Holocene Soil Evolution in South Siberia Based on Phytolith Records and Genetic Soil Analysis (Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.1.1. Hillltop and Midslope Catena Sites
2.1.2. Toeslope Catena Site
2.2. Methods
2.2.1. Morphologic and Genetic Analyses
2.2.2. Physical and Chemical Analyses
2.2.3. Accelerator Mass Spectrometry 14C Dating of Humic Acids (II) and Phytolith-Occluded Carbon
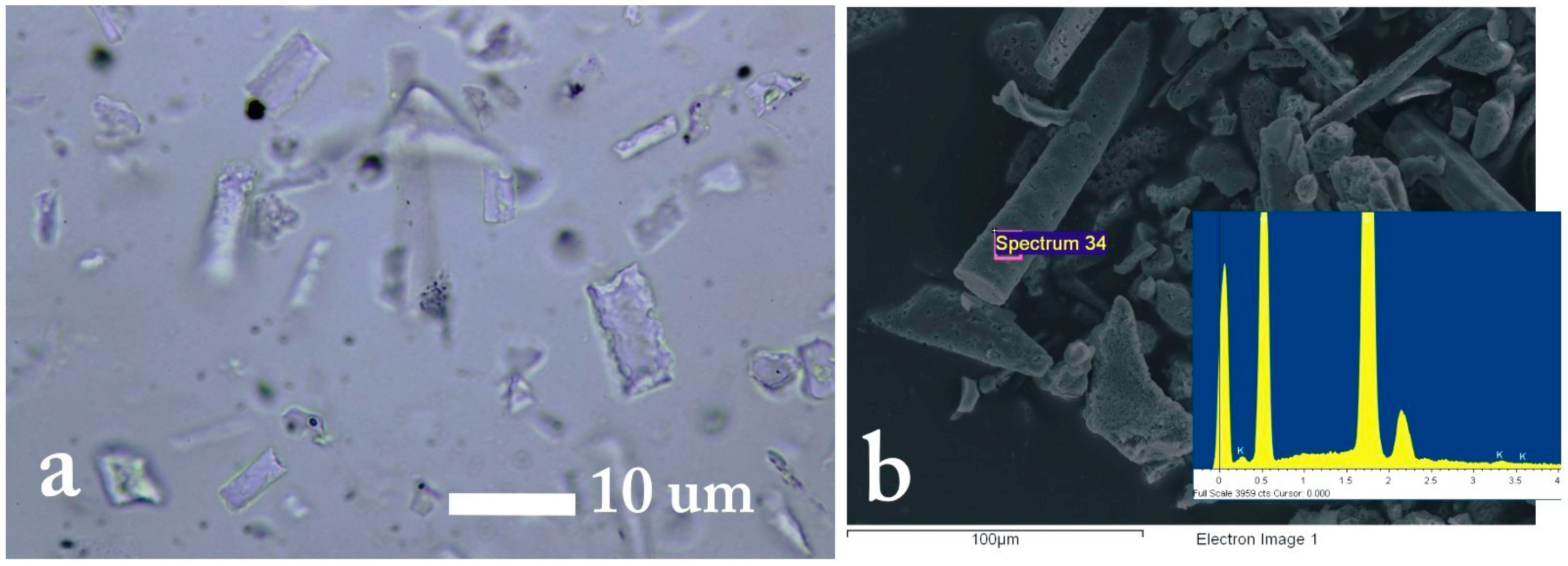
2.2.4. Phytolith Analysis: Procedure and Interpretation
2.2.5. Preservation of Some Features of the Dark-Humus Stage of Soil Formation
3. Results and Discussion
3.1. Soil Morphology and Genesis
3.2. Soil Physical and Chemical Properties
Physical and Chemical Properties Preserved from the Dark-Humus Stage of Soil Formation
3.3. Phytolith Analysis
3.3.1. Endocalcic Stagnic Albic Luvisol (Siltic, Abruptic, Cutanic, Epiloamic) (Eluvial Position)
3.3.2. Endocalcic Stagnic Albic Luvisols (Siltic, Abruptic, Cutanic, Epiloamic) (Transit Position)
3.3.3. Calcic Stagnic Greyzemic Epidystric Umbrisols (Siltic) (Toeslope Site)
3.4. AMS 14C Dating of the Eluvial and Humus Horizons
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dukarev, A.G.; Pologova, N.N. Soils with complex organic profiles on the Vasyugan plain. Eurasian Soil Sci. 2011, 44, 480–492. [Google Scholar] [CrossRef]
- Lashchinsky, N.N.; Korolyuk, A.Y. Syntaxonomy of zonal dark-coniferous forests of southern taiga of the West Siberian plain and of humid low-mountains of the Altai-Sayan mounain region. Veg. Russia 2015, 26, 85–107, (In Russian, Summary in English). [Google Scholar]
- Gadzhiev, I.M. Evolution of Taiga Soils in Western Siberia; Nauka: Novosibirsk, Russia, 1982; pp. 1–455. (In Russian) [Google Scholar]
- Dyukarev, A.G. Landscape and Dynamic Aspects of Taiga Soil Formation in Western Siberia; NTL Publishing: Tomsk, Russia, 2005; pp. 1–284. (In Russian) [Google Scholar]
- Ponomareva, V.V.; Tochelnikov, Y.S. Some data on the composition and properties of humus and the genesis of soil with the second humus horizon of the southern taiga of Western Siberia. Proc. Inst. Geogr. Sib. Far East 1968, 20, 23–32. (In Russian) [Google Scholar]
- Nechaeva, E.G.; Laivinish, M.I. Spatial distribution patterns of podzolic soils with the second humus horizon. Proc. Inst. Geogr. Sib. Far East 1970, 25, 38–48. (In Russian) [Google Scholar]
- Budina, L.P.; Erokhina, A.A. Genetic features of sod-podzolic gleysolic cold soils with second humus horizon of Krasnoyarsk Kray. Pochvovedenie 1969, 10, 13–28. (In Russian) [Google Scholar]
