Hydrochemistry and 222Rn Concentrations in Spring Waters in the Arid Zone El Granero, Chihuahua, Mexico
Abstract
:1. Introduction
2. Materials and Methods
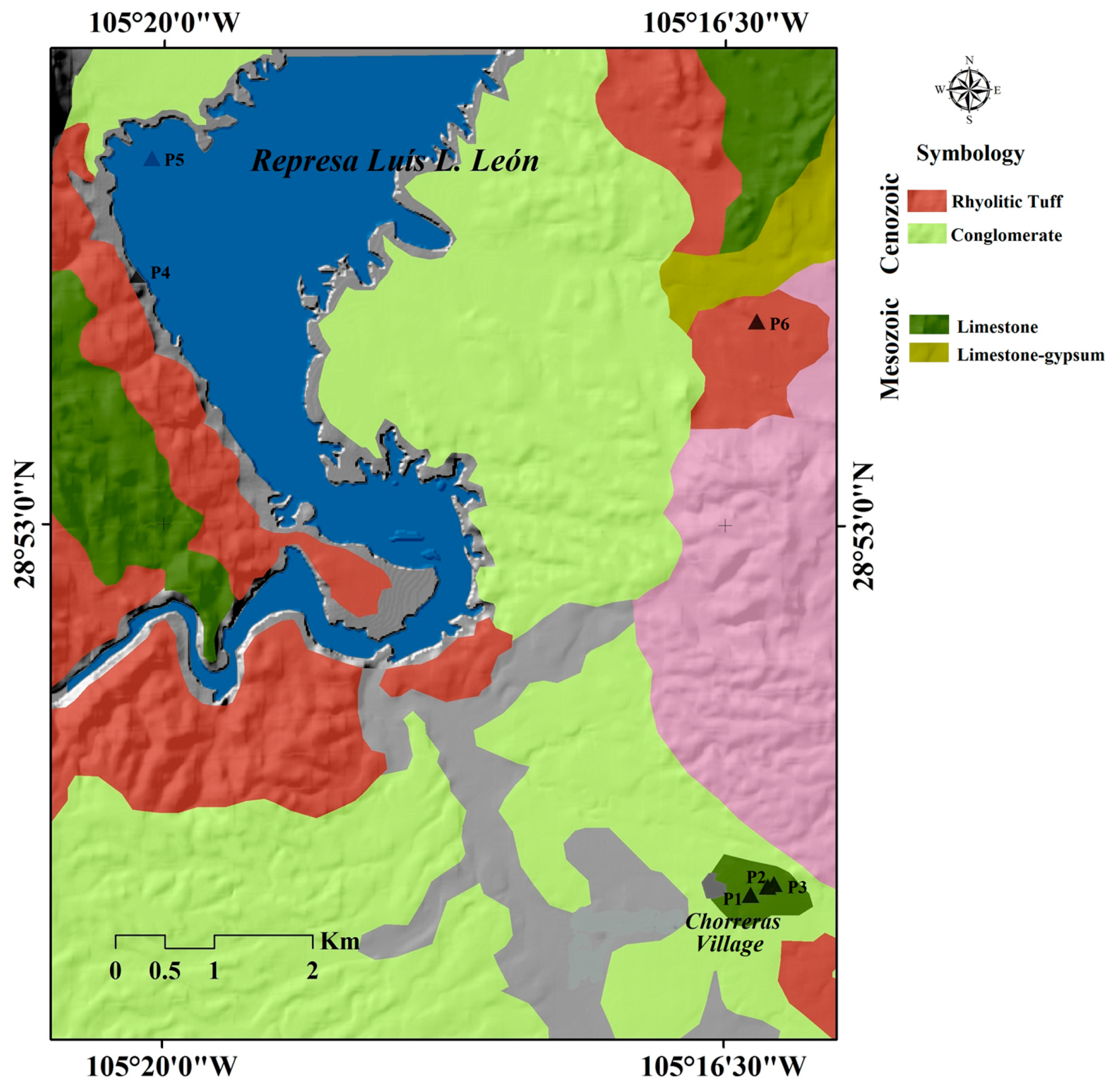
2.1. Study Area
2.2. Sampling
2.3. Determination of Dissolved Ions
2.4. Determination of 222Rn
2.5. Statistical Analysis
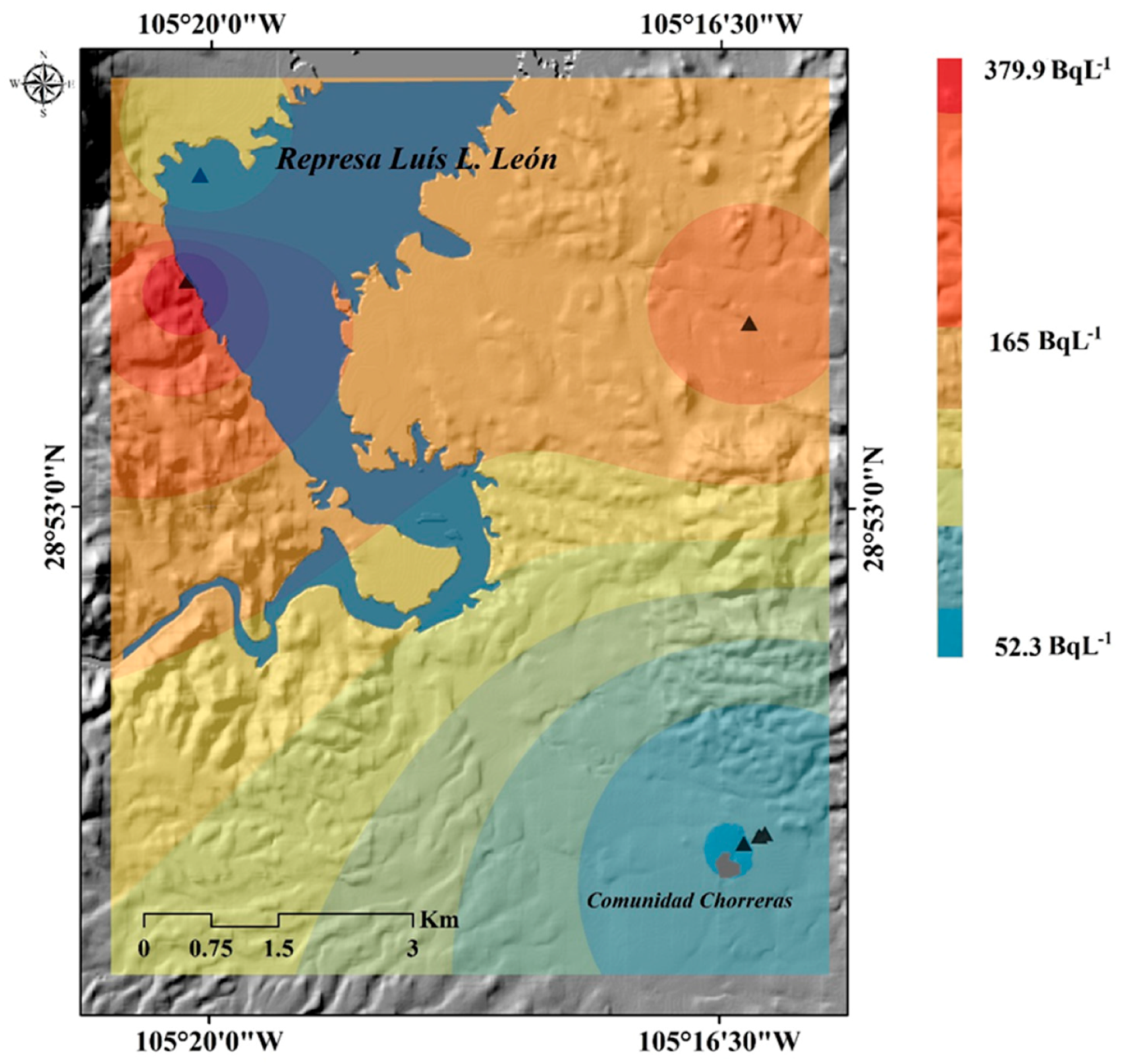
2.6. Generation of the Spatial Distribution for the Inverse of the Distance (IDW)
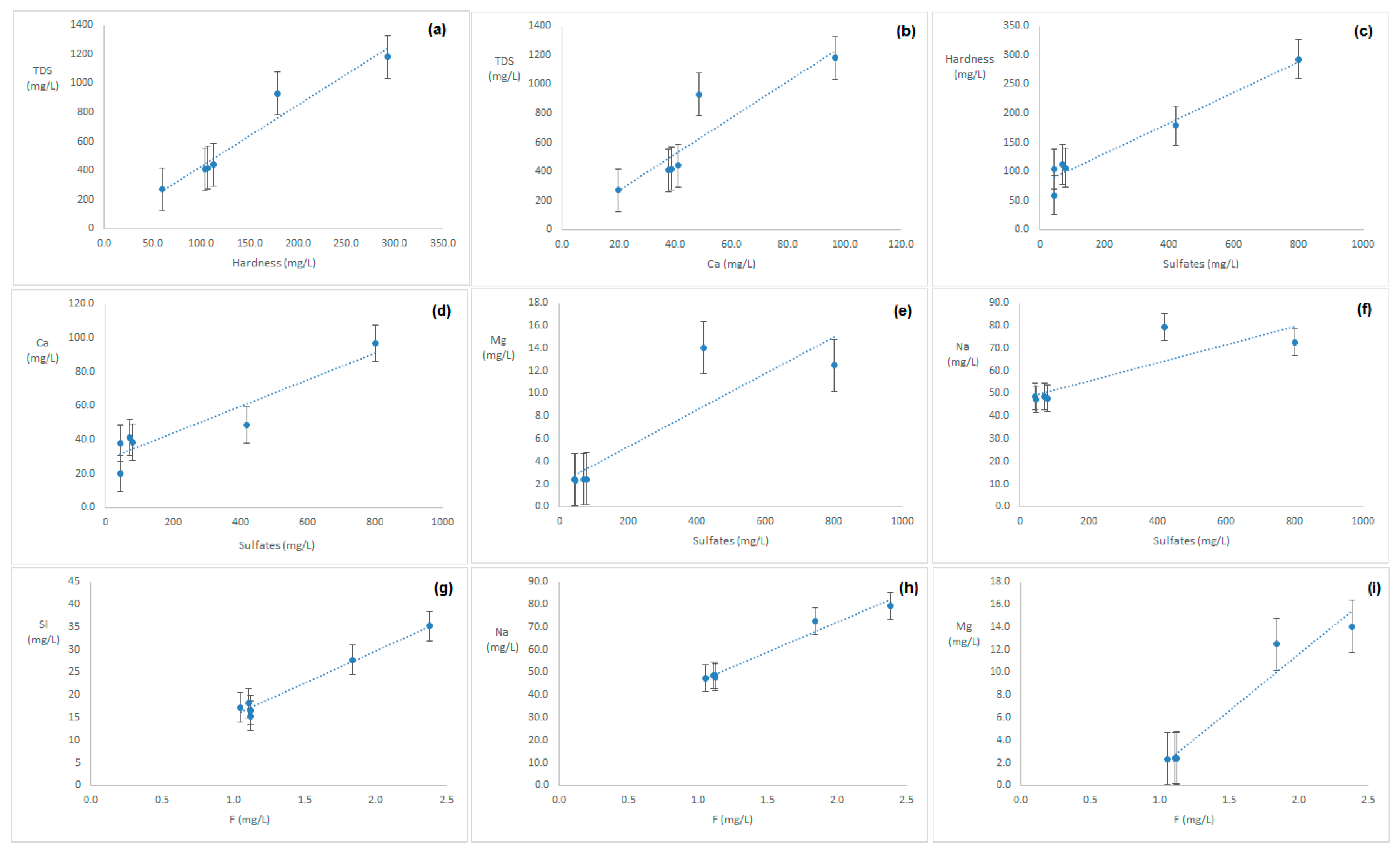
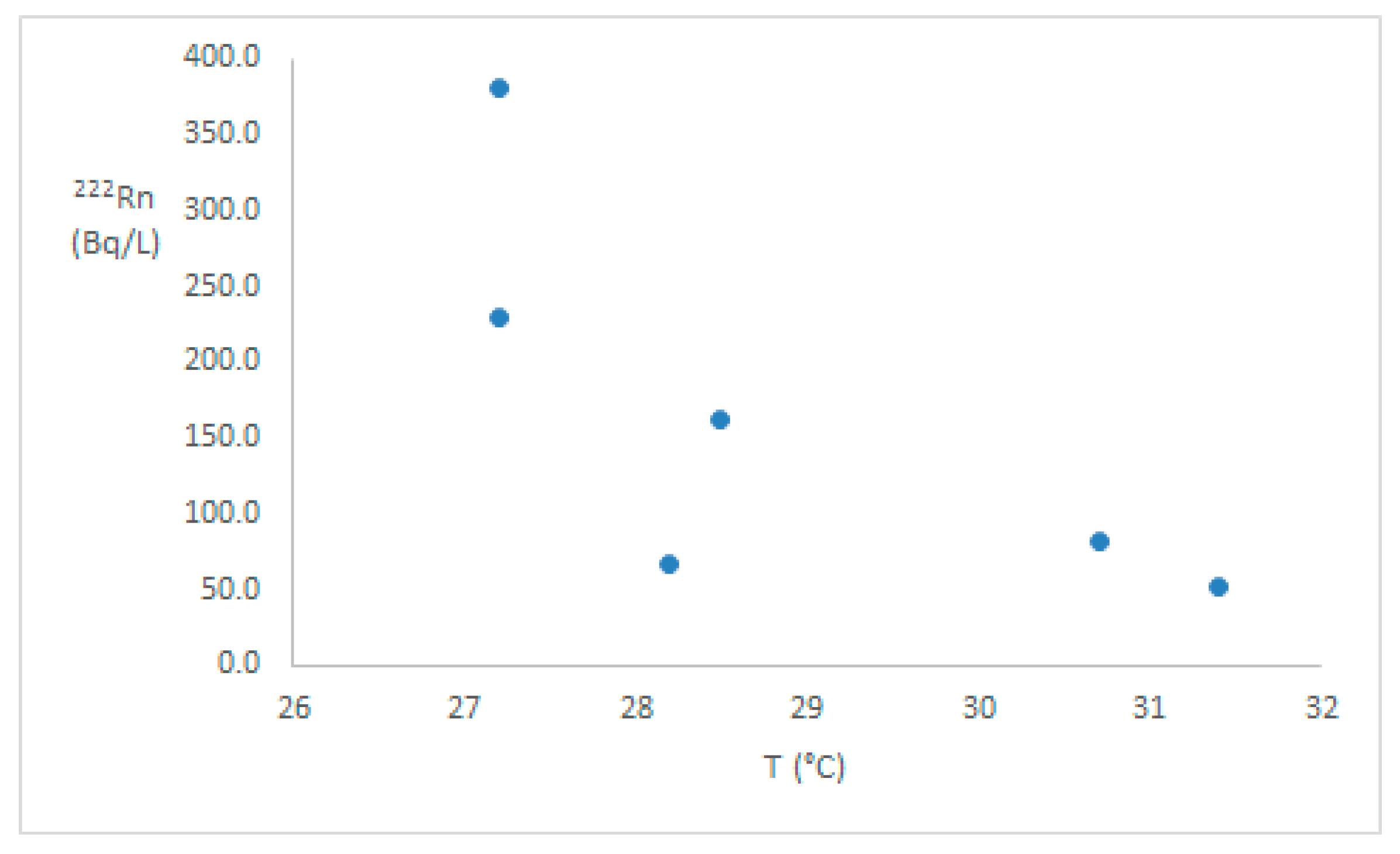
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osmond, J.K.; Ivanovich, M. Uranium-series mobilization and surface hydrology. In Uranium Series Disequilibrium. Application to Earth, Marine and Environmental Sciences; Ivanovich, M., Harmon, R.S., Eds.; Oxford University: New York, NY, USA, 1992; pp. 259–289. [Google Scholar]
