Environment and Human Health: The Challenge of Uncertainty in Risk Assessment
Abstract
:1. Introduction
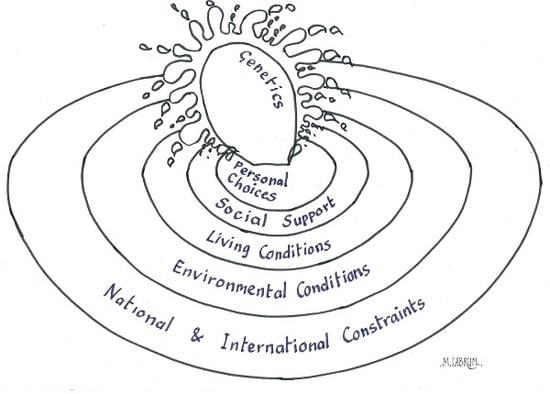
2. Causes of Ill Health
3. The Impact of Lead Poisoning
“Water-supply by earthenware pipes has these advantages. First, if any fault occurs in the work, anybody can repair it. Again, water is much more wholesome from earthenware pipes than from lead pipes. For it seems to be made injurious by lead, because white lead is produced by it; and this is said to be harmful to the human body. Thus, if what is produced by anything is injurious, it is not doubtful but that the thing is not wholesome in itself. We can take example by the workers in lead who have complexions affected by pallor. For, when, in casting, the lead receives the current of air, the fumes from it occupy the members of the body and burning them thereupon, rob the limbs of the virtues of the blood. Therefore, it seems that water should not be brought in lead pipes if we desire to have it wholesome. Our daily table may show that the flavour from earthenware pipes is better, because everybody, even when they pile up their tables with silver vessels for all that, uses earthenware to preserve the flavour of water”.[60]
4. Receptors: Measurement of Disease and Risk
5. Pathways: Exposure, Dose and Response
6. Sources
7. Putting It All Together: Risk Assessment
- Hazard identification—recognition and characterisation of the toxin(s) present
- Exposure assessment—measurement or estimation of the intensity, frequency and duration of human exposure to the agent
- Dose-response assessment—characterisation of the relationships between varying doses and adverse effects in exposed populations
- Risk characterisation—estimation of the incidence of health effects under the various actual conditions of human exposure
- Risk communication—informing those affected or responding to the issue of the size of the risk and appropriate responses
8. Putting It All Together: Preventing Adverse Health Outcomes
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghebrehewet, S.; Stewart, A.G.; Baxter, D.; Shears, P.; Conrad, D.; Kliner, M. (Eds.) Health Protection: Principles and Practice; OUP: Oxford, UK, 2016; ISBN 9780198745471. [Google Scholar]
- Hursthouse, A.; Kowalczyk, G. Transport and dynamics of toxic pollutants in the natural environment and their effect on human health: Research gaps and challenge. Environ. Geochem. Health 2009, 31, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.H.; Argyraki, A. Estimation of measurement uncertainty from field sampling: Implications for the classification of contaminated land. Sci. Total Environ. 1997, 198, 243–257. [Google Scholar] [CrossRef]
- De Zorzi, P.; Barbizzi, S.; Belli, M.; Barbina, M.; Fajgelj, A.; Jacimovic, R.; Jeran, Z.; Menegon, S.; Pati, A.; Petruzzelli, G.; et al. Estimation of uncertainty arising from different soil sampling devices: The use of variogram parameters. Chemosphere 2008, 70, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM). Environmental Decisions in the Face of Uncertainty; National Academies Press: Washington, DC, USA, 2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK200848/ (accessed on 20 September 2017).
- US EPA. Uncertainty and Variability. Collections and Lists. United States Environment Protection Agency. 14 May 2015. Available online: https://www.epa.gov/expobox/uncertainty-and-variability (accessed on 8 January 2018).
