Escape Burrowing of Modern Freshwater Bivalves as a Paradigm for Escape Behavior in the Devonian Bivalve Archanodon catskillensis
Abstract
:1. Introduction
1.1. Taxonomy
1.2. Paleoecology
1.3. Paleoethology
1.4. Aims of the Research Discussed Here
2. Bivalve Burrowing Behavior
2.1. Downward Burrowing
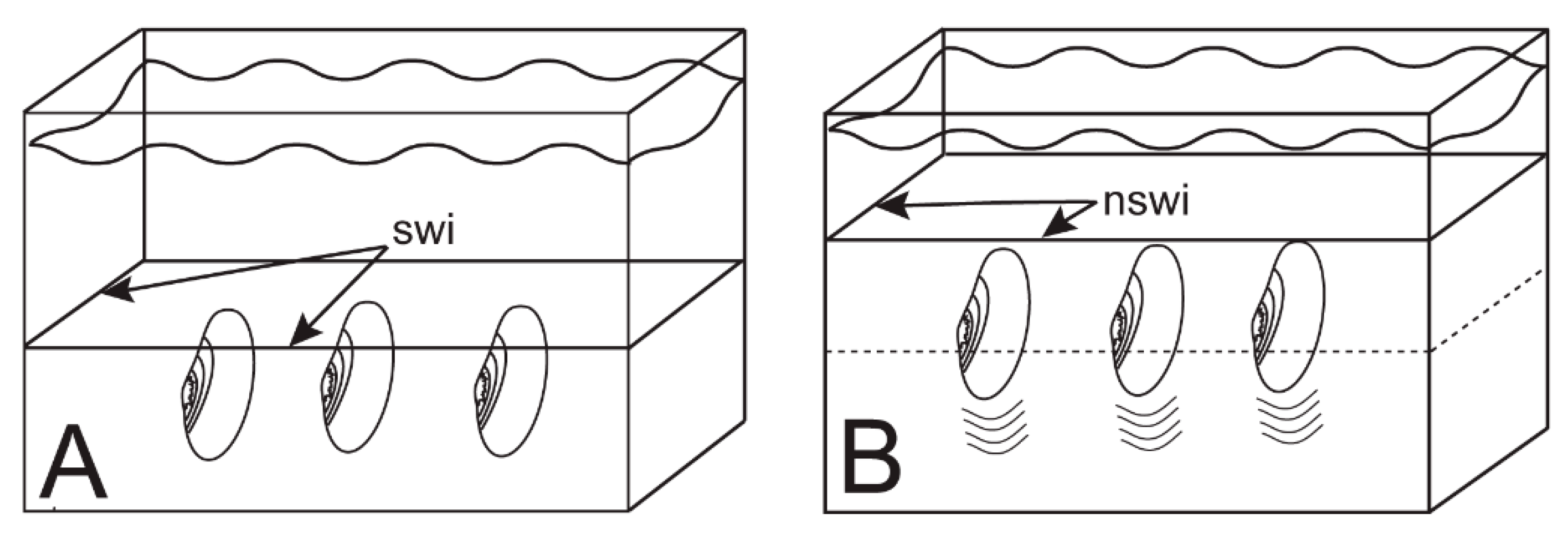
2.2. Escape Burrowing
2.2.1. Marine Bivalves
2.2.2. Freshwater Bivalves
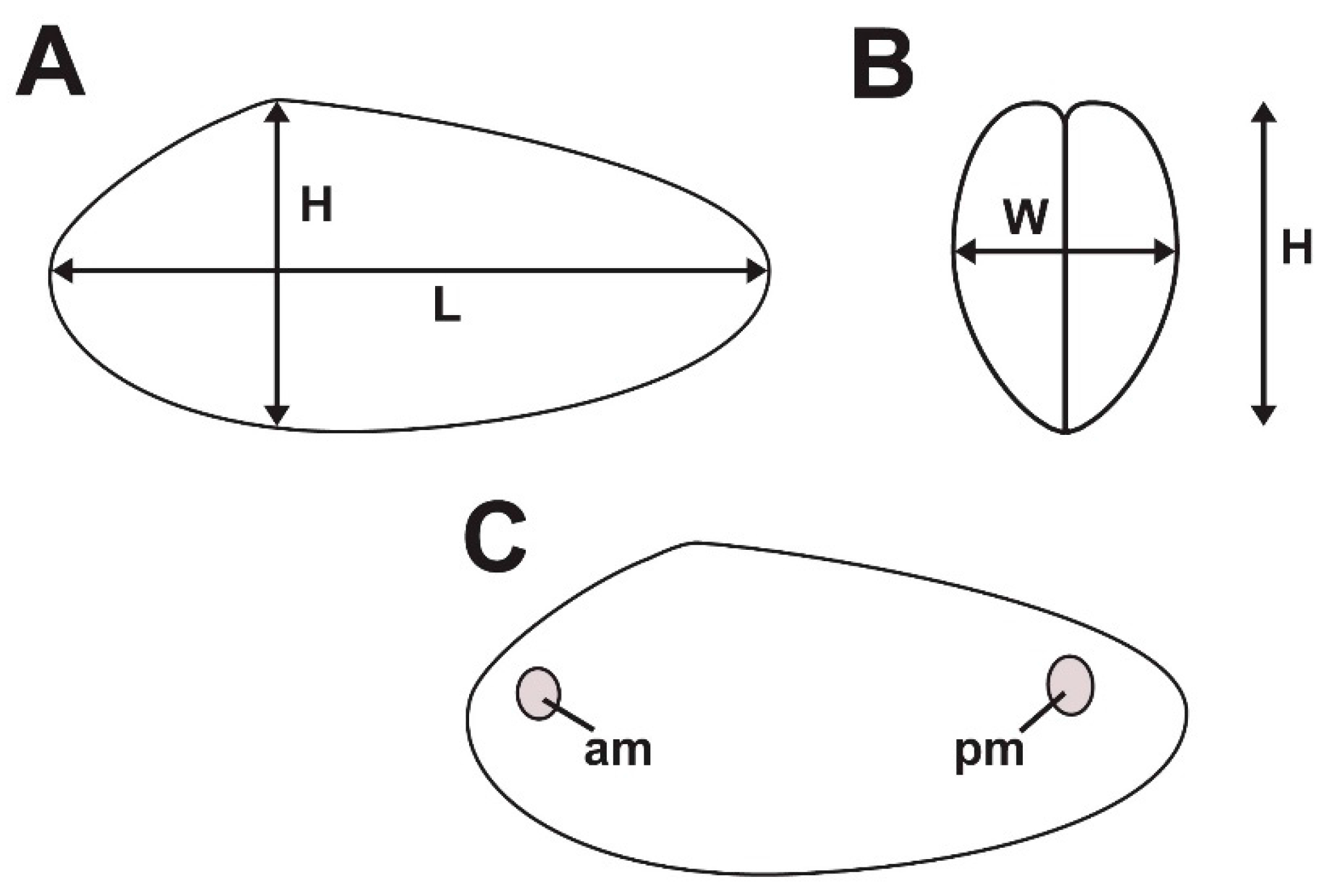
3. Comparative Shell Form
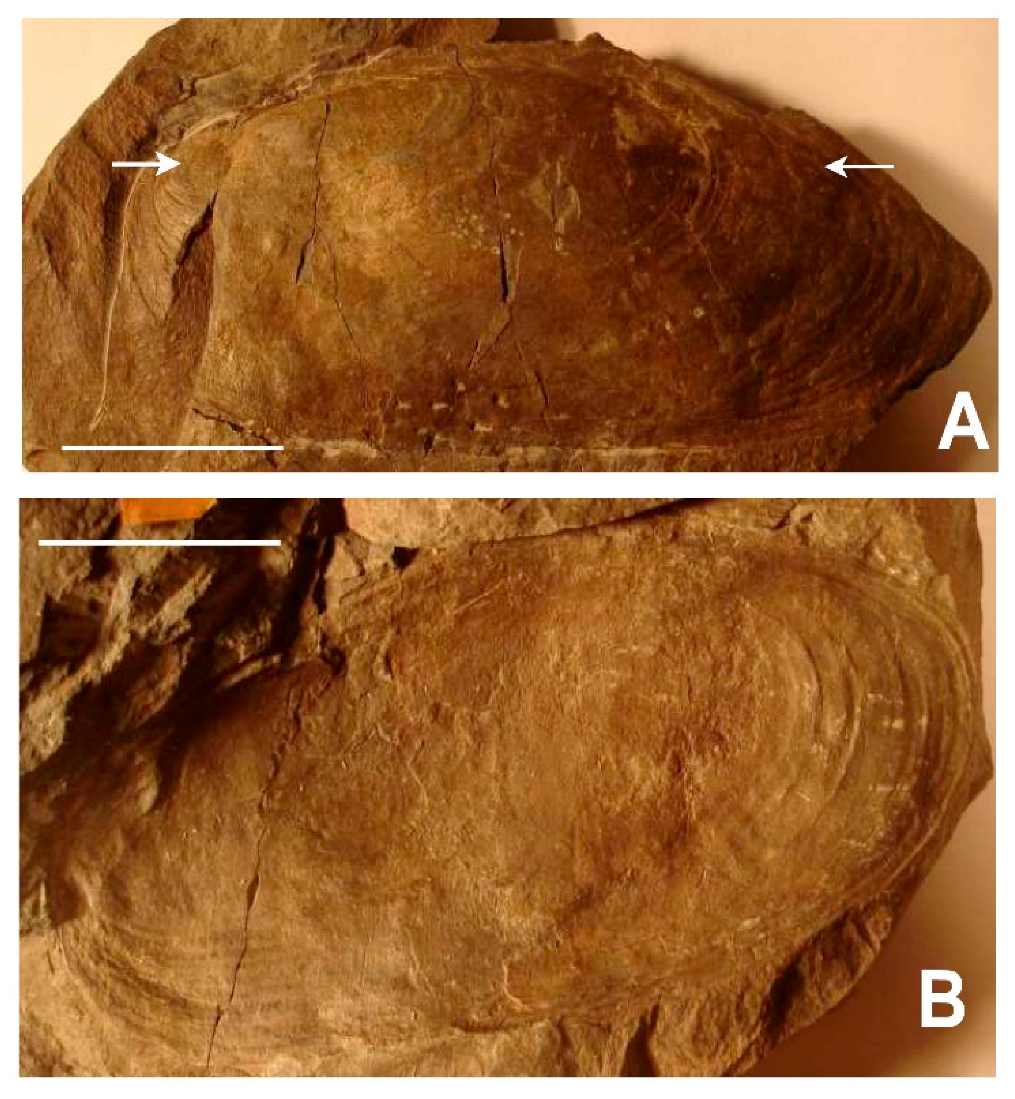
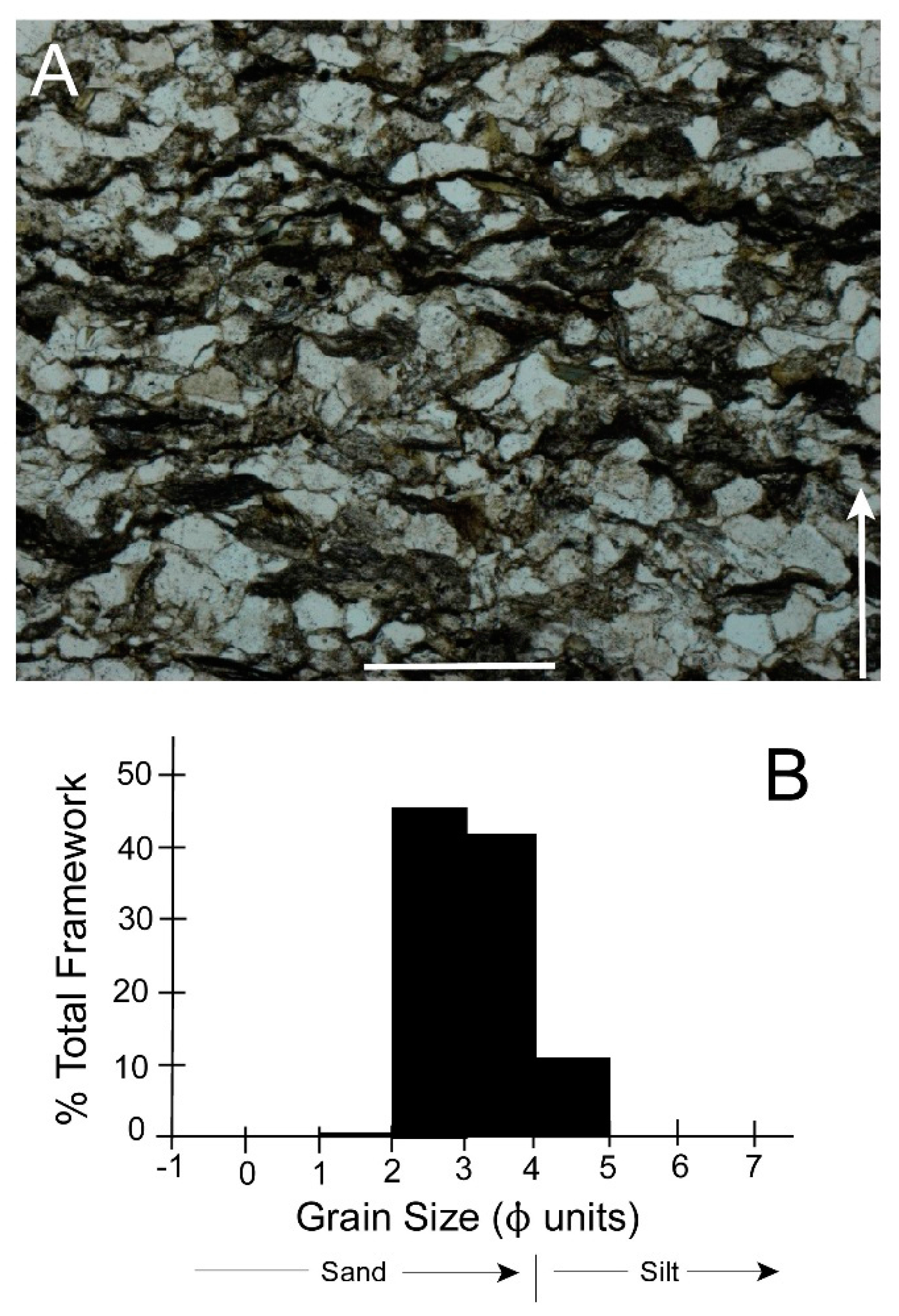
3.1. Shell Form of Archanodon Catskillensis
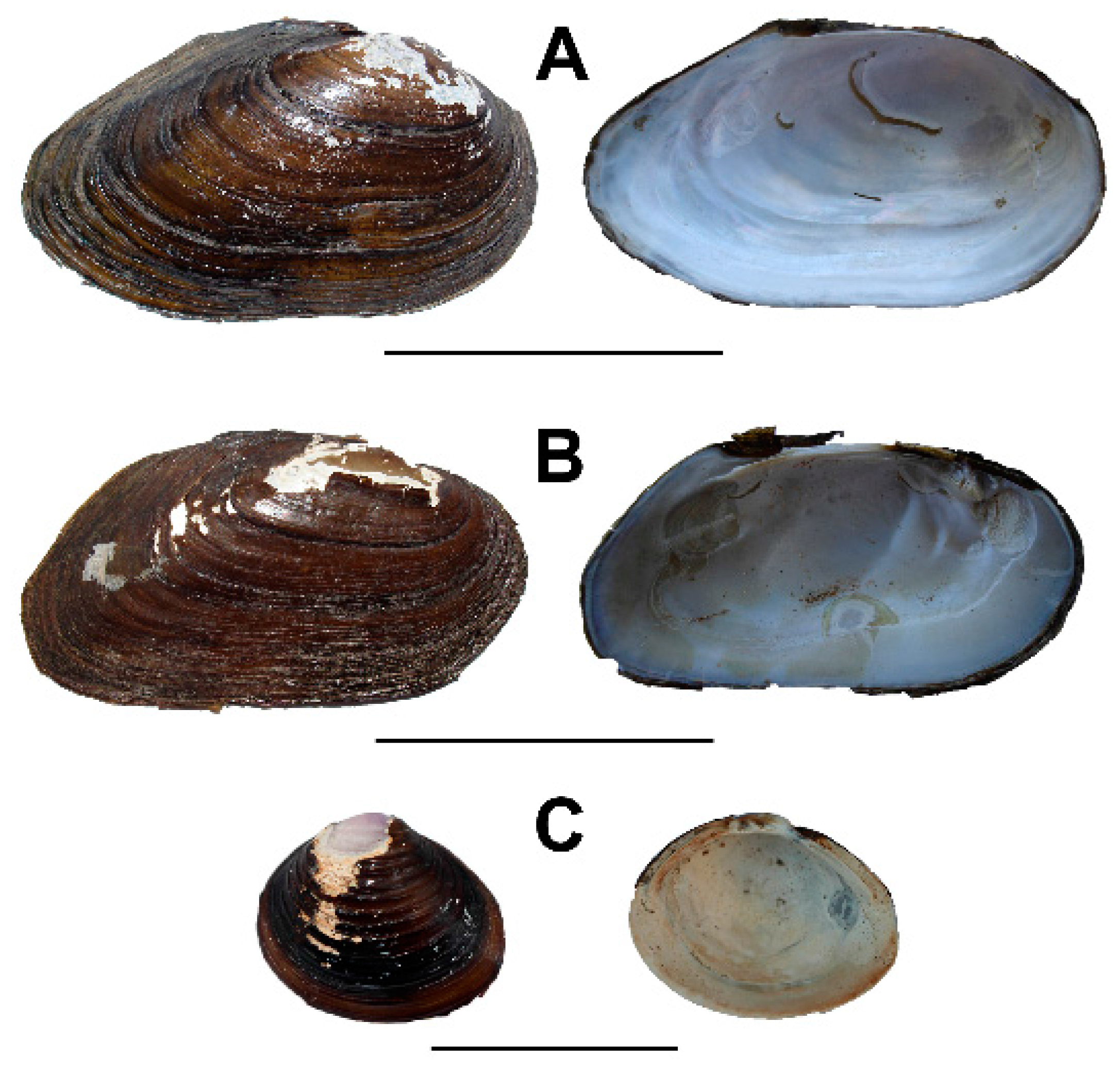
3.2. Archanodon Catskillensis Analogues
3.2.1. Pyganodon Cataracta
3.2.2. Corbicula Fluminea
4. Experimental Methods
4.1. Specimen Collection
4.2. Animal Maintenance
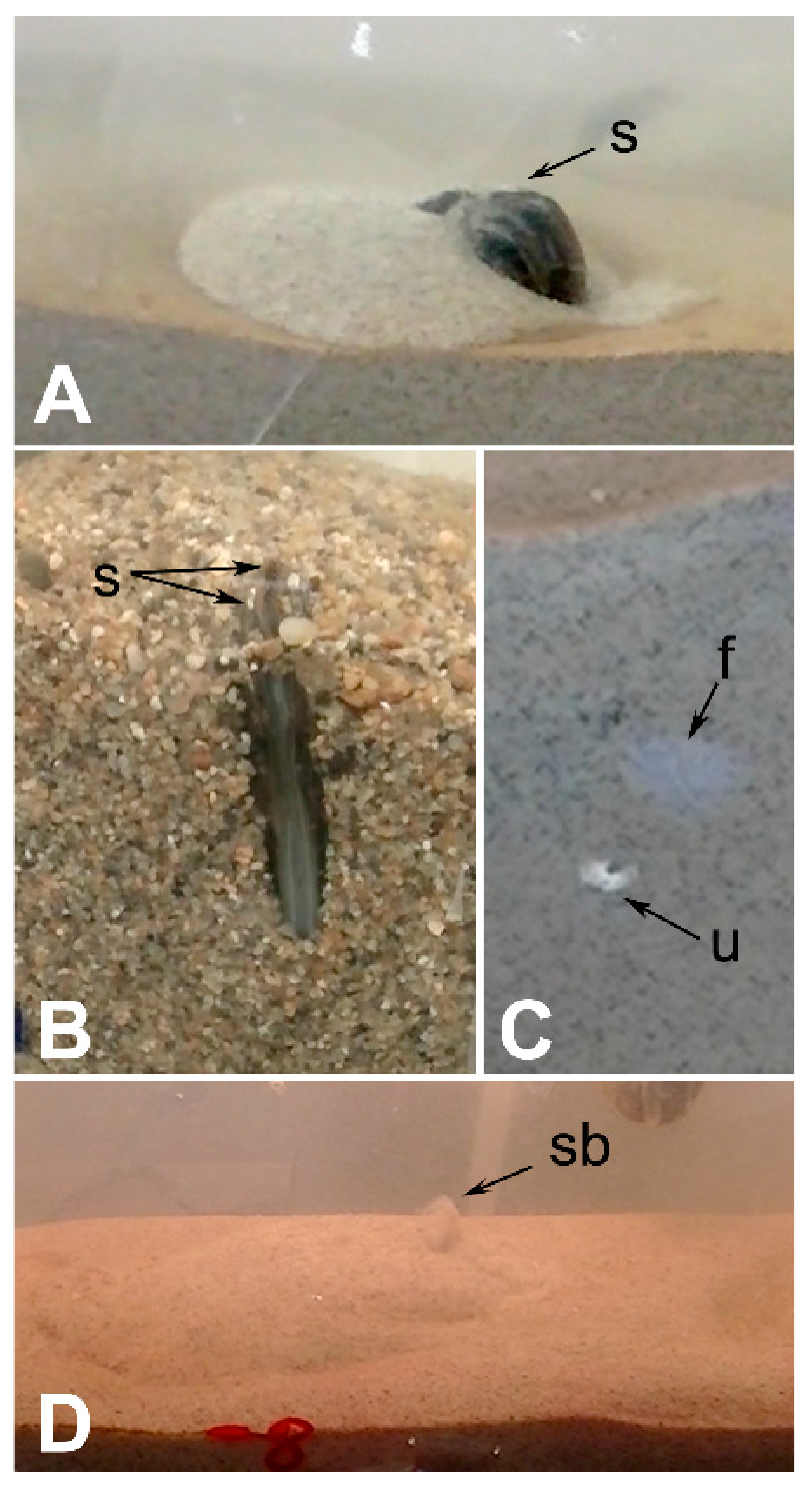
4.3. Escape Burrowing Tests
4.4. Burrow Structure Tests
5. Field Methods
6. Experimental Results
6.1. Escape Orientation
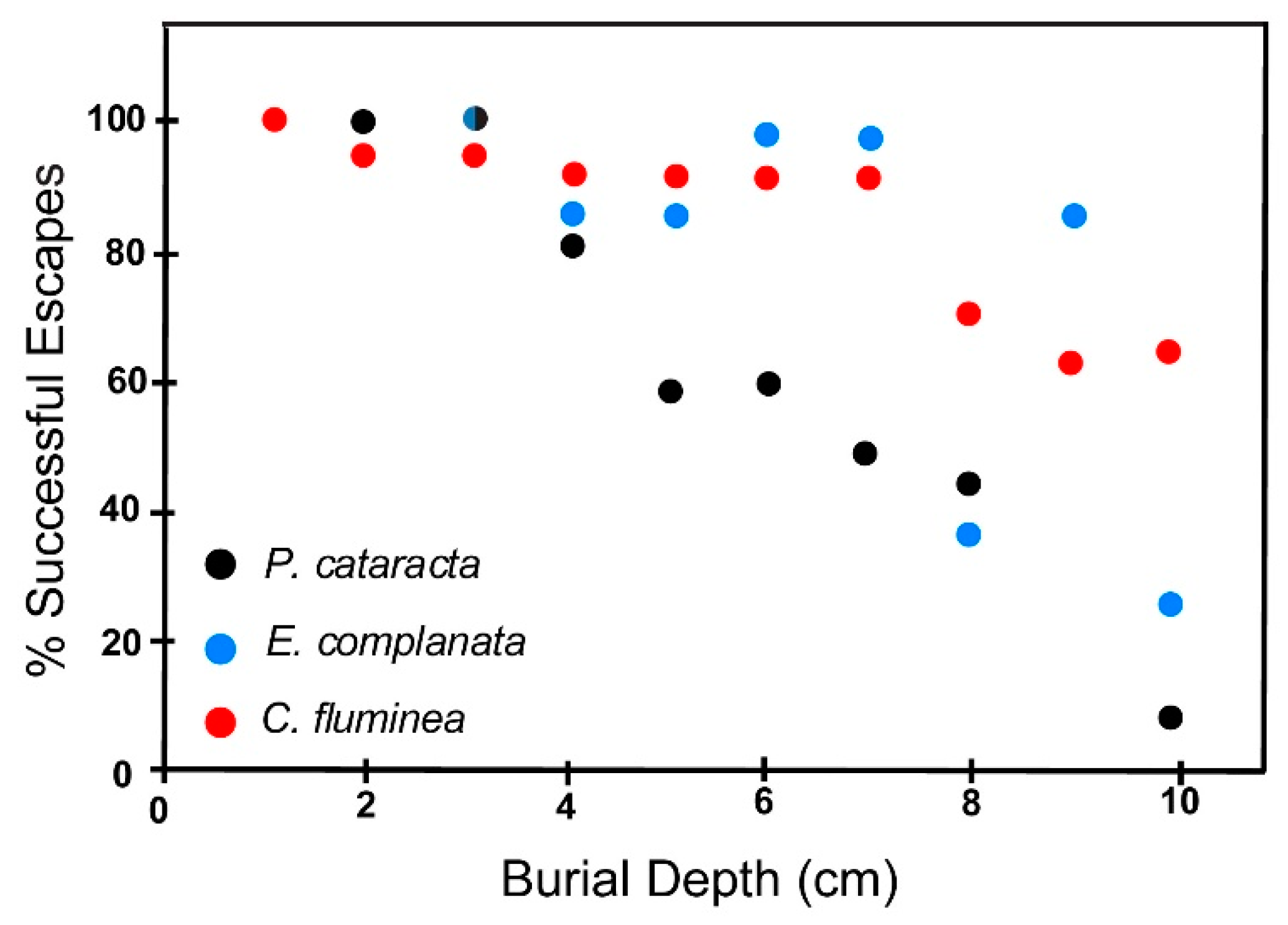
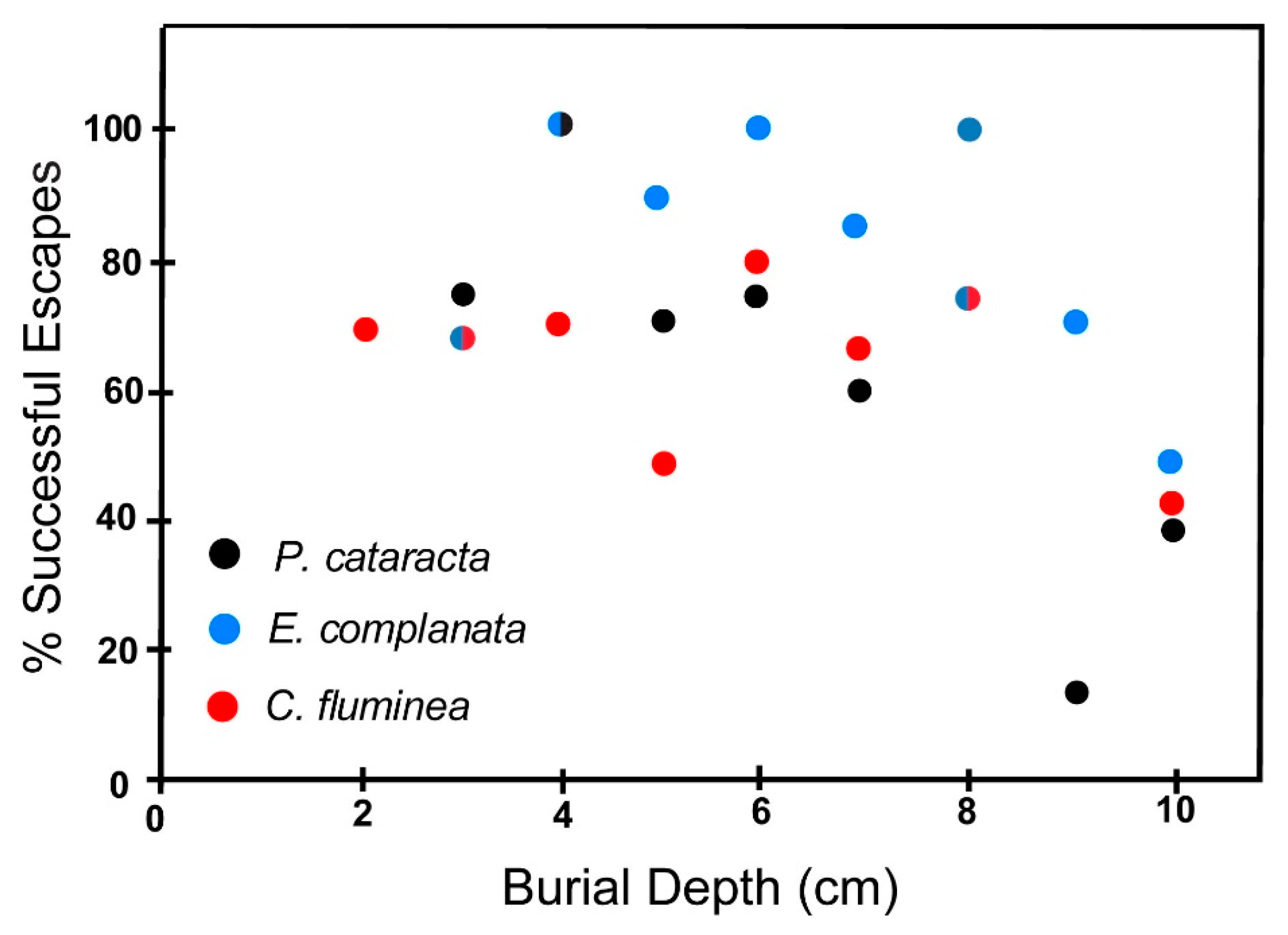
6.2. Escape Potential
6.2.1. Fine Sand
6.2.2. Coarse Sand
6.3. Effect of Shell Size on Escape Potential
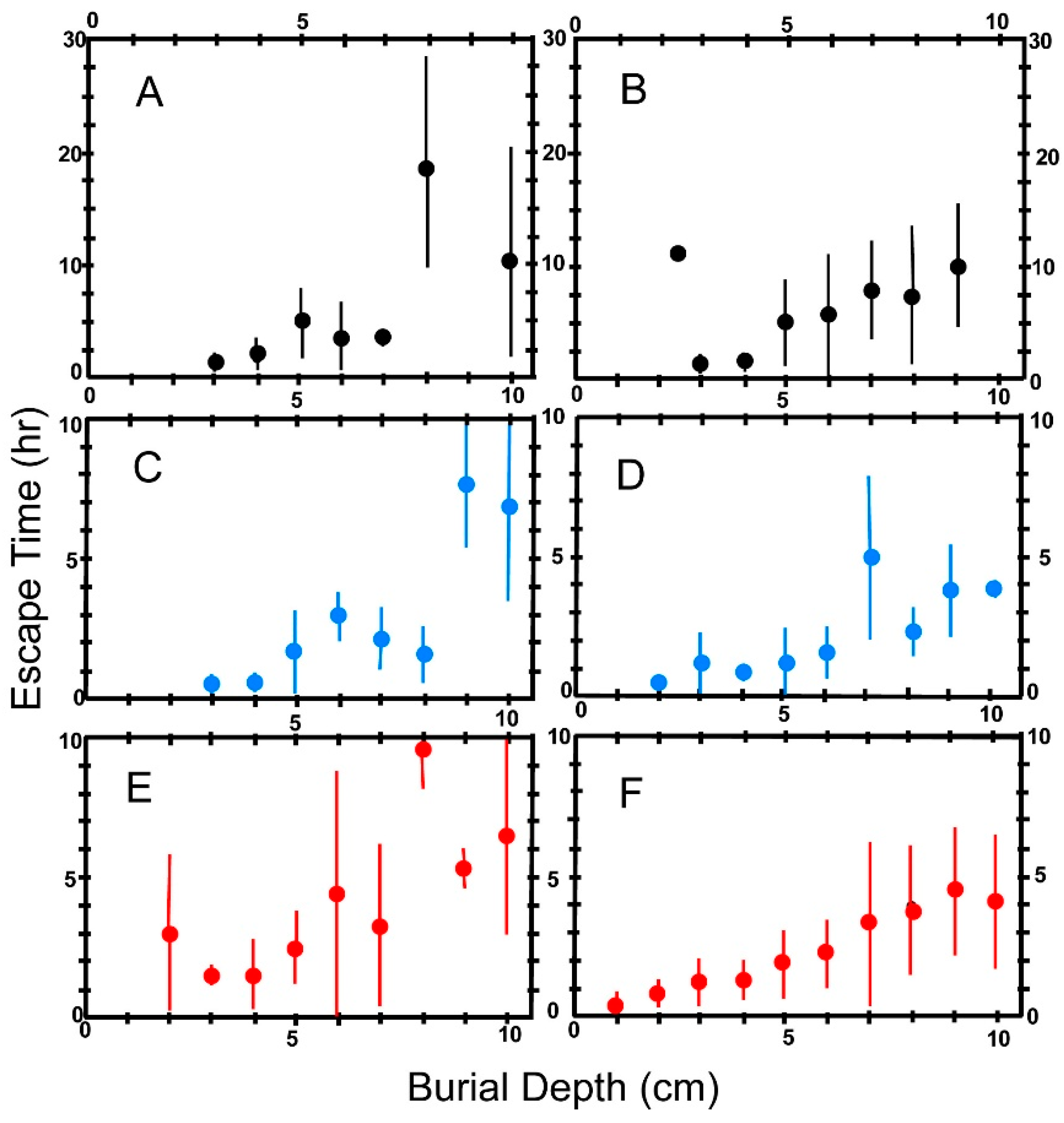
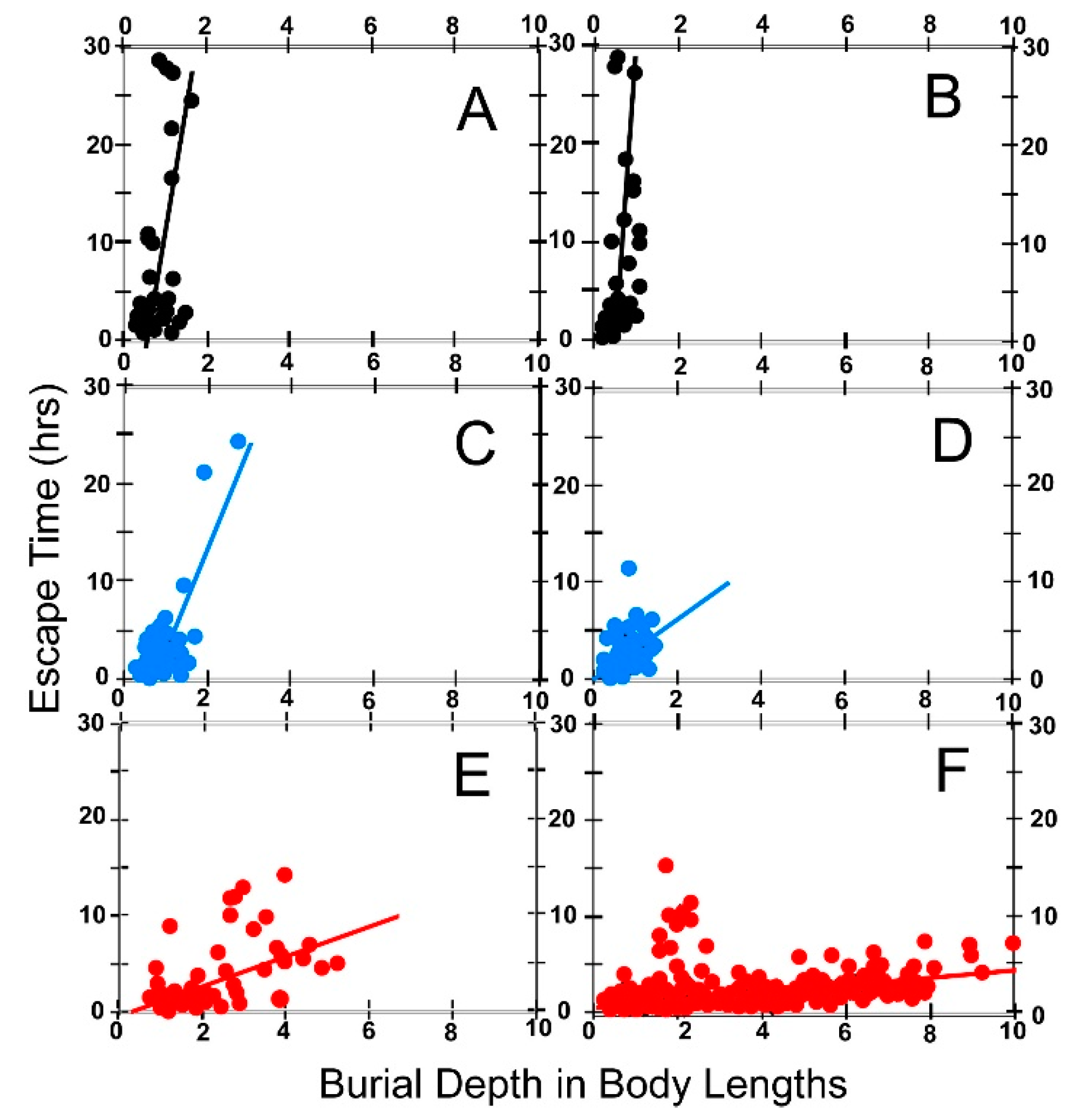
6.4. Escape Time
6.5. Effect of Body Size on Escape Time
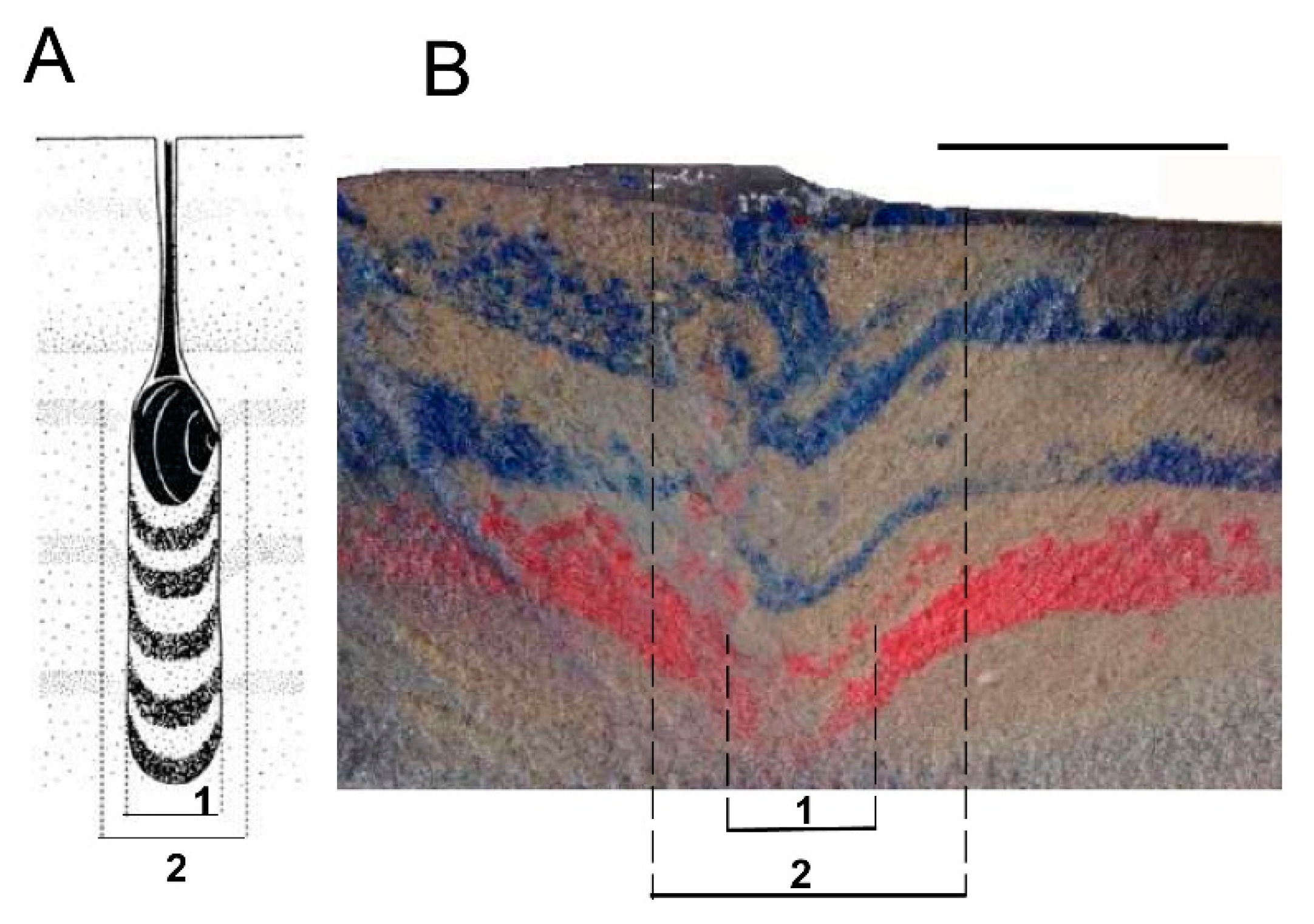
6.6. Burrow Structure
7. Field Results
8. Discussion
8.1. Escape Capacity of Modern Archanodon catskillensis Analogues
8.1.1. Escape Potential
(a) Effect of Animal Size on Escape Potential
(b) Effect of Shell Characteristics on Escape Potential
(c) Effect of Soft Anatomy on Escape Potential
(d) Effect of Life Habit on Escape Potential
8.1.2. Escape Time
(a) Effect of Foot Size on Escape Time
(b) Effect of Reaction Time and Sediment Compaction on Escape Time
(c) Effect of Oxygen Availability on Escape Rate
(d) Effect of Sexual Dimorphism on Escape Rate
8.2. Which Species Is the Better Escape Burrower
8.3. Escape Capacity of Archanodon catskillensis
8.4. Burrow Structure
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vanuxem, L. Geology of New York. Part 3. In Comprising the Survey of the Third Geological District; W. and A. White and J. Visscher: Albany, NY, USA, 1842; p. 306. [Google Scholar]
- Hall, J. Lamellibranchiata II. Dimyaria from the upper Helderberg, Hamilton, Portage, and Chemung groups. In Natural History of New York: Palaeontology; Part I.; Charles Van Benthuysen and Sons: Albany, NY, USA, 1885; Volume 5, pp. 516–518. [Google Scholar]
- Clarke, J.L. Value of Amnigenia as an indicator of fresh-water deposits during the Devonic of New York. N. Y. State Mus. Bull. 1901, 49, 199–203. [Google Scholar]
- Weir, J. Superfamily Archanodontacea. In Treatise on Invertebrate Paleontology; Part N, Mollusca 6; Moore, R.C., Ed.; Geological Society of America and University of Kansas: Lawrence, KS, USA, 1969; Volume 1, pp. 402–404. [Google Scholar]