- Salih, I.; Pettersson, H.; Lund, E. Determination of 222Rn and 226Ra in water using a large volume ionisation chamber. J. Environ. Radioact. 2000, 48, 235–245. [Google Scholar] [CrossRef]
- Cook, P.G.; Herczeg, A.L. Green, Radon-222. In Environmental Tracers in Subsurface Hydrology; Springer Science & Business Media: New York, NY, USA, 2000; pp. 175–194. [Google Scholar]
- Monnin, M.; Seidel, J.L. Pressure response of radon detecting devices placed at depth in acuifers. Geofís. Int. 2002, 41, 229–232. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assemby, Annex B: Exposures from Natural Radiation Sources; United Nations Scientific Committee on the Effect of Atomic Radiation; United Nations Sales Publications: New York, NY, USA, 2000. [Google Scholar]
- International Commission on Radiological Protection (ICRP). ICRP Publication No. 60; Annals of the ICRP: Ottawa, ON, Canada, 1991. [Google Scholar]
- Villalba, L.; Montero-Cabrera, M.E.; Manjon-Collado, G.; Colmenero-Sujo, L.; Renteria-Villalobos, M.; Cano-Jimenez, A.; Rodriguez-Pineda, A.; Davila-Rangel, I.; Quirino-Torres, L.; Herrera-Peraza, E.F. Natural radioactivity in groundwater and estimates of committed effective dose due to water ingestion in the state of Chihuahua (Mexico). Radiat. Prot. Dosim. 2006, 121, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Villalba, L.; Colmenero, S.L.; Montero, C.M.E.; Cano, J.A.; Renteria, V.M.; Delgado, M.C.J.; Jurado, T.L.A.; Davila, R.I.; Herrera, P.E.F. Radon concentrations in ground and drinking water in the state of Chihuahua, Mexico. J. Environ. Radioact. 2005, 80, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Montero-Cabrera, M.E.; Colmenero-Sujo, L.C.; Villalba, L.; de La Cruz Gandara, S.; Saenz-Peinado, J.; Renteria-Villalobos, M.; Sanin-Aguirre, L.H.; Herrera-Peraza, E.F.; Lopez, J.; Gardea-Torresdey, J.L. Rn-222 air concentrations in Chihuahua State (Mexico) dwellings and in the U.S./Mexico border. Microchem. J. 2005, 81, 28–34. [Google Scholar] [CrossRef]
- Colmenero, S.L.; Montero, C.M.E.; Villalba, L.; Renteria, V.M.; Torres, M.E.; Garcia, L.M.; Garcia-Tenorio, R.; Mireles, G.F.; Herrera, P.E. F.; Sanchez, A.D. Uranium-238 and thorium-232 series concentrations in soil, radon-222 indoor and drinking water concentrations and dose assessment in the city of Aldama, Chihuahua, Mexico. J. Environ. Radioact. 2004, 77, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Villalba, L.; Villalba, L.; Martínez, A.; Colmenero, L.; Montero, M.E. Determinación de Uranio y Radio en muestras de agua de los alrededores de la ciudad de Chihuahua. In Proceedings of the XII Congreso Anual de la Sociedad Nuclear Mexicana y XXV Congreso de la Sociedad Mexicana de Seguridad Radiológica, Zacatecas, Mexico, 7–10 October 2001.