- Dobrovolsky, G.V.; Afanaseva, T.V.; Vasilenko, V.I.; Remezova, G.L. About genesis and geography of soils of Tomsk Priobje. Pochvovedenie 1969, 10, 3–12. (In Russian) [Google Scholar]
- Afanasyeva, T.V.; Remezova, G.L. On relict signs of secondary podzolic soils of the southern taiga of Western Siberia. Moscow State Univ. Bull. Ser. 15 Soil Sci. 1974, 1, 118–123. [Google Scholar]
- Ufimtseva, K.A. Soils of the Southern Taiga Zone of the West Siberian Plain; Kolos: Moscow, Russia, 1974; pp. 1–203. (In Russian) [Google Scholar]
- Karavaeva, N.A. Genesis and evolution of the second humus horizon in the soils of southern taiga of Western Siberia. In Pedogenesis and Weathering in Humid Landscapes; Targulian, V.O., Ed.; Nauka: Moscow, Russia, 1978; pp. 133–157. (In Russian) [Google Scholar]
- Gavrilov, D.A.; Golyeva, A.A. Microbiomorphic research of soils with the second humus horizon of the West Siberian southern taiga subzone (Russia). Tomsk State Univ. J. Biol. 2014, 2, 5–22, (In Russian, Summary in English). [Google Scholar] [CrossRef] [PubMed]
- Dobrovolsky, G.V.; Afanasyeva, T.V.; Vasilenko, V.I. On the age and relict features of soils in the Tomsk Ob region. In Materials for the Symposium 4th Meetings of Geographers of Siberia and the Far East; Nauka: Novosibirsk, Russia, 1969; pp. 117–119. (In Russian) [Google Scholar]
- Tochelnikov, Y.S. To the characteristic of the absolute age of the second humus horizon of sod-podzolic soils of Western Siberia. Proc. Acad. Sci. SSSR Ser. Geol. 1970, 191, 1151–1152. (In Russian) [Google Scholar]
- Jones, R.L.; Beavers, A.H. Aspects of caternary and depth distribution of opal phytoliths in Illinois soils. Soil Sci. Soc. Am. Proc. 1964, 28, 413–416. [Google Scholar] [CrossRef]
- Bobrova, E.; Bobrov, A. Phytoliths in soils: Species composition, distribution along a soil profile, and value as environmental indicators. In Monografías del Centro de Ciencias Medioambientales, CSCI (4), The State of-the-Art of Phytholits in Soils and Plants; Pinilla, A., Juane Tresseras, J., Machado, M.J., Eds.; CSCI: Madrid, Spain, 1997; pp. 5–13. [Google Scholar]
- Golyeva, A.A. Content and distrubution of phytoliths in the main types of soils in Eastern Europe. In Monografías del Centro de Ciencias Medioambientales, CSCI (4), The State of-the-Art of Phytholits in Soils and Plants; Pinilla, A., Juane Tresseras, J., Machado, M.J., Eds.; CSCI: Madrid, Spain, 1997; pp. 15–22. [Google Scholar]
- Golyeva, A.A. Biomorphic analysis as a part of soil morphological investigations. Catena 2001, 43, 217–230. [Google Scholar] [CrossRef]
- Golyeva, A.A.; Alexandrovskiy, A.L.; Tselishcheva, L.K. Phytolithic analysis of Holocene paleosoils. Eurasian Soil Sci. 1995, 27, 46–56. [Google Scholar]