- Chamberlain, J.A., Jr.; Chamberlain, R.B. The Devonian bivalve, Archanodon catskillensis: A status report on the first freshwater mussel from New Jersey. In Contributions to the Paleontology of New Jersey; Rainforth, E., Ed.; Geological Association of New Jersey: Trenton, NJ, USA, 2007; Volume 24, pp. 24–40. [Google Scholar]
- Chamberlain, J.A., Jr.; Friedman, G.M.; Chamberlain, R.B. Devonian archanodont unionoids from the Catskill Mountains of New York: Implications for the paleoecology and biogeography of the first freshwater bivalves. Northeast. Geol. Environ. Sci. 2004, 26, 211–229. [Google Scholar]
- Newell, N.D. Classification of the Bivalvia. Am. Mus. Nat. Hist. Novit. 1965, 2206, 1–25. [Google Scholar]
- Watters, G.T. The evolution of the Unionacea in North America, and its implications for the worldwide fauna. In Ecology and Evolution of the Freshwater Mussels Unionoida; Bauer, G., Wächtler, K., Eds.; Ecological Studies Springer: Berlin, Germany, 2001; Volume 145, pp. 281–307. [Google Scholar]
- Howse, R. Preliminary notice of the occurrence of Archanodon (Anodonta) jukesii, Forbes, in the Lower Carboniferous rocks of north Northumberland. Nat. Hist. Trans. Northumberl. Durh. Newctle.-Upon-Tyne 1878, 7, 173–175. [Google Scholar]
- Forbes, E. On the fossils of the Yellow Sandstone of the south of Ireland. Rep. Br. Assoc. Adv. Sci. (22nd Meet.) 1853, 22, 43. [Google Scholar]
- Beushausen, L. Amnigenia rhenana n. sp., ein Anodonta ähnlicher Zweischaler aus dem rheinsischen Mitteldevon. Jahrb. Königlich Preuss. Geol. Landesanst. Bergakad. Berlin Jahrb. Abhand. Mitarbeiten. 1890, Band XI, II, 1–10. [Google Scholar]
- Whiteaves, J.F. Note on the recent discovery of large Unio-like shells in the Coal Measures at South Joggins, Nova Scotia. Trans. R. Soc. Can. Sec. 4 1893, 11, 21–24. [Google Scholar]
- Bridge, J.S.; Gordon, E.A.; Titus, R.C. Non-marine bivalves and associated burrows in the Catskill magnafacies (Upper Devonian) of New York State. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 55, 65–77. [Google Scholar] [CrossRef]
- Berg, T.M. Bivalve burrow structures in the Bellvale Sandstone, New Jersey and New York. Bull. N. J. Acad. Sci. 1977, 22, 1–5. [Google Scholar]
- Friedman, G.M.; Chamberlain, J.A., Jr. Archanodon catskillensis (Vanuxem): Freshwater clams from one of the oldest back-swamp fluvial facies (upper Middle Devonian), Catskill Mountains, New York. Northeast. Geol. Environ. Sci. 1995, 17, 431–443. [Google Scholar]
- Thoms, R.E.; Berg, T.M. Interpretation of bivalve trace fossils in fluvial beds of the basal Catskill Formation (late Devonian), Eastern USA. In Biogenic Structures: Their Use in Interpreting Depositional Environments; Curran, H.A., Ed.; Society for Sedimentary Geology: Tulsa, OK, USA, 1985; Volume 35, pp. 13–20. [Google Scholar]
- Knox, L.; Gordon, E.L. Ostracodes as indicators of brackish water environments in the Catskill Magnafacies (Devonian) of New York State. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 148, 9–22. [Google Scholar] [CrossRef]
- Friedman, G.M.; Lundin, R.F. Freshwater ostracodes from Middle Devonian fluvial facies, Catskill Mountains, New York. J. Paleontol. 1998, 72, 485–490. [Google Scholar] [CrossRef]