- Wells, J.R.; Schoemaecker, C.; Carslaw, N.; Waring, M.S.; Ham, J.E.; Nelissen, I.; Wolkoff, P. Reactive Indoor Air Chemistry and Health—A Workshop Summary. Int. J. Hyg. Environ. Health 2017, 220, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Han, P.K.J.; Klein, W.M.P.; Arora, N.J. Varieties of uncertainty in health care: A conceptual taxonomy. Med. Decis. Mak. 2011, 31, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Ascough, J.C., II; Maier, H.R.; Ravalico, J.K.; Strudley, M.W. Future research challenges for incorporation of uncertainty in environmental and ecological decision-making. Ecol. Modell. 2008, 219, 383–399. [Google Scholar] [CrossRef]
- Dellarco, M.; Zaleski, R.; Gaborek, B.J.; Qian, H.; Bellin, C.A.; Egeghy, P.; Heard, N.; Jolliet, O.; Lander, D.R.; Sunger, N.; et al. Using exposure bands for rapid decision making in the RISK21 tiered exposure assessment. Crit. Rev. Toxicol. 2017, 47, 317–341. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rapid Risk Assessment of Acute Public Health Events; WHO/HSE/GAR/ARO/2012.1; World Health Organization: Geneva, Switzerland, 2012; Available online: http://apps.who.int/iris/bitstream/10665/70810/1/WHO_HSE_GAR_ARO_2012.1_eng.pdf (accessed on 20 September 2017).
- Mahoney, G.; Stewart, A.G.; Kennedy, N.; Whitely, B.; Turner, L.; Wilkinson, E. Achieving attainable outcomes from good science in an untidy world: Case studies in land and air pollution. Environ. Geochem. Health 2015, 37, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Karalliede, L.; Murray, V.; Maynard, R.; Parkinson, N. (Eds.) Essentials of Toxicology for Health Protection: A Handbook for Field Professionals, 2nd ed.; OUP: Oxford, UK, 2012; ISBN 9780199652549. [Google Scholar]
- Connolly, M.A.; Heymann, D.A. Deadly comrades: War and infectious diseases. Lancet 2002, 360, s23–s24. [Google Scholar] [CrossRef]
- Onalaja, A.O.; Claudio, L. Genetic susceptibility to lead poisoning. Environ. Health Perspect. 2000, 108 (Suppl. 1), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Talsania, K.; Mettil, W.; Anderson, D.W. Genetic diversity influences the response of the brain to developmental lead exposure. Toxicol. Sci. 2014, 141, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Claus Henn, B.; Wang, C.; Wei, Y.; Su, L.; Sun, R.; Chen, H.; Wagner, P.J.; Lu, Q.; Lin, X.; et al. Genome-wide gene by lead exposure interaction analysis identifies UNC5D as a candidate gene for neurodevelopment. Environ. Health 2017, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B.; Dy, V.; McQuilty, R.; Zhu, G.; Montgomery, G.; Ferreira, M.; Duffy, D.; Neale, M.; Heijmans, B.; Heath, A.; et al. Evidence of genetic effects on blood lead concentration. Environ. Health Perspect. 2007, 115, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Heredia, N.; Senut, M.C.; Hess, M.; Land, S.; Qu, W.; Hollacher, K.; Dereski, M.O.; Ruden, D.M. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics 2015, 7, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, R.V. Leaded eye cosmetics: A cultural cause of elevated lead levels in children. J. Fam. Pract. 1995, 40, 358–363. [Google Scholar] [PubMed]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Gouitaa, H.; Bellaouchou, A.; Fekhaoui, M.; El Abidi, A.; Mahnine, N.; Ben Aakame, R. Assessment of lead levels in traditional eye cosmetic “kohl” frequently used in Morocco and health hazard. J. Mater. Environ. Sci. 2016, 7, 631–637. [Google Scholar]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed]
- Geltman, P.L.; Brown, M.J.; Cochran, J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics 2001, 108, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashban, R.M.; Aslam, M.; Shah, A.H. Kohl (surma): A toxic traditional eye cosmetic study in Saudi Arabia. Public Health 2004, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Catherin Cartwright-Jones. Kohl as Traditional Women’s Adornment in North Africa and the Middle East; TapDancing Lizard Publications: Stow, OH, USA, 2005; pp. 1–9. Available online: http://www.hennapage.com/harquuspdfs/kohlintro.pdf (accessed on 20 September 2017).
