, E. Hydrogen Isotope Studies of Water-bearing Post-Stishovite Silica Phase and Feldspathic Glass in the Martian Meteorites Shergotty and Zagami. In Proceedings of the Lunar and Planetary Institute Science Conference Abstracts, Houston, TX, USA, 12–16 March 2001; p. 1309. [Google Scholar]
- El Goresy, A.; Gillet, P.; Miyahara, M.; Ohtani, E.; Ozawa, S.; Beck, P.; Montagnac, G. Shock-induced deformation of Shergottites: Shock-pressures and perturbations of magmatic ages on Mars. Geochim. Cosmochim. Acta 2013, 101, 233–262. [Google Scholar] [CrossRef]
- Usui, T.; McSween, H.Y.; Floss, C. Petrogenesis of olivine-phyric shergottite Yamato 980459, revisited. Geochim. Cosmochim. Acta 2008, 72, 1711–1730. [Google Scholar] [CrossRef]
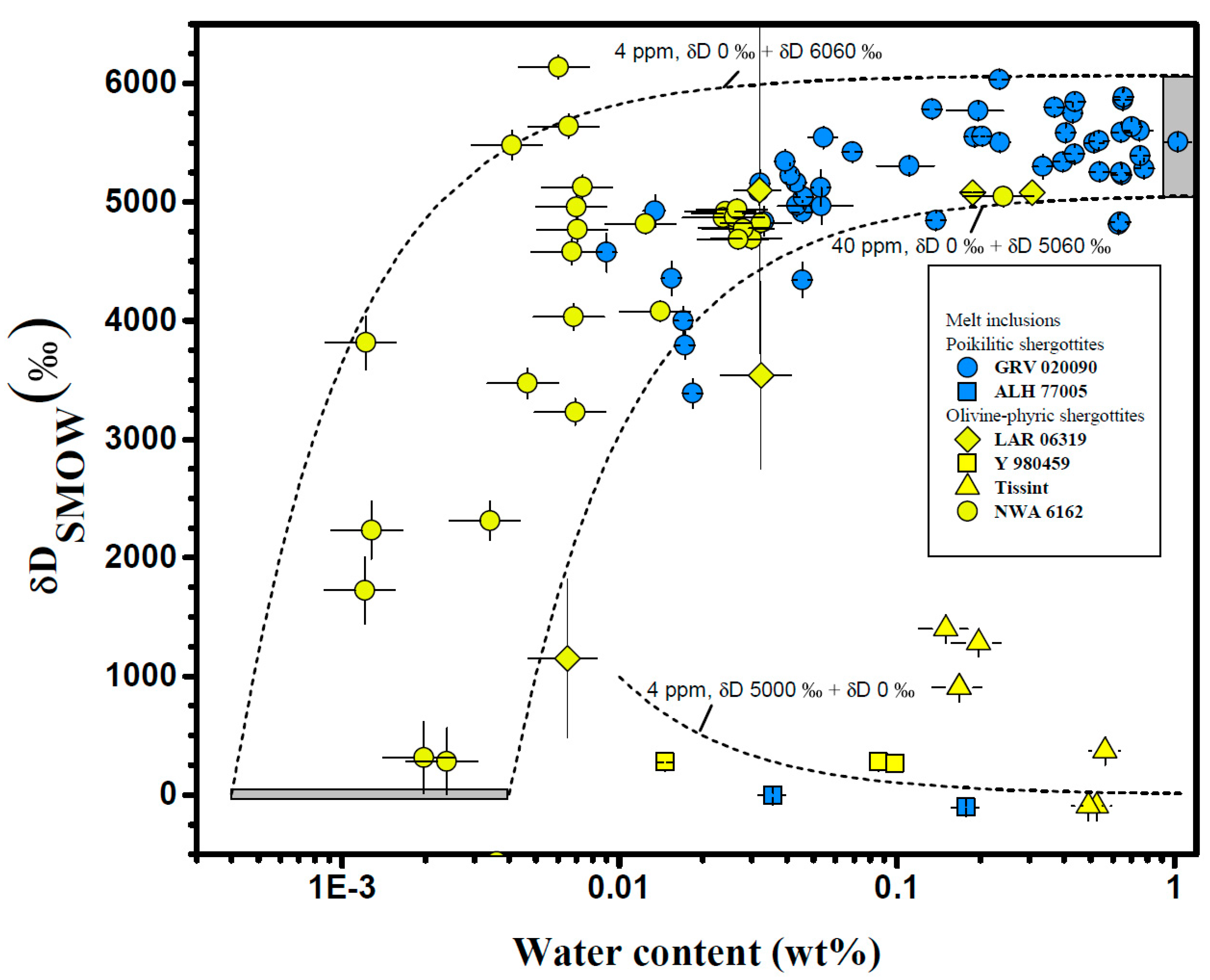
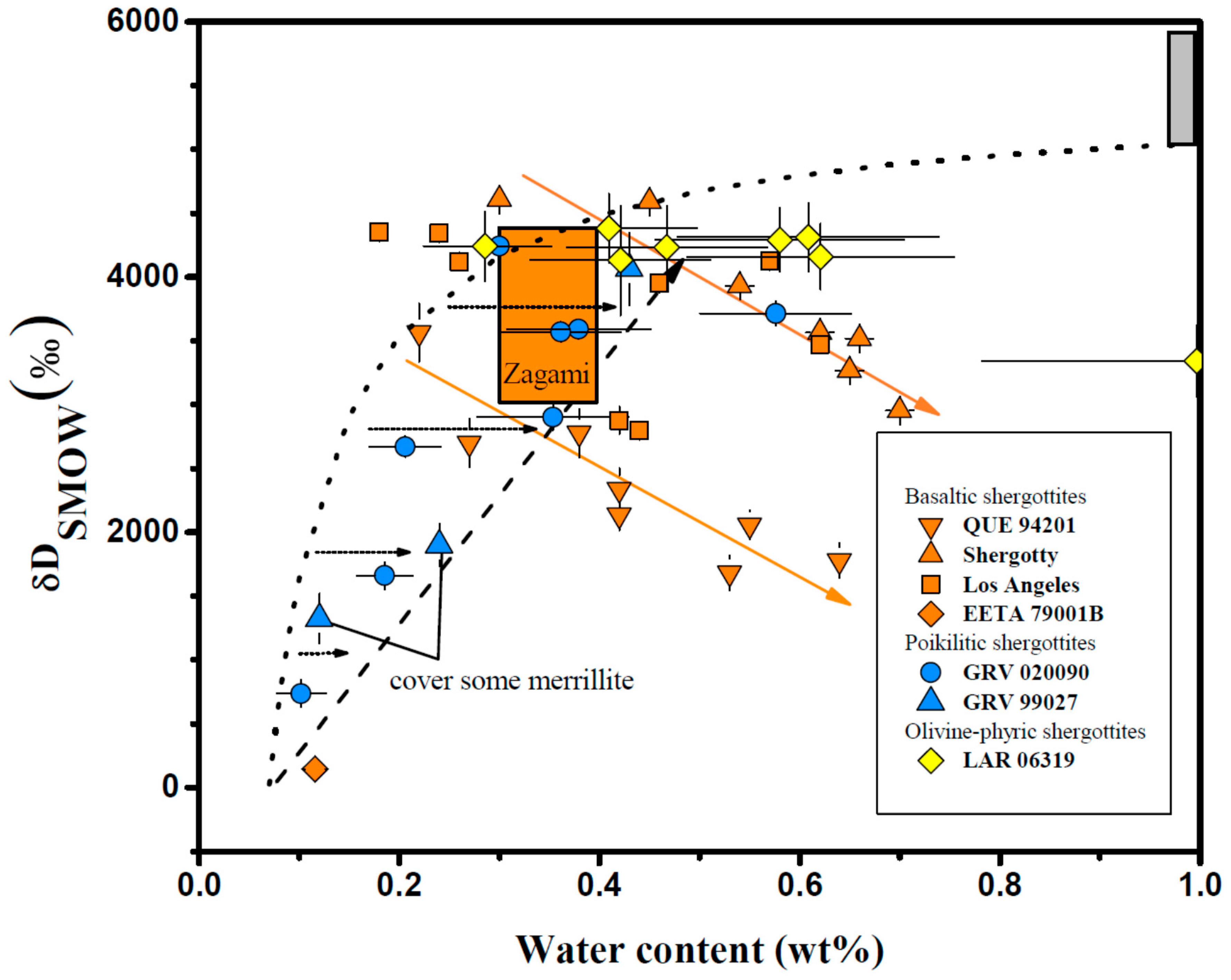
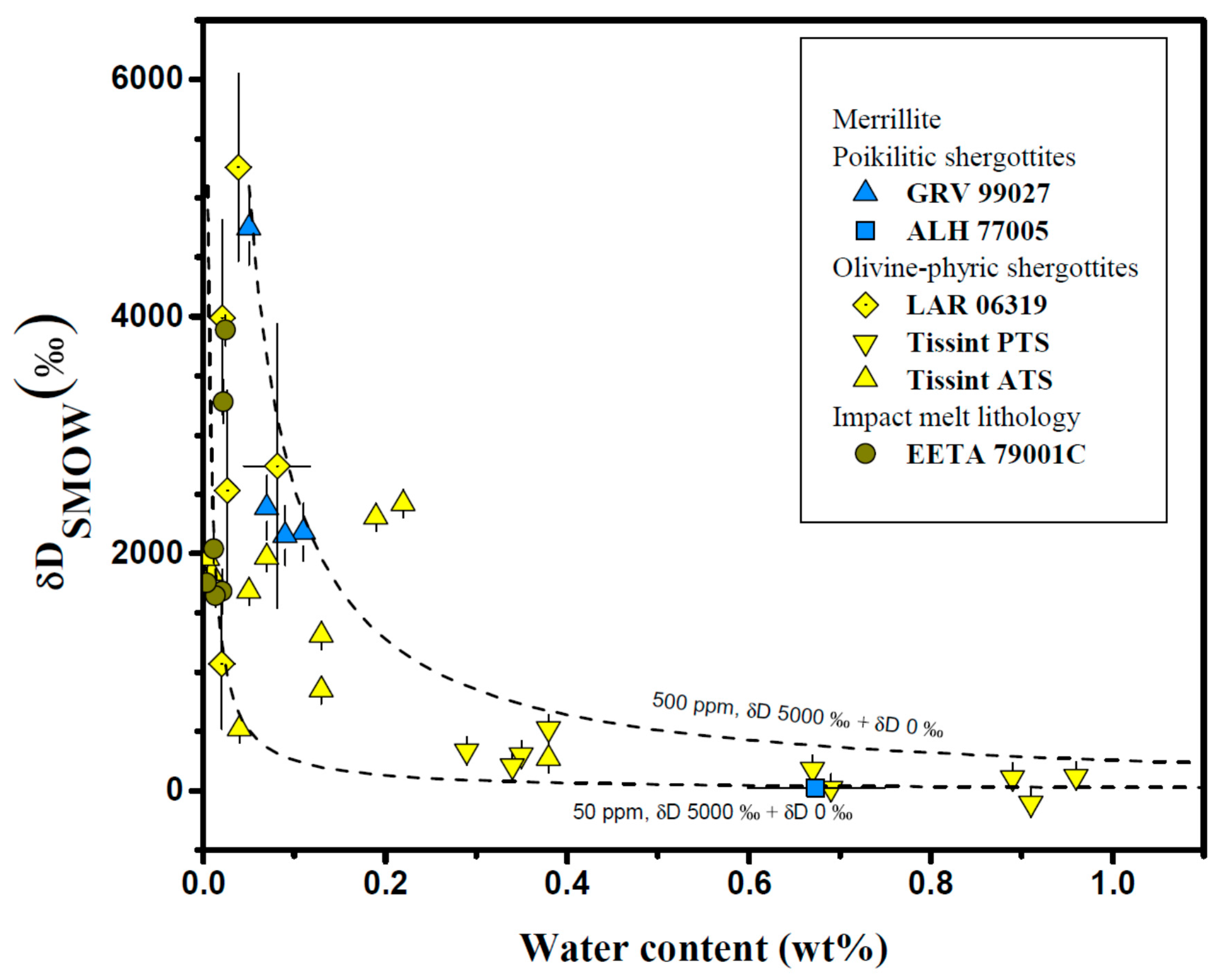
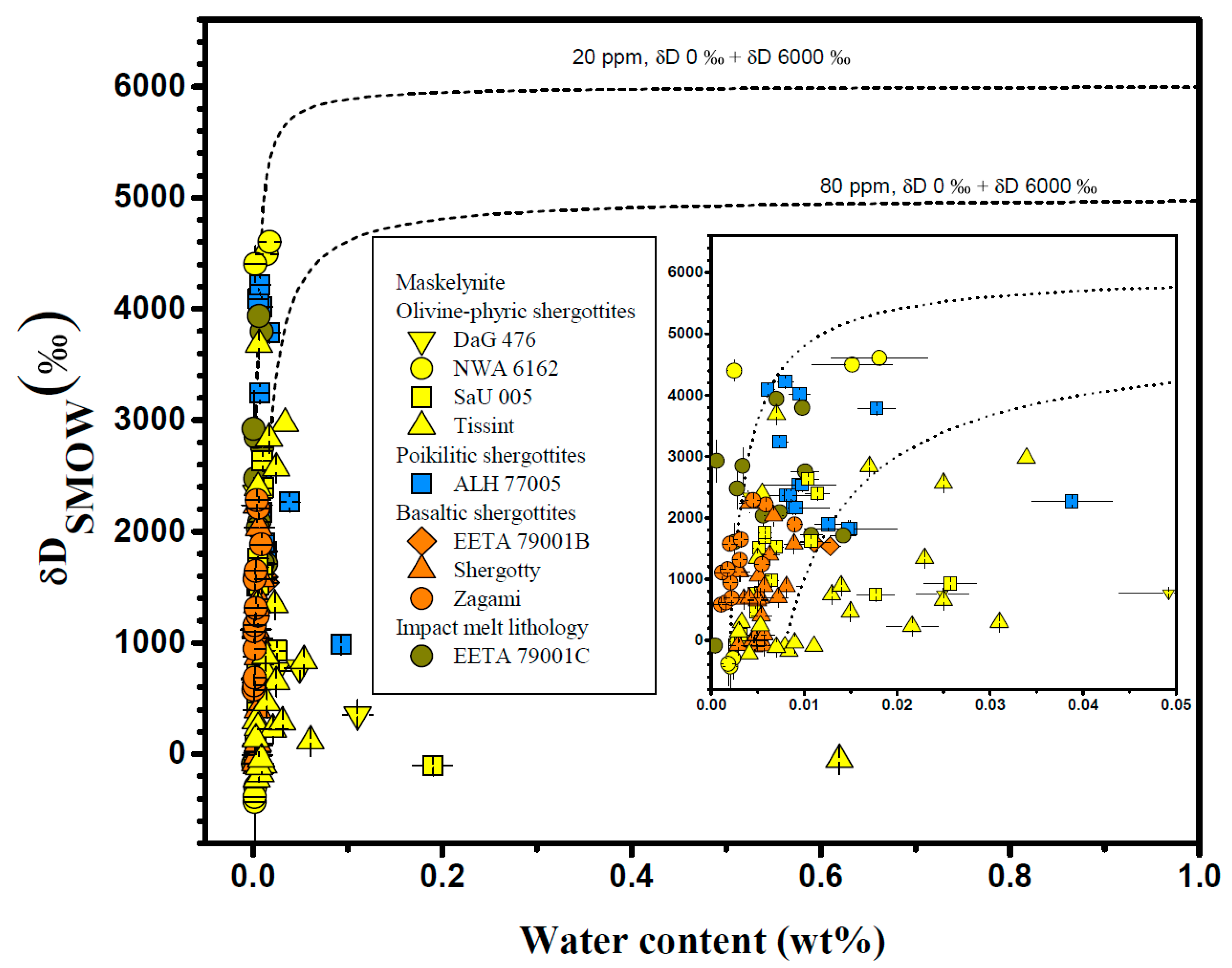

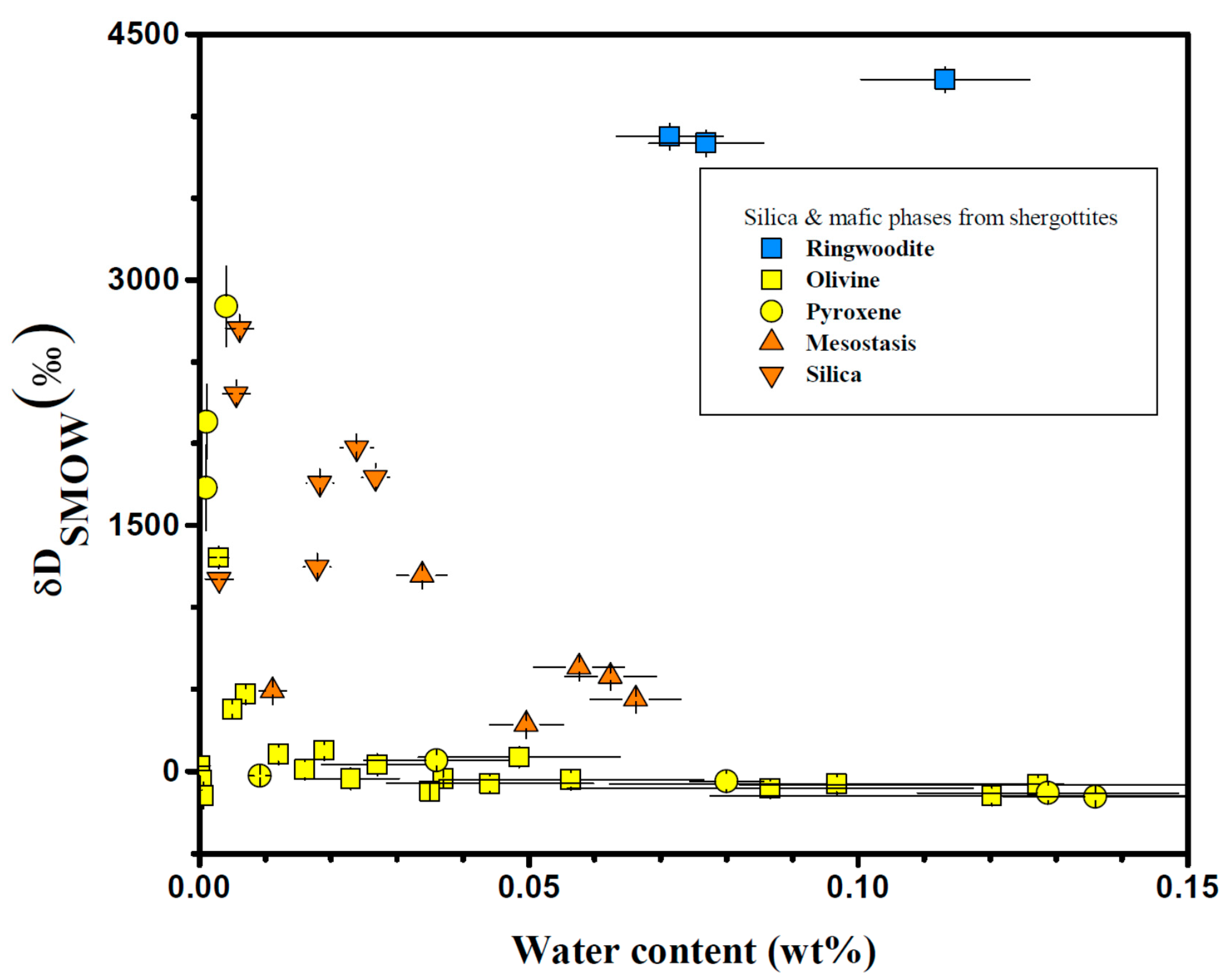
| Meteorite | Groups 1 | Mineral/Phases | H2O (ppm) | δD (‰) | Ref. |
|---|---|---|---|---|---|
| Shergotty | BS | Apatite | 3000–7000 | 2953–4606 | [51] |
| Kaersutite | 1000–2000 | 512 | [58] | ||
| Augite | 323 | [51] | |||
| Olivine | 12–86 | [51] | |||
| Pigeonite | 800–1360 | −153–−60 | [59] | ||
| Silica | 179–267 | 1246–1975 | [59] | ||
| Mesostasis | 111–662 | 441–490 | [59] | ||
| Post-stishovite | 30–39 | 1246–1975 | [111] | ||
| Zagami | BS | Kaersutite | 1000–2000 | 1498–1672 | [58] |