- Falcon-Lang, H.J.; Rygel, M.C.; Calder, J.H.; Gibling, M.R. An early Pennsylvanian waterhole deposit and its fossil biota in a dryland alluvial plain setting, Joggins, Nova Scotia. J. Geol. Soc. Lond. 2004, 161, 209–222. [Google Scholar] [CrossRef]
- Hebert, B.L.; Calder, J.H. On the discovery of a unique terrestrial faunal assemblage in the classic Pennsylvanian section at Joggins, Nova Scotia. Can. J. Earth Sci. 2004, 41, 247–254. [Google Scholar] [CrossRef]
- Rygel, M.C.; Gibling, M.R. Natural geomorphic variability recorded in a high-accommodation setting: Fluvial architecture of the Pennsylvanian Joggins Formation of Atlantic Canada. J. Sediment. Res. 2006, 76, 1230–1251. [Google Scholar] [CrossRef]
- Trueman, E.R. The Locomotion of the freshwater clam Margaritifera margaritifera (Unionacea: Margaritanidae). Malacologia 1968, 6, 401–410. [Google Scholar]
- Strayer, D.L.; Jirka, K.J. The Pearly Mussels of New York State. In New York State Museum; Memoir 26; New York State Education Department: Albany, NY, USA, 1997; p. 113. [Google Scholar]
- Haag, W.R. North American Freshwater Mussels; Cambridge University Press: Cambridge, UK, 2012; p. 505. [Google Scholar]
- Tevesz, M.J.S.; Cornelius, D.W.; Fisher, J.B. Life habits and distribution of riverine Lampsilis radiata luteola (Mollusca: Bivalvia). Kirtlandia 1985, 41, 27–33. [Google Scholar]
- Di Maio, J.; Corkum, L.D. Patterns of orientation in unionids as a function of rivers with differing hydrological variability. J. Molluscan Stud. 1997, 63, 531–539. [Google Scholar] [CrossRef]
- Perles, S.J.; Christian, A.D.; Berg, D.J. Vertical Migration, Orientation, Aggregation, and Fecundity of the Freshwater Mussel Lampsilis Siliquoidea. Ohio J. Sci. 2003, 103, 73–78. [Google Scholar]
- Gordon, E.A. Body and trace fossils from the Middle-Upper Devonian Catskill Magnafacies, southeastern New York, USA. In Devonian of the World, Volume II: Sedimentation; McMillan, N.J., Embry, A.F., Glass, D.J., Eds.; Canadian Society of Petroleum Geologists: Calgary, AB, Canada, 1988; pp. 139–155. [Google Scholar]
- Chamberlain, J.A., Jr.; Chamberlain, R.B. Archanodon catskillensis: The life and times of a bivalve pioneer. N. Am. Paleont. Conv. Halifax NS Can. Programme Abstr. PaleoBios 2005, 25 (Suppl. 2), 29. [Google Scholar]
- Johnson, K.G.; Friedman, G.M. The Tully clastic correlatives (Upper Devonian) of New York State: A model for the recognition of alluvial, dune (?), tidal, nearshore (bar and lagoon), and offshore sedimentary environments in a tectonic delta complex. J. Sediment. Petrol. 1969, 39, 451–485. [Google Scholar]
- Chamberlain, J.A., Jr. Hydromechanical design of fossil cephalopods. In The Ammonoidea; House, M.R., Senior, J.R., Eds.; Academic Press: New York, NY, USA, 1981; pp. 289–335. [Google Scholar]
- Chamberlain, J.A., Jr. Locomotion of Nautilus. In Nautilus: Biology and Paleobiology of a Living Fossil; Landman, N.H., Saunders, W.B., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 489–525. [Google Scholar]