- Oberle, M.W. Lead poisoning: A preventable childhood disease of the slums. Science 1969, 165, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Bodeau-Livinec, F.; Glorennec, P.; Cot, M.; Dumas, P.; Durand, S.; Massougbodji, A.; Ayotte, P.; Le Bot, B. Elevated blood lead levels in infants and mothers in Benin and potential sources of exposure. Int. J. Environ. Res. Public Health 2016, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Jacobziner, H. Lead poisoning in childhood: Epidemiology, manifestations, and prevention. Clin. Pediatr. (Phila.) 1966, 5, 277–286. [Google Scholar] [CrossRef]
- Bellinger, D.; Leviton, A.; Waternaux, C.; Needleman, H.; Rabinowitz, M. Low-level lead exposure, social class, and infant development. Neurotoxicol. Teratol. 1988, 10, 497–503. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Sharaby, C.M.; El-Kholy, N.G.; Elsherif, I.Y.; El-Wakeel, S.T. Factors influencing lead and iron release from some Egyptian drinking water pipes. J. Hazard. Mater. 2008, 160, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Troesken, W. Lead water pipes and infant mortality at the turn of the Twentieth Century. J. Hum. Resour. 2008, 43, 553–575. [Google Scholar] [CrossRef]
- Hanna-Attisha, M.; LaChance, J.; Sadler, R.C.; Champney Schnepp, A. Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2015, 106, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Walraven, N.; Bakker, M.; van Os, B.; Klaver, G.; Middelburg, J.J.; Davies, G. Pollution and oral bioaccessibility of Pb in soils of villages and cities with a long habitation history. Int. J. Environ. Res. Public Health 2016, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Bradham, K.D.; Nelson, C.M.; Kelly, J.; Pomales, A.; Scruton, K.; Dignam, T.; Misenheimer, J.C.; Li, K.; Obenour, D.R.; Thomas, D.J. Relationship between total and bioaccessible lead on children’s blood lead levels in urban residential Philadelphia. Soils Environ. Sci. Technol. 2017, 51, 10005–10011. [Google Scholar] [CrossRef] [PubMed]
- Schoolman, E.D.; Ma, C. Migration, class and environmental inequality: Exposure to pollution in China’s Jiangsu Province. Ecol. Econ. 2012, 75, 140–151. [Google Scholar] [CrossRef]
- Taylor, J.; Lovell, S. Urban home gardens in the Global North: A mixed methods study of ethnic and migrant home gardens in Chicago, IL. Renew. Agric. Food Syst. 2015, 30, 22–32. [Google Scholar] [CrossRef]
- Ahmed, K.; Ayana, G.; Engidawork, E. Lead exposure study among workers in lead acid battery repair units of transport service enterprises, Addis Ababa, Ethiopia: A cross-sectional study. J. Occup. Med. Toxicol. 2008, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Lead exposures from car batteries—A global problem. Environ. Health Perspect. 2009, 117, A535. [Google Scholar] [CrossRef] [PubMed]
- WHO. Childhood Lead Poisoning; World Health Organization: Geneva, Switzerland, 2010; Available online: http://www.who.int/ceh/publications/leadguidance.pdf (accessed on 20 September 2017).