| Apatite | 3000–4000 | 2962–4358 | [58] | ||
| Mesostasis | 338–624 | 285–1195 | [59] | ||
| Maskelynite | 12–71 | 579–2532 | [59] | ||
| Silica | 30–183 | 1173–2704 | [59] | ||
| Pigeonite | 3360 | –204 | [59] | ||
| QUE 94201 | BS | Apatite | 2200–6400 | 1683–3565 | [62,73] |
| Los Angeles | BS | Apatite | 1800–6200 | 2794–4348 | [61,62] |
| EETA 79001B | BS | Apatite | 1160 | 146 | [59] |
| Maskelynite | 111–128 | 1540–1589 | [59] | ||
| Olivine | 29 | 1303 | [59] | ||
| Pyroxene | 92 | −26 | [59] | ||
| EETA 79001C | IMG | Mafic glass | 101–556 | 2289–2901 | [59,71] |
| Maskelynite | 4–98 | −91–3938 | [59,71] | ||
| Impact melt glass | 90–646 | 3368–4639 | [71] | ||
| Olivine | 4–7 | −146–−55 | [71] | ||
| Pyroxene | 10–41 | 1729–2837 | [71] | ||
| EETA 79001A | OS | Impact melt glass | 232–393 | 1454–1644 | [72,112] |
| Tissint | OS | Melt inclusions | 1500–5629 | −98–1397 | [52,65] |
| Maskelynite | 30–6200 | −222–3682 | [52,60] | ||
| Olivine | 50–1273 | −149–470 | [52,65] | ||
| Clinopyroxene | 1288 | −131 | [65] | ||
| Merrillite | 50–9600 | −105–2418 | [52] | ||
| Impact melt glass | 179–2388 | 45–4867 | [60,70] | ||
| Ringwoodite | 714–1132 | 3834–4224 | [65] | ||
| DaG 476 | OS | Maskelynite | 40–1110 | 352–2347 | [59] |
| SaU 005 | OS | Maskelynite | 32–76200 | −105–3260 | [59] |
| Y 980459 | OS | Melt inclusions | 146–841 | −95–285 | [53,72] |
| Groundmass glass | 17.7–257.4 | −71–1562 | [53,72] | ||