- Chamberlain, J.A., Jr. Jet propulsion of Nautilus: A surviving example of early Paleozoic cephalopod locomotor design. Can. J. Zool. 1990, 68, 806–814. [Google Scholar] [CrossRef]
- Reineck, H.-E. Wühlbau-Gefüge in Abhängigkeit von Sediment-Umlagerungen. Senckenberg. Lethaea 1958, 39, 1–24. [Google Scholar]
- Schäfer, W. Aktuo-Paläontologie Nach Studien in der Nordsee; Verlag Waldemar Kramer: Frankfurt am Main, Germany, 1962; p. 666. [Google Scholar]
- Raup, D.M.; Seilacher, A. Fossil foraging behavior: Computer simulation. Science 1969, 166, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Bromley, R.G. Trace Fossils: Biology, Taphonomy and Applications; Chapman and Hall: New York, NY, USA, 1996; p. 361. [Google Scholar]
- Plotnick, R.E. Behavioral biology of trace fossils. Paleobiology 2012, 38, 459–473. [Google Scholar] [CrossRef]
- Sims, D.W.; Reynolds, A.M.; Humphries, N.E.; Southall, E.J.; Wearmouth, V.J.; Metcalfe, B.; Twitchett, R.J. Hierarchical random walks in trace fossils and the origin of optimal search behavior. Proc. Natl. Acad. Sci. USA 2014, 111, 11073–11078. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.M. Why clams have the shape they have. Paleobiology 1975, 1, 48–58. [Google Scholar] [CrossRef]
- Winter, A.G.; Hosois, A.E. Identification and evaluation of the Atlantic razor clam (Ensis directus) for biologically inspired subsea burrowing systems. Integr. Comp. Biol. 2011, 51, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.G.; Hosoi, A.E.; Slocum, A.H.; Deits, R.L.H. The design and testing of Roboclam: A machine used to investigate and optimize razor clam-inspired burrowing mechanisms for engineering applications. In Proceedings of the ASME 2009 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, San Diego, CA, USA, 30 August–2 September 2009; pp. 1–6. [Google Scholar]
- Winter, A.G.; Deits, R.L.H.; Dorsch, D.S.; Hosoi, A.E.; Slocum, A.H. Teaching Roboclam to dig: The design, testing, and genetic algorithm optimization of a Biomimetic robot. In Proceedings of the International Conference on Intelligent Robots and Systems (IROS), Taipei, Taiwan, 18–22 October 2009; pp. 4231–4235. [Google Scholar]
- Koller-Hodac, A.; Germann, D.P.; Gilgen, A.; Dietrich, K.; Hadorn, M.; Schatz, W.; EggenbergerHotz, P. Actuated bivalve robot study of the burrowing locomotion in sediment. In Proceedings of the International Conference on Robots and Automation (ICRA), Anchorage, AK, USA, 3–7 May 2010; pp. 1209–1214. [Google Scholar]
- Germann, D.P.; Schatz, W.; EggenbergerHotz, P. Artificial bivalves—The biomimetics of underwater burrowing. Procedia Comp. Sci. 2011, 7, 169–172. [Google Scholar] [CrossRef]
- Winter, A.G.; Deits, R.L.H.; Dorsch, D.S.; Slocum, A.H.; Hosoi, A.E. Razor clam to RoboClam: Burrowing drag reduction mechanisms and their robotic adaptation. Bioinspir. Biomim. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raup, D.M. Geometric analysis of shell coiling: General problems. J. Paleontol. 1966, 40, 1178–1190. [Google Scholar]
- Raup, D.M. Geometric analysis of shell coiling: Coiling in ammonoids. J. Paleontol. 1967, 41, 43–65. [Google Scholar]