- Instituto Nacional de Estadística y Geografía. Diccionario de datos Climáticos. Base de datos Geográficos. Escalas 1:250 000 y 1:1 000 000 (VECTORIAL); Instituto Nacional de Estadística y Geografía INEGI: Aguascalientes, Mexico, 2000. [Google Scholar]
- CONAPESCA, Comisión Nacional de Acuacultura y Pesca. Informe Final del Proyecto Diagnostico Socioeconómico de la Presa Luis. L. León “El Granero”, Chihuahua, México; Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa: Mazatlán, Sinaloa, Mexico, 2004; pp. 1–82. [Google Scholar]
- Comisión Nacional del Agua (CONAGUA). Actualización. Determinación de la Disponibilidad de Agua en el Acuífero Potrero del Llano (0841), Estado de Chihuahua; Comisión Nacional del Agua: Mexico City, Mexico, 2015; pp. 1–30. [Google Scholar]
- Cantaloub, M.; Higginbotham, J.; Semprini, L. The Determination of Rn Partition Coefficients for Several Liquid Scintillation Cocktails Proceedings. In Proceedings of the 43rd Annual Conference on Bioassay, Analytical and Environmental Radiochemistry, Charleston, SC, USA, 9–13 November 1997.
- Comisión Nacional del Agua (CONAGUA). NMX-AA-051-SCFI-2001. Water Analysis-Determination of Metals by Atomic Absorption in Natural, Drinking, Wastewaters and Wastewaters Treated-Testethod. Análisis de Agua-Determinación de Metales por Absorción Atómica en Aguas Naturales, Potables, Residuales y Residuales Tratadas; Diario Oficial de la Federación (DOF): Mexico City, Mexico, 2001; pp. 1–47. [Google Scholar]
- Somaratne, N. Characterization of the effects of redox condition on Fe(III)/Fe(II) transformation in a small karstic aquifer: Poocher swamp freshwater lens, South Australia. Environ. Nat. Resour. Res. 2016, 6, 134–145. [Google Scholar] [CrossRef]
- Salud, S.D. Norma Oficial Mexicana Nom-127-Ssa1–1994, "Salud Ambiental, Agua Para Uso Y Consumo Humano-Limites Permisibles de Calidad Y Tratamientos A que Debe Someterse el Agua Para Su Potabilizacion; Diario Oficial de la Federación: Mexico City, Mexico, 2000. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization (WHO): Geneva, Switzerland, 2008; pp. 1–492. [Google Scholar]
- Environmental Protection Agency (EPA). National Primary Drinking Water Regulations, Radionuclides; 40 CFR Parts 141 and 142; EPA: Washington, DC, USA, 2000; Volume 65, pp. 21575–21628.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation. Report to General Assembly with Scientific Annexes 2008; United Nations Scientific Committee on the Effect of Atomic Radiation; United Nations Sales Publications: New York, NY, USA, 2008. [Google Scholar]
- EU, European Commission. Commission recommendation of 20th December 2001 on the protection of the public against exposure to radon in drinking water. In Official Journal of the European Commission; 2001/982/Euratom, Editor.; European Commission: Brussels, Belgium, 2001. [Google Scholar]
- Baioumy, H. Geochemistry and geothermometry of non-volcanic hot springs in West Malaysia. J. Volcanol. Geotherm. Res. 2015, 290, 12–22. [Google Scholar] [CrossRef]
- Sracek, O.; Wanke, H.; Ndakunda, N.N.; Mihaljevič, M.; Buzek, F. Geochemistry and fluoride levels of geothermal springs in Namibia. J. Geochem. Explor. 2006, 148, 96–104. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía. Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos Aldama, Chihuahua. Clave Geoestadística 08002. Marco Geoestadístico Municipal; INEGI: Mexico City, Mexico, 2005. [Google Scholar]