- Shah, F.; Kazi, T.G.; Afridi, H.I.; Naeemullah; Arain, S.S. Exposures of lead to adolescent workers in battery recycling workshops and surrounding communities. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Were, F.H.; Kamau, G.N.; Shiundu, P.M.; Wafula, G.A.; Moturi, C.M. Air and blood lead levels in lead acid battery recycling and manufacturing plants in Kenya. J. Occup. Environ. Hyg. 2012, 9, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Karim, N.; Munshi, A.B. Occupational lead toxicity in battery workers. Pak. J. Med. Sci. 2015, 31, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Piacitelli, G.M.; Whelan, E.A.; Sieber, W.K.; Gerwel, B. Elevated lead contamination in homes of construction workers. Am. Ind. Hyg. Assoc. J. 1997, 58, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Van der Kuijp, T.J.; Huang, L.; Cherry, C.R. Health hazards of China’s lead-acid battery industry: A review of its market drivers, production processes, and health impacts. Environ. Health 2013, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, J.-M.; Huang, Y.; Duan, Y.-Y.; Huang, R.-X.; Hu, J.-A. Dose-response relationship between cumulative occupational lead exposure and the associated health damages: A 20-year cohort study of a smelter in China. Int. J. Environ. Res. Public Health 2016, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; McWeeney, G.; Kim, R.; Tahirukaj, A.; Bulat, P.; Syla, S.; Savic, Z.; Amitai, Y.; Dignam, T.; Kaluski, D.N. Lead poisoning among internally displaced Roma, Ashkali and Egyptian children in the United Nations-Administered Province of Kosovo. Eur. J. Public Health 2010, 20, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, A.; Li, X.; Ramsey, M.H.; Thornton, I. Chemical speciation and bioaccessibility of lead in surface soil and house dust, Lavrion urban area, Attiki, Hellas. Environ. Geochem. Health 2010, 32, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, R.J.; Gittleman, J.L.; Deddens, J.A.; Petersen, M.R.; Halperin, W.E. Blood lead levels among children of lead-exposed workers: A meta-analysis. Am. J. Ind. Med. 1999, 36, 475–481. [Google Scholar] [CrossRef]
- Hursthouse, A.S.; Leitão, T.E. Environmental pressures on and the status of urban allotments. In Urban Allotment Gardens in Europe; Bell, S., Fox-Kämper, R., Keshavarz, N., Benson, M., Caputo, S., Noori, S., Voigt, A., Eds.; Routledge: Abingdon, UK, 2016; Chapter 6; pp. 142–164. ISBN 9781138921092. [Google Scholar]
- Riva, M.A.; Lafranconi, A.; D’Orso, M.I.; Cesana, G. Lead poisoning: Historical aspects of a paradigmatic “Occupational and Environmental Disease”. Saf. Health Work 2012, 3, 11–16. [Google Scholar] [CrossRef] [PubMed]
- World Atlas. Countries That Still Use Leaded Gasoline. 2017. Available online: http://www.worldatlas.com/articles/countries-that-still-use-leaded-gasoline.html (accessed on 20 September 2017).
- Basel Convention. Controlling Transboundary Movements of Hazardous Waste and Their Disposal. 2011. Available online: http://www.basel.int/ (accessed on 20 September 2017).
- Asante-Duah, D.K.; Nagy, I.V. International Trade in Hazardous Wastes; Routledge: Abingdon, UK, 2002; ISBN 9780419218906 1998-03-12. [Google Scholar]
- US Geological Survey. Mineral Commodity Summaries 2017; US Geological Survey: Reston, VA, USA, 2017. Available online: https://minerals.usgs.gov/minerals/pubs/mcs/2017/mcs2017.pdf (accessed on 20 September 2017).