- Raup, D.M.; Michelson, A. Theoretical morphology of the coiled shell. Science 1965, 147, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- McGhee, G.R., Jr. Theoretical Morphology: Its Concepts and Its Applications; Columbia University Press: New York, NY, USA, 1999; p. 323. [Google Scholar]
- Trueman, E.R. Bivalve molluscs: Fluid dynamics of burrowing. Science 1966, 153, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Trueman, E.R. The burrowing activities of bivalves. Symp. Zool. Soc. Lond. 1968, 22, 167–186. [Google Scholar]
- Stanley, S.M. Relation of Shell Form to Life Habits of the Bivalvia (Mollusca); Geological Society of America: Boulder, CO, USA, 1970; p. 296. [Google Scholar]
- Trueman, E.R.; Brand, A.R.; Davis, P. Dynamics of burrowing of some common littoral bivalves. J. Exp. Biol. 1966, 44, 469–492. [Google Scholar]
- Eagar, R.M.C. Shape and function of the shell: A comparison of some living and fossil bivalve molluscs. Biol. Rev. Camb. Philos. Soc. 1978, 53, 169–210. [Google Scholar] [CrossRef]
- Lewis, J.B.; Riebel, P.N. The effect of substratum on burrowing in freshwater mussels (Unionidae). Can. J. Zool. 1984, 62, 2023–2025. [Google Scholar] [CrossRef]
- Watters, G.T. Form and function of unionoidean shell sculpture and shape Bivalvia). Am. Malacol. Bull. 1994, 11, 1–20. [Google Scholar]
- Campos, J.; Van der Veer, H.W. Autoecology of Crangon crangon (L.) with an emphasis on latitudinal trends. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 65–104. [Google Scholar] [CrossRef]
- Glude, J.B. Survival of soft-shell clams, Mya arenaria, buried at various depths. Res. Bull. Maine Dept. Sea Shore Fish. 1954, 22, 1–26. [Google Scholar]
- Morton, J.E. Locomotion. In Physiology of Mollusca, 1; Wilbur, K.M., Yonge, C.M., Eds.; Academic Press: New York, NY, USA, 1964; pp. 383–423. [Google Scholar]
- Trueman, E.R. The Dynamics of Burrowing in Ensis (Bivalvia). Proc. R. Soc. Lond. Ser. B Biol. Sci. 1967, 166, 459–476. [Google Scholar] [CrossRef]
- Shulenberger, E. Responses of Gemma gemma (Mollusca: Pelecypoda) to a catastrophic burial. Veliger 1970, 13, 163–170. [Google Scholar]
- Kranz, P.M. The anastrophic burial of bivalves and its paleoecological significance. J. Geol. 1974, 82, 237–265. [Google Scholar] [CrossRef]
- Alexander, R.R.; Stanton, R.J., Jr.; Dodd, J.R. Influence of sediment grain size on the burrowing of bivalves: Correlation with distribution and stratigraphic persistence of selected Neogene clams. Palaios 1993, 8, 289–303. [Google Scholar] [CrossRef]
- Nichols, S.J. Burrowing saves Lake Erie clams. Nature 1997, 389, 921. [Google Scholar]
- Nichols, S.J. Factors protecting unionids from zebra-mussel induced mortality at Metzger Marsh, Lake Erie, Appendix 2. In Reestablishing the Freshwater Unionid Population of Metzger Marsh, Lake Erie; Nichols, S.J., Wilcox, D., Eds.; United States Geological Survey—Great Lakes Science Center: Ann Arbor, MI, USA, 2002. Available online: http://www.epa.gov/glnpo/ecopage/wetlands/metzger/apndx2.pdf (accessed on 2 October 2017).