- Servicio Geológico Mexicano, SGM. Carta Geológico-Minera Chorreras H13-C59, Chihuahua; Secretaría de Economía: Mexico City, Mexico, 2000. [Google Scholar]
- Banks, D.; Frengstad, B.; Midtgård, A.K.; Krog, J.R.; Strand, T. The chemistry of Norwegian groundwaters: I. The distribution of radon, major and minor elements in 1604 crystalline bedrock groundwaters. Sci. Total Environ. 1998, 222, 71–91. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Nordstrom, D.K.; Bove, D.J.; Plumlee, G.S.; Runkel, R.L. Naturally acidic surface and ground waters draining porphyry-related mineralized areas of the Southern Rocky Mountains, Colorado and New Mexico. Appl. Geochem. 2009, 24, 255–267. [Google Scholar] [CrossRef]
- Chang, R. Chemistry, 7th ed.; McGraw Hill: Boston, MA, USA, 2002. [Google Scholar]
- Pauling, L. General Chemistry; Dover: Mineola, NY, USA, 2014. [Google Scholar]
- Servicio Geológico Mexicano, SGM. Panorama Minero del Estado de Chihuahua; Secretaria de Economia: Mexico City, Mexico, 2015; pp. 20–23. [Google Scholar]
- Abdelgawad, A.M.; Watanabe, K.; Takeuchi, S.; Mizuno, T. The origin of fluoride-rich groundwater in Mizunami area, Japan-Mineralogy and geochemistry implications. Eng. Geol. 2009, 108, 76–85. [Google Scholar] [CrossRef]
- Berger, T.; Mathurin, F.A.; Drake, H.; Åström, M.E. Fluoride abundance and controls in fresh groundwater in Quaternary deposits and bedrock fractures in an area with fluorine-rich granitoid rocks. Sci. Total Environ. 2016, 569–570, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A. Fluoride toxicity to aquatic organisms: A review. Chemosphere 2003, 50, 251–264. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua (CONAGUA). NMX-AA-077-SCFI-2001. Waters Analysis-Determination of Fluoride in Natural, Wastewaters and Wastewaters Treated-Test Method. Análisis de Aguas. Determinación de Fluoruros en Aguas Naturales, Residuales y Residuales; Diario Oficial de la Federación (DOF): Mexico City, Mexico, 2001. [Google Scholar]
- Chae, G.T.; Yun, S.T.; Kwon, M.J.; Kim, Y.S.; Mayer, B. Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochemistry 2006, 40, 95–102. [Google Scholar] [CrossRef]
- Lima, M.A.; Santos, W.D.; Geraldo, L.P. Direct measurements of radon activity in water from various natural sources using nuclear track detectors. Appl. Radiat. Isot. 2004, 60, 801–804. [Google Scholar]
- Cho, J.S.; Ahn, J.K.; Kim, H.C.; Lee, D.W. Radon concentrations in groundwater in Busan measured with a liquid scintillation counter method. J. Environ. Radioact. 2004, 75, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.M.R.; Lauria, D.C.; Ferreira, A.C.; Sracek, O. Groundwater radon, radium and uranium concentrations in Região dos Lagos, Rio de Janeiro State, Brazil. J. Environ. Radioact. 2004, 73, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, D.; Swarzenski, P.W. The Behavior of U-and Th-series Nuclides in Groundwater. Rev. Mineral. Geochem. 2003, 52, 317–361. [Google Scholar] [CrossRef]
- Pérez del Villar, L.; Crespo, M.T.; Pardillo, J.; Pelayo, M.; Galán, M.P. U and Th series disequilibrium in unaltered and hydrothermally-altered granites from the El Berrocal site (Spain): Weathering effects. Appl. Radiat. Isot. 1996, 47, 1115–1119. [Google Scholar] [CrossRef]
- Gunn, B.M. Geochemistry of Igneous Rocks. 2004. Available online: http://geokem.com (accessed on 9 March 2017).