- Massadeh, A.M.; El-khateeb, M.Y.; Ibrahim, S.M. Evaluation of Cd, Cr, Cu, Ni, and Pb in selected cosmetic products from Jordanian, Sudanese, and Syrian markets. Public Health 2017, 149, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Retief, F.P.; Cilliers, L. Lead poisoning in ancient Rome. Acta Theol. 2006, 26, 147–164. [Google Scholar] [CrossRef]
- Boni, M.; Maio, G.D.; Frei, R.; Villa, I.M. Lead isotopic evidence for a mixed provenance for Roman water pipes from Pompeii. Archaeometry 2000, 42, 201–208. [Google Scholar] [CrossRef]
- Hodge, A.T. Vitruvius, lead pipes and lead poisoning. Am. J. Archaeol. 1981, 85, 486–491. [Google Scholar] [CrossRef]
- Vitruvius. On Architecture (15AD); Granger, F., Translator; Harvard University Press: Cambridge, MA, USA, 1943. [Google Scholar]
- Bellinger, D.C.; Bellinger, A.M. Childhood lead poisoning: The torturous path from science to policy. J. Clin. Investig. 2006, 116, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Settle, D.M.; Patterson, C.C. Lead in albacore: Guide to lead pollution in Americans. Science 1980, 207, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Renberg, I.; Bigler, C.; Bindler, R.; Norberg, M.; Rydberg, J.; Segerström, U. Environmental history: A piece in the puzzle for establishing plans for environmental management. J. Environ. Manag. 2009, 90, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Benedictow, O.J. The Black Death, 1346-1353: The Complete History; Boydell & Brewer: Woodbridge, UK, 2004; p. 454. ISBN 9780851159430. [Google Scholar]
- More, A.F.; Spaulding, N.E.; Bohleber, P.; Handley, M.; Hoffmann, H.; Korotkikh, E.; Kurbatov, A.; Loveluck, C.; Sneed, S.; McCormick, M.; et al. Next-generation ice core technology reveals true minimum natural levels of lead (Pb) in the atmosphere: Insights from the Black Death. GeoHealth 2017, 1, 211–219. [Google Scholar] [CrossRef]
- Koller, K.; Brown, T.; Spurgeon, A.; Levy, L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ. Health Perspect. 2004, 112, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, L.; Kaufmann, R.; Prüss-Ustün, A. Lead: Assessing the Environmental Burden of Disease at National and Local Levels; WHO Environmental Burden of Disease Series, No. 2; World Health Organization: Geneva, Switzerland, 2003; Available online: http://www.who.int/quantifying_ehimpacts/publications/en/leadebd2.pdf (accessed on 20 September 2017).
- Pure Earth; Green Cross Switzerland. 2016 World’s Worst Pollution Problem; The Toxics Beneath Our Feet; Pure Earth: New York, NY, USA, 2016; Available online: http://www.worstpolluted.org/2016-report.html (accessed on 20 September 2017).
- WHO. Lead Poisoning and Health; World Health Organization: Geneva, Switzerland, 2017; Available online: http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed on 20 September 2017).
- Nevin, R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ. Res. 2000, 83, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.N.; Chen, A.; Succop, P.R.P.; Wright, J.; Dietrich, K.N. Exposure to airborne metals and particulate matter and risk for youth adjudicated for criminal activity. Environ. Res. 2011, 111, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Boutwell, B.B.; Nelson, E.J.; Emo, B.; Vaughn, M.G.; Schootman, M.; Rosenfeld, R.; Lewis, R. The intersection of aggregate-level lead exposure and crime. Environ. Res. 2016, 148, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-González, M.; Osorio-Yáñez, C.; Gaspar-Ramírez, O.; Pavković, M.; Ochoa-Martínez, A.; López-Ventura, D.; Medeiros, M.; Barbier, O.C.; Pérez-Maldonado, I.N.; Sabbisetti, V.S.; et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ. Res. 2016, 150, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A.; Jelonek-Waliszewska, A. As and Pb and Their Potential Source in the Hair of Residents of Cracow. J. Elementol. 2017, 22, 517–528. [Google Scholar] [CrossRef]
- Tam, C.C.; Rodrigues, L.C.; Viviani, L.; Dodds, J.P.; Evans, M.R.; Hunter, P.R.; Gray, J.J.; Letley, L.H.; Rait, G.; Tompkins, D.S.; et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut 2012, 61, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). Cardiovascular disease. In How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53012/ (accessed on 20 September 2017).
- Schane, R.E.; Ling, P.M.; Glantz, S.A. Health effects of light and intermittent smoking: A review. Circulation 2010, 121, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. The Global Burden of Disease. A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 Projected to 2020; Summary Report; Harvard School of Public Health: Boston, MA, USA, 1996; Available online: http://apps.who.int/iris/bitstream/10665/41864/1/0965546608_eng.pdf (accessed on 20 September 2017).