- Amyot, J.-P.; Downing, J.A. Seasonal variation in vertical and horizontal movement of the freshwater bivalve Elliptio complanata (Mollusca: Unionidae). Freshw. Biol. 1997, 37, 345–354. [Google Scholar] [CrossRef]
- Amyot, J.-P.; Downing, J.A. Locomotion of Elliptio complanata (Mollusca: Unionidae): A reproductive function? Freshw. Biol. 1998, 39, 351–358. [Google Scholar] [CrossRef]
- Watters, G.T.; O’Dee, S.H.; Chordas, S. Patterns of vertical migration in freshwater mussels (Bivalvia: Unionoida). J. Freshw. Ecol. 2001, 16, 541–549. [Google Scholar] [CrossRef]
- Saarinen, M.; Taskinen, J. Burrowing and crawling behaviour of three species of Unionidae in Finland. J. Moll. Stud. 2003, 69, 81–86. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Burrowing behavior of freshwater mussels in experimentally manipulated communities. J. N. Am. Benthol. Soc. 2009, 28, 93–100. [Google Scholar] [CrossRef]
- Yeager, M.M.; Cherry, D.S.; Neves, R.J. Feeding and burrowing behaviors of juvenile rainbow mussels, Villosa iris (Bivalvia: Unionidae). J. N. Am. Benthol. Soc. 1994, 13, 217–222. [Google Scholar] [CrossRef]
- Chamberlain, J.A., Jr. Two Catskill Freshwater clams, Archanodon (Devonian) and Margaritifera (Recent): What they tell us about the origin and evolution of unionoid bivalves. Northeast. Geol. Environ. Sci. 2004, 26, 5–21. [Google Scholar]
- Knoll, K.; Chamberlain, J.A., Jr.; Chamberlain, R.B. Escape burrowing of modern freshwater bivalves as a paradigm for escape capabilities of Archanodon catskillensis (Devonian), the oldest known freshwater bivalve. Geol. Soc. Am. Abstr. Progr. 2014, 46, 81. [Google Scholar]
- McMahon, R.F. Mollusca: Bivalvia. In Ecology and Classification of North American Freshwater Invertebrates; Thorp, J.H., Covich, A.P., Eds.; Academic Press: New York, NY, USA, 1991; pp. 315–399. [Google Scholar]
- Epstein, J.B.; Sevon, W.D.; Glaeser, J.D. Geology and mineral resources of the Lehighton and Palmerton Quadrangles, Carbon and Northampton Counties, Pennsylvania. In Atlas of the Geological Survey of Pennsylvania; Pennsylvania Geological Survey: Harrisburg, PA, USA, 1974; Volume 195, p. 460. [Google Scholar]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1942; p. 1116. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood, NJ, USA, 1974; p. 620. [Google Scholar]
- Tada, R.; Siever, R. Pressure solution during diagenesis. Ann. Rev. Earth Planet. Sci. 1989, 17, 89–118. [Google Scholar] [CrossRef]
- Gibson, M.A.; Broadhead, T.W. Species specific growth responses of favositid corals to soft-bottom substrates. Lethaia 1989, 22, 287–299. [Google Scholar] [CrossRef]
- Alexander, R.R. Comparative hydrodynamic stability of brachiopod shells on current-scoured arenaceous substrates. Lethaia 1984, 17, 17–23. [Google Scholar] [CrossRef]
- Posenato, R. The gen. Comelicania Frech, 1901 (Brachiopoda) from the southern Alps: Morphology and classification. Riv. Italiana Paleontol. Stratigr. 1998, 104, 43–68. [Google Scholar] [CrossRef]
- Jin, J.; Copper, P. Evolution of the Early Silurian rhynchonellide brachiopod Stegerhynchus, Anticosti Island, eastern Canada. J. Paleontol. 2004, 78, 866–883. [Google Scholar] [CrossRef]
- Meckel, T.A.; Ten Brink, U.S.; Williams, S.J. Sediment compaction rates and subsidence in deltaic plains: Numerical constraints and stratigraphic influences. Basin Res. 2007, 19, 19–31. [Google Scholar] [CrossRef]
- Whitman, R.L.; Clark, W.J. Availability of oxygen in interstitial waters of a sandy Creek. Hydrobiologia 1982, 91, 651–658. [Google Scholar] [CrossRef]
- Chambers, P.A.; Flynn, E.E.; Gibson, K. Temporal and Spatial Dynamics in Riverbed Chemistry: The Influence of Flow and Sediment Composition. Can. J. Fish. Aquat. Sci. 1992, 49, 2128–2140. [Google Scholar]