- Kiro, Y.; Weinstein, Y.; Starinsky, A.; Yechieli, Y. Application of radon and radium isotopes to groundwater flow dynamics: An example from the Dead Sea. Chem. Geol. 2015, 411, 155–171. [Google Scholar] [CrossRef]
- Kiro, Y.; Yechieli, Y.; Voss, C.I.; Starinsky, A.; Weinstein, Y. Modeling radium distribution in coastal aquifers during sea level changes: The Dead Sea case. Geochim. Cosmochim. Acta 2012, 88, 237–254. [Google Scholar] [CrossRef]
- Mittal, S.; Rani, A.; Mehra, R. Radon levels in drinking water and soil samples of Jodhpur and Nagaur districts of Rajasthan, India. Appl. Radiat. Isot. 2016, 113, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.M.; Habib, R.R.; Nuwayhid, R.Y.; Chatila, M.; Katul, G. Radon measurements in well and spring water in Lebanon. Radiat. Meas. 2007, 42, 298–303. [Google Scholar] [CrossRef]
- Al-Bataina, B.A.; Ismail, A.M.; Kullab, M.K.; Abumurad, K.M.; Mustafa, H. Radon measurements in different types of natural waters in Jordan. Radiat. Meas. 1997, 28, 591–594. [Google Scholar] [CrossRef]
- Óskarsson, F.; Ásgeirsdóttir, R.S. Radon in Icelandic Cold Groundwater and Low-Temperature Geothermal Water. Procedia Earth Planet. Sci. 2017, 17, 229–232. [Google Scholar] [CrossRef]
- Horváth, Á.; Bohus, L.O.; Urbani, F.; Marx, G.; Piróth, A.; Greaves, E.D. Radon concentrations in hot spring waters in northern Venezuela. J. Environ. Radioact. 2000, 47, 127–133. [Google Scholar] [CrossRef]
- Song, G.; Zhang, B.; Wang, X.; Gong, J.; Chan, D.; Bernett, J.; Lee, S.C. Indoor radon levels in selected hot spring hotels in Guangdong, China. Sci. Total Environ. 2005, 339, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Freiler, Á.; Horváth, Á.; Török, K.; Földes, T. Origin of radon concentration of Csalóka Spring in the Sopron Mountains (West Hungary). J. Environ. Radioact. 2016, 151, 174–184. [Google Scholar] [CrossRef] [PubMed]
- KozŁowska, B.; Walencik, A.; Dorda, J.; Zipper, W. Radon in groundwater and dose estimation for inhabitants in Spas of the Sudety Mountain area, Poland. Appl. Radiat. Isot. 2010, 68, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Moldovan, M.; Dicu, T.; Kovacs, T. Radon in water from Transylvania (Romania). Radiat. Meas. 2008, 43, 1423–1428. [Google Scholar] [CrossRef]
- Radolić, V.; Vuković, B.; Šmit, G.; Stanić, D.; Planinić, J. Radon in the spas of Croatia. J. Environ. Radioact. 2005, 83, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Fonollosa, E.; Peñalver, A.; Borrull, F.; Aguilar, C. Radon in spring waters in the south of Catalonia. J. Environ. Radioact. 2016, 151, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Segovia, N.; Armienta, M.A.; Valdes, C.; Mena, M.; Seidel, J.L.; Monnin, M.; Peña, P.; Lopez, M.B.E.; Reyes, A.V. Volcanic monitoring for radon and chemical species in the soil and in spring water samples. Radiat. Meas. 2003, 36, 379–383. [Google Scholar] [CrossRef]
- Ramírez-Guzmán, A.; Taran, Y.; Armienta, M.A. Geochemistry and origin of high-pH thermal springs in the Pacific coast of Guerrero, Mexico. Geofís. Int. 2004, 43, 415–425. [Google Scholar]
- Atkins, M.L.; Santos, I.R.; Perkins, A.; Maher, D.T. Dissolved radon and uranium in groundwater in a potential coal seam gas development region (Richmond River Catchment, Australia). J. Environ. Radioact. 2016, 154, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Alabdula’aly, A.I. Occurrence of radon in groundwater of Saudi Arabia. J. Environ. Radioact. 2014, 138, 186–191. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Censo General de Poblacion y Vivienda, Mexico; Instituto Nacional de Estadısticas y Geografıa: Aguascalientes, Mexico, 2010. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Radon; P.H.S. Department of Health and Human Services: Atlanta, GA, USA, 2012.