- Golyeva, A.A.; Alexandrovskiy, A.L. The application of phytolith analysis for solving problems of soil genesis and evolution. Eurasian Soil Sci. 1999, 32, 884–891. [Google Scholar]
- Kamanina, I.Z. Phytolits data analysis of soils of different landscape zones. In Monografías del Centro de Ciencias Medioambientales, CSCI (4), The State of-the-Art of Phytholits in Soils and Plants; Pinilla, A., Juane Tresseras, J., Machado, M.J., Eds.; CSCI: Madrid, Spain, 1997; pp. 23–32. [Google Scholar]
- Kamanina, I.Z. Accumulation of phytoliths in Southern Taiga soils of different age. In Monografías del Centro de Ciencias Medioambientales, CSCI (4), The State of-the-Art of Phytholits in Soils and Plants; Pinilla, A., Juane Tresseras, J., Machado, M.J., Eds.; CSCI: Madrid, Spain, 1997; pp. 45–47. [Google Scholar]
- Kamanina, I.Z.; Shoba, A. The phytoliths analysis applied to soils of complex formation and paleosoils. In Monografías del Centro de Ciencias Medioambientales, CSCI (4), The State of-the-Art of Phytholits in Soils and Plants; Pinilla, A., Juane Tresseras, J., Machado, M.J., Eds.; CSCI: Madrid, Spain, 1997; pp. 33–43. [Google Scholar]
- Peto, Á. Studying modern soil profiles of different landscape zones in Hungary: An attempt to establish a soil-phytolith identification key. Quat. Int. 2013, 287, 149–161. [Google Scholar] [CrossRef]
- Calegari, M.R.; Lopes Paisani, S.D.; Cecchet, F.A.; Ewald, P.; Osterrieth, M.L.; Paisani, J.C.; Pontelli, M.E. Phytolith signature on the Araucarias Plateau—Vegetation change evidence in Late Quaternary (South Brasil). Quat. Int. 2016, 434, 1–12. [Google Scholar] [CrossRef]
- Silva Neto, E.C.D.; Calegari, M.R.; Pereira, M.G.; Maranhão, D.D.C.; Schiavo, J.A.; Fontana, A.; Fernandes, J.C.F. Phytoliths as indicators of pedogenesis and paleoenvironmental changes in Spodosols of the state of Rio de Janeiro, Brazil. Sci. Total Environ. 2018, 636, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F.; Newell Bourne, C.; Fisher, W.F. Opal phytoliths as an indicator of the floristics of prehistoric grasslands. Geoderma 1995, 68, 243–255. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; Alta Mira Press: Lanham, MD, USA, 2006; pp. 1–238. [Google Scholar]
- Piperno, D.R. Quaternary environmental history and agricultural impact on vegetation in Central America. Ann. Mo Bot. Gard. 2006, 93, 274–296. [Google Scholar] [CrossRef]
- Electronic Version of the National Atlas of Soils of the Russian Federation. Available online: https://soilatlas.ru/ (accessed on 20 October 2018). (In Russian).