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, L.J.; Prüss-Ustün, A.; Landrigan, P.; Ayuso-Mateos, J.L. Estimating the global burden of disease of mild mental retardation and cardiovascular diseases from environmental lead exposure. Environ. Res. 2004, 94, 120–133. [Google Scholar] [CrossRef]
- Cleary, P.; Ghebrehewet, S.; Shears, P. Essential statistics and epidemiology. In Health Protection: Principles and Practice; Ghebrehewet, S., Stewart, A.G., Baxter, D., Shears, P., Conrad, D., Kliner, M., Eds.; OUP: Oxford, UK, 2016; pp. 228–239. ISBN 9780198745471. [Google Scholar]
- Stewart, A.G.; Luria, P.; Reid, J.; Lyons, M.; Jarvis, R. Real or Illusory? Case studies on the public perception of environmental health risks in the North West of England. Int. J. Environ. Res. Public Health 2010, 7, 1153–1173. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A. Health risk assessment related to an effect of sample size fractions: Methodological remarks. Stoch. Environ. Res. Risk Assess. 2017, 1–21. [Google Scholar] [CrossRef]
- Public Health England. Lead: Health Effects, Incident Management and Toxicology. 2017. Available online: https://www.gov.uk/government/publications/lead-properties-incident-management-and-toxicology (accessed on 20 September 2017).
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med. Sci. Monit. 2005, 11, RA329–RA336. [Google Scholar] [PubMed]
- Swartjes, F.A. Dealing with Contaminated Sites: From Theory towards Practical Application; Springer Science & Business Media: Dordrecht, The Netherlands, 2011; ISBN 978-94-017-7811-4. [Google Scholar]
- BARGE. The BARGE Unified Bioaccessibility Method. 2016. Available online: https://www.bgs.ac.uk/barge/ubm.html (accessed on 20 September 2017).
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Chemical Hazards Compendium. 2017. Available online: https://www.gov.uk/government/collections/chemical-hazards-compendium (accessed on 20 September 2017).
- ATSDR. Toxic Substances Portal. 2017. Available online: https://www.atsdr.cdc.gov/toxprofiles/index.asp (accessed on 20 September 2017).
- Mineralogy Database. Mineral Species Containing Lead. 1997–2014. Available online: http://webmineral.com/chem/Chem-Pb.shtml#.WeXGZXRJlhE (accessed on 20 September 2017).
- Cave, M.R.; Wragg, J.; Denys, S.; Jondreville, C.; Feidt, C. Oral bioavailability. In Dealing with Contaminated Sites: From Theory towards Practical Application; Swartjes, F.A., Ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2011; pp. 287–324. ISBN 978-94-017-7811-4. [Google Scholar]
- Li, J.; Li, K.; Cave, M.; Li, H.-B.; Ma, L.Q. Lead bioaccessibility in 12 contaminated soils from China: Correlation to lead relative bioavailability and lead in different fractions. J. Hazard. Mater. 2015, 295 (Suppl. C), 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, X.; Chen, X. Happiness in the air: How does a dirty sky affect mental health and subjective well-being? J. Environ. Econ. Manag. 2017, 85 (Suppl. C), 81–94. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.; Baxter, J.; Litva, A.; Staples, B. The social and psychological impact of the chemical contamination incident in Weston Village, UK: A qualitative analysis. Soc. Sci. Med. 2002, 55, 2227–2241. [Google Scholar] [CrossRef]
- Barnes, G.J.; Litva, A.; Tuson, S. The social impact of land contamination: Reflections on the development of a community advocacy and counselling service following the Weston village incident. J. Public Health (Oxf.) 2005, 27, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C. A personal therapeutic journey. BMJ 1996, 313, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Boase, N; White, M.; Gaze, W.; Redshaw, C. Evaluating the mental models approach to developing a risk communication: A scoping review of the evidence. Risk Anal. 2017, 37, 2132–2149. [Google Scholar] [CrossRef] [PubMed]
- Rose, G. Strategy of prevention: Lessons from cardiovascular disease. BMJ 1981, 282, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 2001, 30, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T.R. A framework for public health action: The health impact pyramid. Am. J. Public Health 2010, 100, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Chmiel, J.F.; Ferkol, T. The impact of the Clean Air Act. J. Pediatr. 2012, 161, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Schimmel, H.; Higgins, I.T.T. Relation of daily mortality to air pollution: An analysis of 14 London winters, 1958/59–1971/72. Arch. Environ. Health 1982, 37, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gowers, A.M.; Miller, B.G.; Stedman, J.R. Estimating Local Mortality Burdens Associated with Particulate Air Pollution; Public Health England: London, UK, 2014; ISBN 978-0-85951-753-9. [Google Scholar]
- Scutchfield, F.D.; Hall, L.; Ireson, C.L. The public and public health organizations: Issues for community engagement in public health. Health Policy 2006, 77, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Faculty of Public Health. Learning Outcomes Framework. Faculty of Public Health. 2010. Available online: http://www.fph.org.uk/learning_outcomes_framework (accessed on 20 September 2017).