- Flynn, K.; Belopolsky Wedin, M.; Bonventre, J.A.; Dillon-White, M.; Hines, J.; Weeks, B.S.; André, C.; Schreibman, M.P.; Gagné, F. Burrowing in the Freshwater Mussel Elliptio complanata is Sexually Dimorphic and Feminized by Low Levels of Atrazine. J. Toxicol. Environ. Health Part A 2013, 76, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Hendelberg, J. The freshwater pearl mussel Margaritifera margaritifera (L.). Rept. Inst. Freshw. Res. Drottingholm 1961, 41, 149–171. [Google Scholar]
- Bauer, G. Variation in the life span and size of the freshwater Pearl Mussel. J. Anim. Ecol. 1992, 61, 425–436. [Google Scholar] [CrossRef]
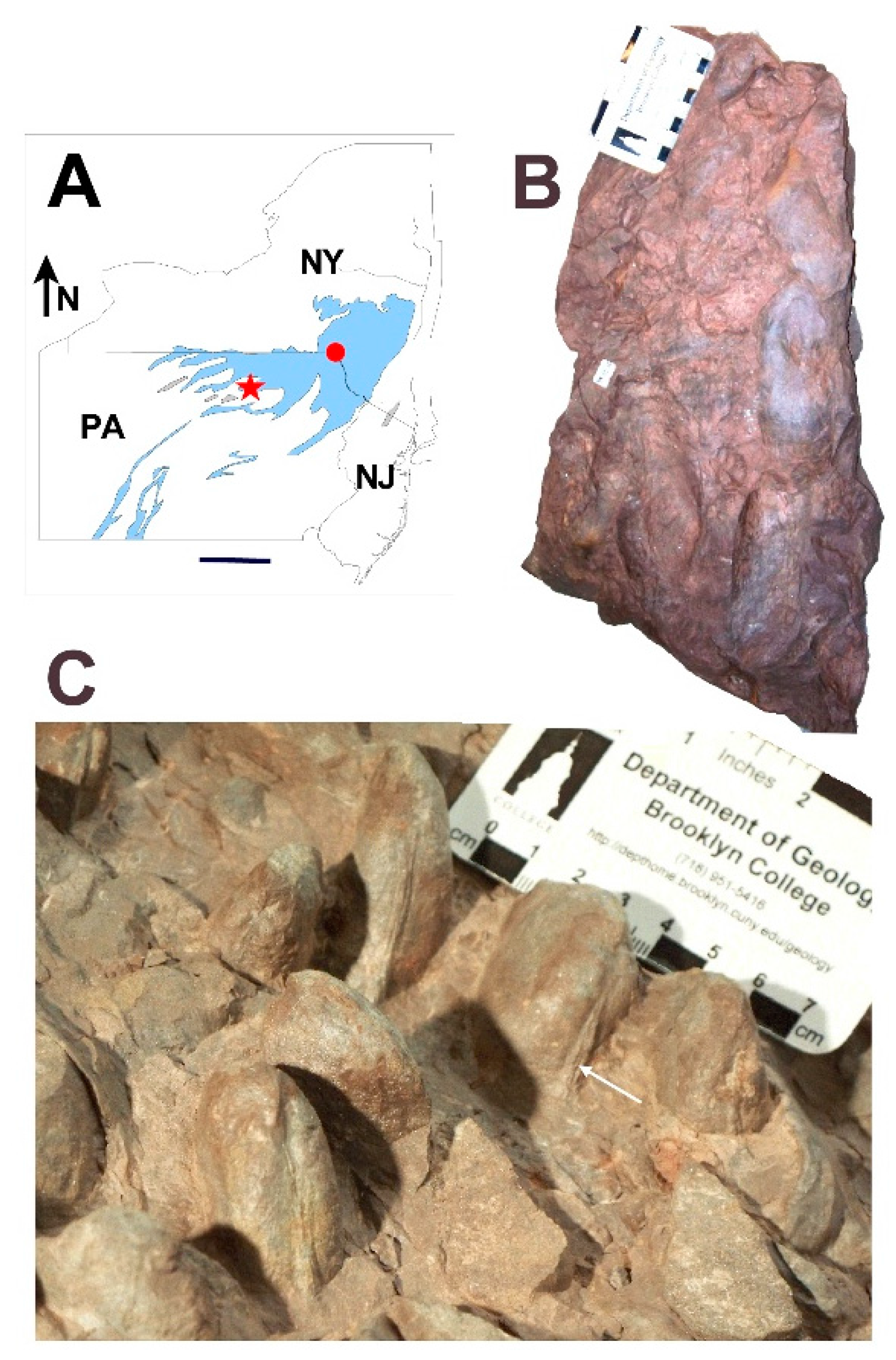
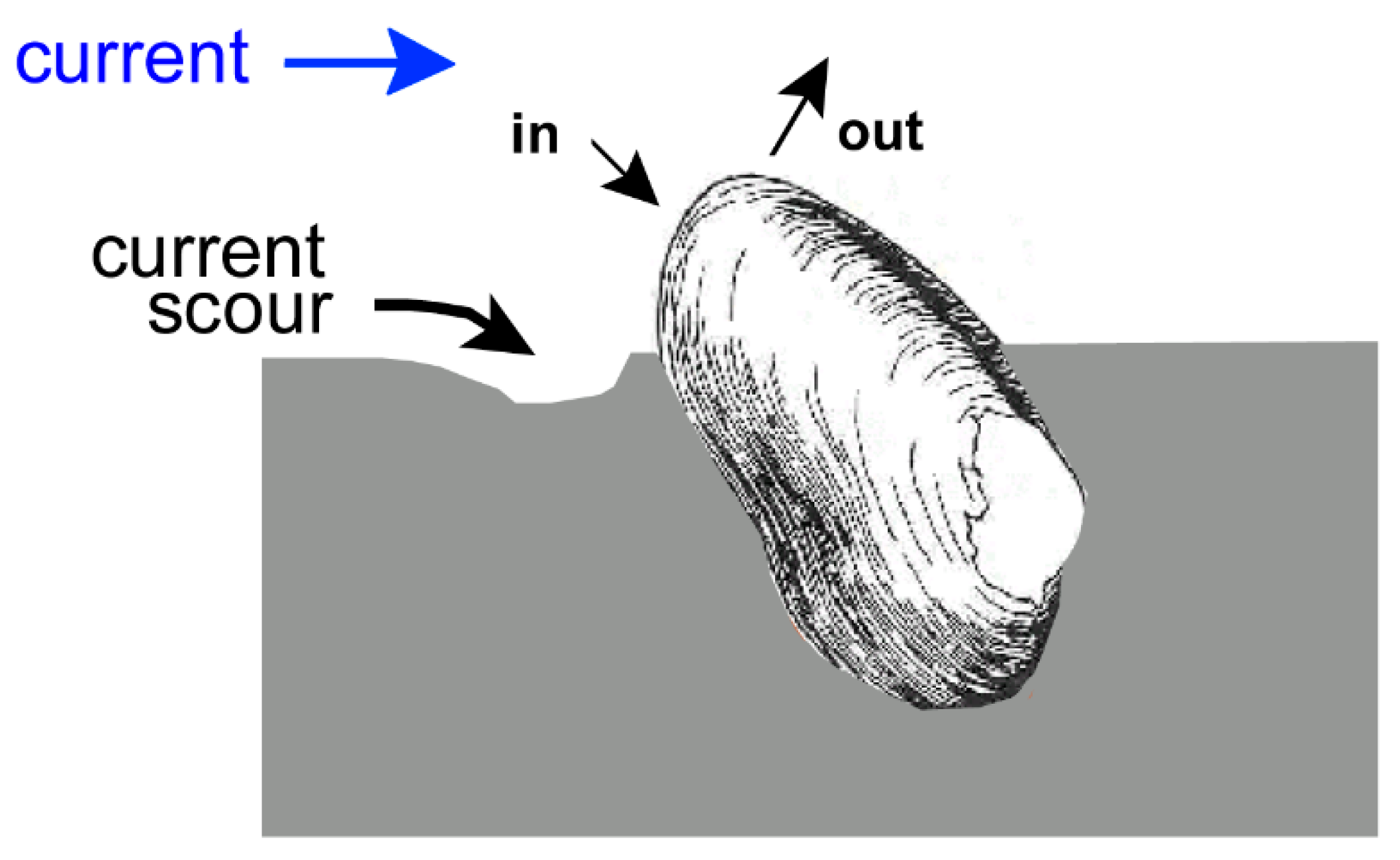
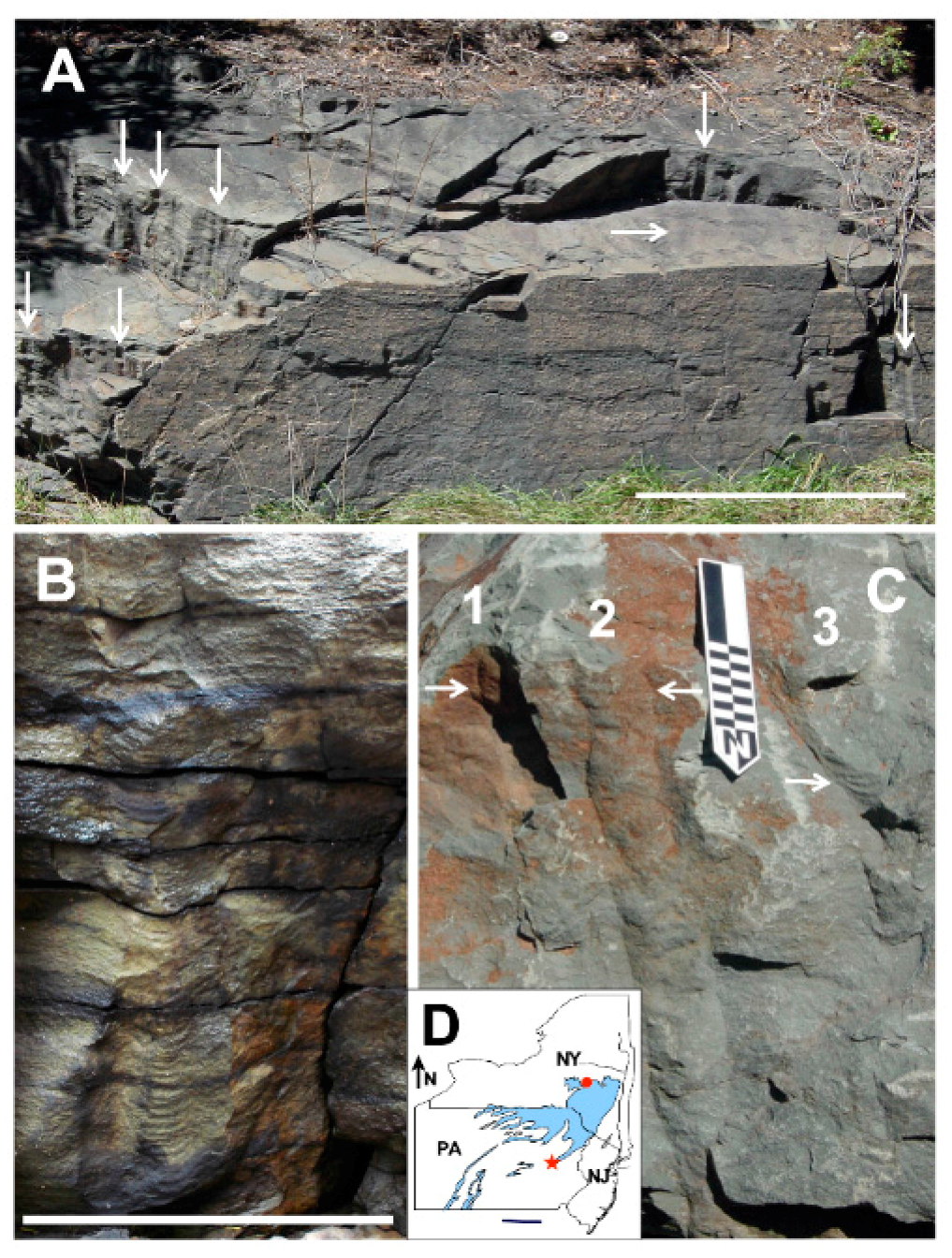
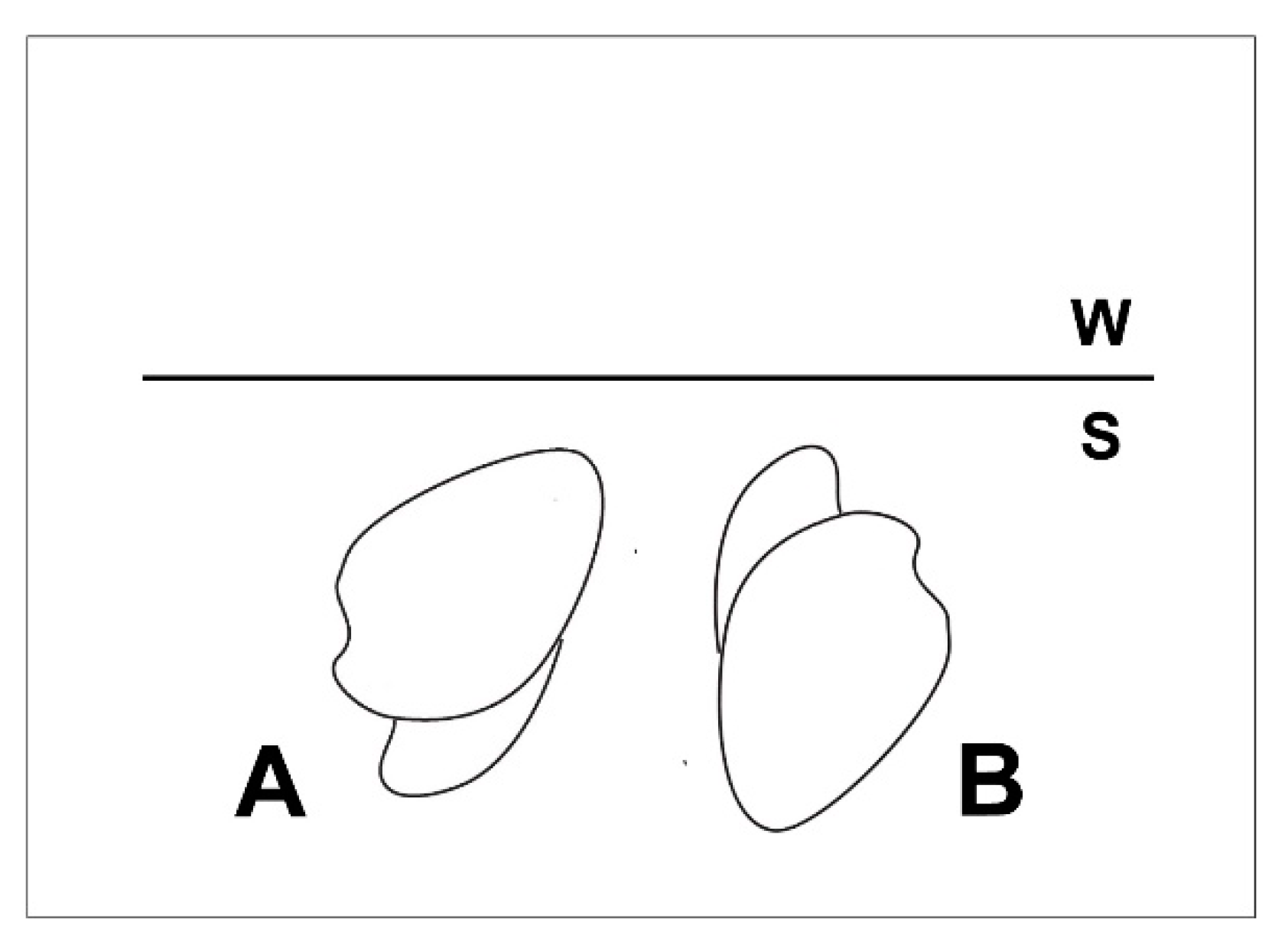

| Parameter | Character |
|---|---|
| Size | X-sectional view: max diameter = 5–10 cm; |
| Longitudinal view: max length up to ≈ 1 m | |
| Shape | X-sectional view: ovate (long axis/short axis ≈ 1.5) |
| Longitudinal view: unbranched, cylindrical tubes | |
| Orientation | Usually perpendicular to bedding, but sometimes with lower portion inclined to bedding |
| Structure | Halo: bedding planes down-turned near burrow |
| Wall: unlined; sometimes with a rim of dark mineral grains | |
| Core: concave up menisci in longitudinal view: arcuate dark lines in X-sectional view | |
| Fill | Same sediment inside burrow as outside: no fecal pellets unless present also in sediment outside burrow |
| Organization | Usually occur in clusters with long axes of burrow X-sections subparallel |
| Geologic Age | Middle Devonian to Pennsylvanian |
| Paleoenvironment | Fluviolacustrine; also tidal flat, possibly brackish early in history of the genus |
| Species | Sample Size | Size Range (cm) | Mean Elongation | Mean Obesity | Mean Muscle Scar Size | |
|---|---|---|---|---|---|---|
| Archanodon catskillensis 1 | Population 1 | 13 | 4.9–18.0 | 2.212 ± 0.2834 | ------- | -------- |
| Population 2 | 14 | ------- | ------- | 0.548 ± 0.1101 | ------- | |
| Population 3 | 1 | 11.3 | ------- | ------- | 0.015 | |
| Pyganodon cataracta | 12 | 4.9–13.4 | 1.925 ± 0.0866 | 0.675 ± 0.0629 | 0.061 ± 0.0151 | |
| Elliptio complanata | 13 | 3.5–10.6 | 2.090 ± 0.0216 | 0.547 ± 0.0697 | 0.069 ± 0.0213 | |
| Corbicula fluminea | 12 | 1.2–4.8 | 1.123 ± 0.0665 | 0.718 ± 0.0707 | 0.033 ± 0.0108 | |
| Shell Elongation | ||||
| ARC | PYG | ELL | COR | |
| ARC | 0 | + | + | + |
| PYG | + | 0 | — | + |
| ELL | + | — | 0 | + |
| COR | + | + | + | 0 |
| Shell Obesity | ||||
| ARC | PYG | ELL | COR | |
| ARC | 0 | + | — | + |
| PYG | + | 0 | + | — |
| ELL | — | + | 0 | + |
| COR | + | — | + | 0 |
| Relative Muscle Scar Size | ||||
| ARC | PYG | ELL | COR | |
| ARC | 0 | 0 | 0 | 0 |
| PYG | 0 | 0 | — | + |
| ELL | 0 | — | 0 | + |
| COR | 0 | + | + | 0 |
| Burial Depth (cm) | PYG | ELL | COR |
|---|---|---|---|
| 1 | — | — | 28, 0 |
| 2 | 1, 0 | — | 55, 0 |
| 3 | 10, 0 | 7, 0 | 35, 0 |
| 4 | 10, 0 | 7, 0 | 28, 0 |
| 5 | 14, 0 | 7, 0 | 42, 0 |
| 6 | 10, 1 | 7, 0 | 44, 0 |
| 7 | 12, 1 | 8, 0 | 37, 0 |
| 8 | 9, 2 | 8, 2 | 17, 0 |
| 9 | — | 8, 0 | 16, 0 |
| 10 | 12, 1 | 8, 0 | 17, 0 |
| Burial Depth (cm) | PYG | ELL | COR |
|---|---|---|---|
| 1 | — | — | — |
| 2 | — | — | 10, 0 |
| 3 | 4, 0 | 6, 0 | 9, 0 |
| 4 | 4, 0 | 5, 0 | 7, 0 |
| 5 | 7, 0 | 9, 0 | 8, 0 |
| 6 | 8, 0 | 10, 0 | 10, 0 |
| 7 | 5, 1 | 7, 0 | 6, 0 |
| 8 | 8, 2 | 6, 0 | 8, 0 |
| 9 | 8, 2 | 7, 0 | — |
| 10 | 8, 0 | 10, 1 | 12, 1 |
| Pyganodon Cataracta | |||||||
|---|---|---|---|---|---|---|---|
| Grain Size | Burrowing Results | Total Data Set | Shallow Burial | Deep Burial | |||
| Average L ± 1σ | T-Test Results | Average L ± 1σ | T-Test Results | Average L ± 1σ | T-Test Results | ||
| Coarse | Escape | 8.10 ± 1.878 | p < 0.002 | 8.27 ± 1.941 | p < 0.70 | 7.98 ± 1.88 | p < 0.002 |
| No Escape | 9.89 ± 1.800 | 8.85 ± 2.137 | 9.99 ± 1.69 | ||||