| Sample | pH | T (°C) | ORP (mV) | TDS (mg/L) | North (N) | West (O) | Altitude (MSL +) |
|---|---|---|---|---|---|---|---|
| P1 | 6.9 | 28.2 | 158 | 410 | 28°51′2.11″ | 105°16′11.49″ | 1168 |
| P2 | 6.6 | 30.7 | 149 | 440 | 28°51′1.23″ | 105°16′13.85″ | 1168 |
| P3 | 6.5 | 31.4 | 153 | 420 | 28°50′58.52″ | 105°16′20.16″ | 1160 |
| P4 | 6.6 | 27.2 | 132 | 270 | 28°54′22.10″ | 105°20′9.86″ | 1029 |
| P5 | 6.8 | 28.5 | 138 | 930 | 28°55′0.45″ | 105°20′4.66″ | 1043 |
| P6 | 6.4 | 27.2 | 158 | 1180 | 28°54′7.25″ | 105°16′18.36″ | 1084 |
| Sample | SO4−2 | F− | Ca+2 | Mg+2 | Na+ | K+ | Si | Hardness * |
|---|---|---|---|---|---|---|---|---|
| NORM | 400 | 1.5 | - | - | 200 | - | - | 500 |
| WHO | - | 1.5 | 75 | 50 | - | - | - | 500 |
| P1 | 45 (0.592) | 1.05 (0.001) | 37.7 (0.001) | 2.3 (0.004) | 47.4 (0.019) | 6.3 (0.039) | 17.3 (0.133) | 104 (0.016) |
| P2 | 71 (0.295) | 1.11 (0.002) | 41.2 (0.001) | 2.4 (0.010) | 48.6 (0.006) | 5.9 (0.036) | 18.2 (0.362) | 113 (0.042) |
| P3 | 80 (0.505) | 1.12 (0.002) | 38.6 (0.001) | 2.5 (0.006) | 47.7 (0.004) | 6.4 (0.012) | 16.6 (0.202) | 107 (0.028) |
| P4 | 44 (0.092) | 1.12 (0.001) | 19.8 (0.002) | 2.4 (0.016) | 48.6 (0.006) | 5.5 (0.056) | 15.3 (0.054) | 59 (0.061) |
| P5 | 420 (0.367) | 2.38 (0.012) | 48.5 (0.017) | 14.0 (0.001) | 79.3 (0.009) | 2.3 (0.015) | 35.2 (0.097) | 179 (0.042) |
| P6 | 800 (0.182) | 1.84 (0.002) | 96.7 (0.012) | 12.5 (0.001) | 72.6 (0.016) | 6.7 (0.081) | 27.7 (0.014) | 293 (0.029) |
| Sample | 222Rn (Bq/L) | Annual Effective Dose (µSv/y) |
|---|---|---|
| EPA | 11 | - |
| UNSCEAR | 40 | - |
| EU | 100 | - |
| P1 | 67 (2.7) | 14.07 |
| P2 | 83 (4.1) | 17.43 |
| P3 | 52 (2.3) | 10.92 |
| P4 | 380 (11.4) | 79.80 |
| P5 | 161 (6.4) | 33.81 |
| P6 | 229 (6.9) | 48.09 |
| Ion | Coefficients | Coefficients Values | p Value |
|---|---|---|---|
| - | β0 | −5277.78 | <0.0001 |
| Si | β1 | −42.53 | <0.0001 |
| Na+ | β2 | 177.77 | <0.0001 |
| Mg+2 | β3 | 98.37 | <0.0001 |
| F− | β4 | 105.01 | <0.0001 |
| Reference | 222Rn (Bq/L) | Temperature (°C) | Country |
|---|---|---|---|
| [47] | 3.3–10.7 | 21–26 | Jordan |
| [47] | 3.2–5.5 | 30–48 | Jordan |
| [49] | 0.1–0.42 | 27 | Venezuela |
| [49] | 1–576 | 34–78 | Venezuela |
| [46] | 0.46–49.6 | 25–30 | Lebanon |
| [50] | 53.4–292.5 | 40–90 | China |
| [54] | 2.1–93.8 | nm * | Croatia |
| [55] | 1.4–105 | 11.3–18.1 | Spain |
| This study | 52–380 | 27–31 | Mexico |
| [56] | <1–3 | 14–20 | Mexico |
| [57] | 8–25 | 40–43 | Mexico |
| [45] | 0.5–22 | 28–34 | India |
| [58] | 0.14–20.33 | nm | Australia |
| [53] | 2–129 | 10–15 | Romania |
| [52] | 4.2–1703 | nm | Poland |
| [48] | <1–10.8 | 1.7–145 | Iceland |
| [48,59] | <1–67.4 | nm | Saudi Arabia |
| [51] | 153–291 | 6.5–12 | Hungary |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Villalobos, M.; Covarrubias-Muños, A.; Pinedo-Álvarez, A.; Manjon-Collado, G. Hydrochemistry and 222Rn Concentrations in Spring Waters in the Arid Zone El Granero, Chihuahua, Mexico. Geosciences 2017, 7, 12. https://doi.org/10.3390/geosciences7010012
Rentería-Villalobos M, Covarrubias-Muños A, Pinedo-Álvarez A, Manjon-Collado G. Hydrochemistry and 222Rn Concentrations in Spring Waters in the Arid Zone El Granero, Chihuahua, Mexico. Geosciences. 2017; 7(1):12. https://doi.org/10.3390/geosciences7010012
Chicago/Turabian StyleRentería-Villalobos, Marusia, Alejandro Covarrubias-Muños, Alfredo Pinedo-Álvarez, and Guillermo Manjon-Collado. 2017. "Hydrochemistry and 222Rn Concentrations in Spring Waters in the Arid Zone El Granero, Chihuahua, Mexico" Geosciences 7, no. 1: 12. https://doi.org/10.3390/geosciences7010012