- Aderibigbe, A.D.; Stewart, A.G.; Hursthouse, A.S. Seeking evidence of multi-disciplinarity in environmental geochemistry and health: An analysis of arsenic in drinking water research. Environ. Geochem. Health 2017. [Google Scholar] [CrossRef] [PubMed]






| Rank | Industry | DALYs (Number) | DALYs (%) |
|---|---|---|---|
| 1 | Used Lead-Acid Battery Recycling | 2,000,000–4,800,000 | 27–29 |
| 2 | Mining and Ore Processing | 450,000–2,600,000 | 7–15 |
| 3 | Lead Smelting | 1,000,000–2,500,000 | 14 |
| 4 | Tanneries | 1,200,000–2,000,000 | 11–17 |
| 5 | Artisanal Small-Scale Gold Mining | 600,000–1,600,000 | 9 |
| 6 | Industrial Dumpsites | 370,000–1,200,000 | 5–7 |
| 7 | Industrial Estates | 370,000–1,200,000 | 5–7 |
| 8 | Chemical Manufacturing | 300,000–750,000 | 4 |
| 9 | Product Manufacturing | 400,000–700,000 | 4–6 |
| 10 | Dye Industry | 220,000–430,000 | 2–3 |
| Disease | Exposure | Outcome |
|---|---|---|
| Respiratory | Occupational chemicals; traffic exhausts | Chronic obstructive airways disease, asthma, silicosis |
| Peri-natal | Maternal (pesticides et al.) | Low birth weight |
| Congenital abnormalities | Maternal (pesticides, PCBs, PCDFs, Pb, Hg, endocrines) | Various birth defects |
| Cancers | Aflatoxins, smoking, PAH, As, asbestos, benzene, pesticides, dioxins | Many sites e.g., lung, skin, liver, brain, kidney, prostate, bladder, etc. |
| Neuro-psychiatric | Pb, methyl-Hg, PCBs, As, toluene, etc. | Cognitive delay, Parkinsonism, Minimata disease |
| Cardiovascular | PM2.5, Pb, As, Cd, Hg, solvents, pesticides, smoking | Ischaemic heart & cerebrovascular disease |
| Diabetes mellitus | As, N-3-pyridylmethyl-N′-p-nitrophenyl urea (rodenticide), 2,3,7,8-Tetrachlorodibenzo-p-dioxin | Type II diabetes |
| Medicine |
| Environmental geochemistry |
| Geography |
| Toxicology |
| Genomics |
| Politics |
| Social Science |
| Epidemiology |
| Management |
| Statistics |
| Behavioural science |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, A.G.; Hursthouse, A.S. Environment and Human Health: The Challenge of Uncertainty in Risk Assessment. Geosciences 2018, 8, 24. https://doi.org/10.3390/geosciences8010024
Stewart AG, Hursthouse AS. Environment and Human Health: The Challenge of Uncertainty in Risk Assessment. Geosciences. 2018; 8(1):24. https://doi.org/10.3390/geosciences8010024
Chicago/Turabian StyleStewart, Alex G., and Andrew S. Hursthouse. 2018. "Environment and Human Health: The Challenge of Uncertainty in Risk Assessment" Geosciences 8, no. 1: 24. https://doi.org/10.3390/geosciences8010024
APA StyleStewart, A. G., & Hursthouse, A. S. (2018). Environment and Human Health: The Challenge of Uncertainty in Risk Assessment. Geosciences, 8(1), 24. https://doi.org/10.3390/geosciences8010024