| Fine | Escape | 9.27 ± 1.111 | p < 0.07 | 9.49 ± 1.094 | p < 0.90 | 9.06 ± 1.122 | p < 0.10 |
| No Escape | 8.80 ± 1.118 | 9.54 ± 0.666 | 8.39 ± 1.472 | ||||
| Elliptio Complanata | |||||||
| Grain Size | Burrowing Results | Total Data Set | Shallow Burial | Deep Burial | |||
| Average L ± 1σ | T-Test Results | Average L ± 1σ | T-Test Results | Average L ±1σ | T-Test Results | ||
| Coarse | Escape | 8.46 ± 2.177 | p < 0.0005 | 8.30 ± 2.103 | p < 0.01 | 8.57 ± 1.122 | p < 0.02 |
| No Escape | 6.80 ± 0.791 | 6.36 ± 4.205 | 6.90 ± 0.986 | ||||
| Fine | Escape | 7.99 ± 1.948 | p < 0.40 | 7.15 ± 2.103 | p < 0.90 | 8.56 ± 1.122 | p < 0.20 |
| No Escape | 7.47 ± 1.497 | 7.37 ± 1.371 | 7.55 ± 1.685 | ||||
| AVERAGE ESCAPE TIME (h) ± 1σ | ||||
|---|---|---|---|---|
| Shallow Burial | Deep Burial | T-Test Results | ||
| COARSE SAND | PYG | 3.29 ± 2.5994 | 8.98 ± 9.8124 | p < 0.03 |
| ELL | 1.14 ± 1.3960 | 3.12 ± 4.8981 | p < 0.02 | |
| COR | 2.14 ± 1.7569 | 6.15 ± 3.8207 | p < 0.0001 | |
| FINE SAND | PYG | 1.67 ± 2.0391 | 7.51 ± 5.4966 | p < 0.0004 |
| ELL | 1.09 ± 0.9828 | 2.30 ± 4.2328 | p < 0.003 | |
| COR | 1.08 ± 0.9669 | 3.26 ± 2.3458 | p < 0.0001 | |
| AVERAGE ESCAPE TIME (h) ± 1σ | ||||
| Coarse Sand | Fine Sand | T-Test Results | ||
| SHALLOW BURIAL | PYG | 3.29 ± 2.5994 | 1.67 ± 2.0391 | p < 0.08 |
| ELL | 1.14 ± 1.3960 | 1.09 ± 0.9828 | p < 0.90 | |
| COR | 2.14 ± 1.7569 | 1.08 ± 0.9669 | p < 0.02 | |
| DEEP BURIAL | PYG | 8.98 ± 9.8124 | 7.51 ± 5.4966 | p < 0.60 |
| ELL | 3.12 ± 4.8981 | 2.30 ± 4.2328 | p < 0.30 | |
| COR | 6.15 ± 3.8207 | 3.26 ± 2.3458 | p < 0.003 | |
| COARSE SAND | ||||
|---|---|---|---|---|
| SHALLOW BURIAL | DEEP BURIAL | |||
| Average ET ± 1σ | T-Test Results | Average ET ± 1σ | T-Test Results | |
| PYG | 3.29 ± 2.5994 | p < 0.02 | 8.98 ± 9.8124 | p < 0.03 |
| ELL | 1.14 ± 1.3960 | 3.12 ± 4.8981 | ||
| PYG | 3.29 ± 2.5994 | p < 0.07 | 8.98 ± 9.8124 | p < 0.30 |
| COR | 2.14 ± 1.7569 | 6.15 ± 3.8207 | ||
| ELL | 1.14 ± 1.3960 | p < 0.06 | 3.12 ± 4.8981 | p < 0.007 |
| COR | 2.14 ± 1.7569 | 6.15 ± 3.8207 | ||
| FINE SAND | ||||
| SHALLOW BURIAL | DEEP BURIAL | |||
| Average ET ± 1σ | T-Test Results | Average ET ± 1σ | T-Test Results | |
| PYG | 1.67 ± 2.0391 | p < 0.3 | 7.51 ± 5.4966 | p < 0.00 |
| ELL | 1.09 ± 0.9828 | 2.30 ± 4.2328 | ||
| PYG | 1.67 ± 2.0391 | p < 0.20 | 7.51 ± 5.4966 | p < 0.005 |
| COR | 1.08 ± 0.9669 | 3.26 ± 2.3458 | ||
| ELL | 1.09 ± 0.9828 | p < 1.0 | 2.30 ± 4.2328 | p < 0.02 |
| COR | 1.08 ± 1.3960 | 3.26 ± 2.3458 | ||
| Species | Coarse Sand | Fine Sand | T-Test Results |
|---|---|---|---|
| PYG | 10.586 | 11.537 | p < 0.35 |
| ELL | 4.184 | 2.948 | p < 0.25 |
| COR | 1.359 | 0.347 | p < 0.001 |
| PYG–ELL | 10.586–4.184 | NT | p < 0.01 |
| PYG–COR | 10.586–1.359 | NT | p < 0.00001 |
| ELL–COR | 4.184–1.359 | NT | p < 0.005 |
| PYG–ELL | NT | 11.537–2.948 | p < 0.0001 |
| PYG–COR | NT | 11.537–0.347 | p < 0.0001 |
| ELL–COR | NT | 2.948–0.347 | p < 0.0001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoll, K.; Chamberlain, R.B.; Chamberlain, J.A. Escape Burrowing of Modern Freshwater Bivalves as a Paradigm for Escape Behavior in the Devonian Bivalve Archanodon catskillensis. Geosciences 2017, 7, 102. https://doi.org/10.3390/geosciences7040102
Knoll K, Chamberlain RB, Chamberlain JA. Escape Burrowing of Modern Freshwater Bivalves as a Paradigm for Escape Behavior in the Devonian Bivalve Archanodon catskillensis. Geosciences. 2017; 7(4):102. https://doi.org/10.3390/geosciences7040102
Chicago/Turabian StyleKnoll, Katja, Rebecca B. Chamberlain, and John A. Chamberlain. 2017. "Escape Burrowing of Modern Freshwater Bivalves as a Paradigm for Escape Behavior in the Devonian Bivalve Archanodon catskillensis" Geosciences 7, no. 4: 102. https://doi.org/10.3390/geosciences7040102