| LAR 06319 | OS | Melt inclusions | 65–1872 | 1150–6830 | [53,69,72] |
| Impact melt glass | 117.8–163.8 | 2096–2929 | [72] | ||
| Apatite | 2854–9964 | 3340–4380 | [69] | ||
| Merrillite | 204–812 | 1070–5260 | [69] | ||
| NWA 6162 | OS | Melt inclusions | 11–2421 | −560–6137 | [68] |
| Maskelynite | 18–181 | −426–4601 | [68] | ||
| Fusion crust | 13–37 | −728–1889 | [68] | ||
| ALH 77005 | PS | Melt inclusions | 0.74–1770 | −106–304 | [59] |
| Mafic glass | 105–520 | 1301–3030 | [59] | ||
| Maskelynite | 61–930 | 982–4214 | [59] | ||
| Merrillite | 6740 | 22 | [59] | ||
| Pigeonite | 360 | 69 | [59] | ||
| Olivine | 0.53 | 35 | [59] | ||
| GRV 020090 | PS | Melt inclusions | 91–10308 | 3386–6034 | [67] |
| Apatite | 1020–5762 | 737–4239 | [67] | ||
| Y 980428 | PS | Pyroxene | 12–15 | 403–522 | [113] |
| Olivine | 23 | 262 | [113] | ||
| GRV 99027 | PS | Apatite | 1200–4300 | 1326–4064 | [63] |
| Merrillite | 500–1100 | 2153–4745 | [63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hu, S. Hydrogen Isotopic Variations in the Shergottites. Geosciences 2020, 10, 148. https://doi.org/10.3390/geosciences10040148
Wang S, Hu S. Hydrogen Isotopic Variations in the Shergottites. Geosciences. 2020; 10(4):148. https://doi.org/10.3390/geosciences10040148
Chicago/Turabian StyleWang, Shuai, and Sen Hu. 2020. "Hydrogen Isotopic Variations in the Shergottites" Geosciences 10, no. 4: 148. https://doi.org/10.3390/geosciences10040148