- Golyeva, A.A. Phytoliths and Their Information Role in Natural and Archeological Objects; Polteks: Moscow, Russia; Syktyvkar, Russia; Elista, Russia, 2001; pp. 1–140. (In Russian) [Google Scholar]
- IUSS Working Group WRB 2014. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; pp. 1–192. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; pp. 1–97. [Google Scholar]
- Kachinskiy, N.A. The Physics of Soils; Vysshaya Shkola Publishers: Moscow, Russia, 1965; pp. 1–321. (In Russian) [Google Scholar]
- Skjemstad, J.O.; Baldock, J.A. Total and Organic Carbon. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 225–237. [Google Scholar]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Ion Exchange and Exchangeable Cations. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 197–208. [Google Scholar]
- Theory and Practice Chemical Analysis of Soils; Vorobyova, L.F. (Ed.) GEOS: Moscow, Russia, 2006; 400p. (In Russian) [Google Scholar]
- Chichagova, O.A. Radiocarbon Dating of Soil Humus: Its Method and Use in Pedology and Paleogeography; Nauka: Moscow, Russia, 1985; pp. 1–158. [Google Scholar]
- Zuo, X.; Lu, H.; Zhang, J.; Wang, C.; Sun, G.; Zheng, Y. Radiocarbon dating of prehistoric phytoliths: A preliminary study of archaeological sites in China. Sci. Rep. 2016, 6, 26769. [Google Scholar] [CrossRef] [PubMed]
- Bronk Ramsey, C. Radiocarbon calibration and analysis of stratigraphy: The OxCal program. Radiocarbon 1995, 37, 425–430. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Academic Press: San Diego, CA, USA, 1988; pp. 1–280. [Google Scholar]
- Golyeva, A.A. Various phytolith types as bearers of different kinds of ecological information. In Plant, People and Places e Recent Studies in Phytolith Analysis; Madella, M., Debora, Z., Eds.; Oxbow: Oxford, UK, 2007; pp. 196–208. [Google Scholar]
- Golyeva, A.A. Microbiomorphic Analysis as a Tool for Natural and Anthropogenic Landscape: Genesis, Geography, Information; URSS: Moscow, Russia, 2008; pp. 1–256. (In Russian) [Google Scholar]
- Madella, M.; Alexandre, A.; Ball, T. International Code for Phytolith Nomenclature 1.0. Ann. Bot. 2005, 96, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrovsliy, A.L.; Chicagova, O.A. Radiocarbon age of Holocene paleosols of the East European forest-steppe zone. Catena 1998, 34, 197–207. [Google Scholar] [CrossRef]
- Santos, G.M.; Alexandre, A.; Southon, J.R.; Treseder, K.K.; Corbineau, R.; Reyerson, P.E. Possible source of ancient carbon in phytolith concentrates from harvested grasses. Biogeosciences 2012, 9, 1873–1884. [Google Scholar] [CrossRef] [Green Version]
- Reyerson, P.E.; Alexandre, A.; Harutyunyan, A.; Corbineau, R.; Martinez De La Torre, H.A.; Badeck, F.; Cattivelli, L.; Santos, G.M. Unambiguous evidence of old soil carbon in grass biosilica particles. Biogeosciences 2016, 13, 1269–1286. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Lamers, M.; Denysenko, D.; Streck, T. Phytolith transport in soil: A field study using fluorescent labeling. Geoderma 2010, 157, 27–36. [Google Scholar] [CrossRef]




| Horizon | Depth, cm | Field Morphological Description |
|---|---|---|
| Endocalcic Stagnic Albic Luvisols (Siltic, Abruptic, Cutanic, Epiloamic) (L1, hilltop) | ||
| O | 0–7 | Peat-like litter of semi-decomposed needles, cones, standing dead phytomass; brown-coloured, loose, fresh, with sharp transition, the boundary having small waves. |
| A | 7–13 | Brown-grey in colour, silt loam, loose, small lumps, with plenty of plant roots; the boundary is clear, with some waves and tongues. |
| E | 13–30 | Light grey with a brown shadow, fresh, slightly packed, silt, foliaceous, with plenty of plant roots, clear wavy boundary. |
| Eh | 30–40 | Light grey with separate grey humus spots, fresh, silt loam, foliaceous, transition into the lower horizon is gradual, wavy boundary. |
| Btst | 40–70 | Brown-coloured with dark grey humus tongues, moist, silty clay, lumpy, humus cutans on ped edges, gradual transition, wavy boundary. |
| Btk | 70–80 | Brown-coloured with dark grey humus tongues, moist, silty clay, lumpy, humus cutans on ped edges, gradual transition, carbonated from 55 cm and deeper. |
| Endocalcic Stagnic Albic Luvisols (Siltic, Abruptic, Cutanic, Epiloamic) (L2, midslope) | ||
| O | 0–6 | Peat-like litter of semi-decomposed needles, cones, aboveground dead phytomass. Brown-coloured, loose, fresh, with sharp transition, boundary with small waves. |
| A | 6–13 | Light grey, fresh, silt loam, loose, small lumps, plentiful plant roots, clear transition, boundary with mid-sized waves. |
| E | 13–24 | Light grey with a brown shadow, fresh, weakly packed, silt loam, silty, porous, foliaceous, with plentiful plant roots, noticeable transition, boundary with small waves. |
| A’ | 24–42 | Dark grey, fresh, silt clay loam, granular-lamellar, packed, transformed by earthworm passages, gradual transition, boundary with mid-sized waves. |
| Bt, st | 42–70 | Brown-coloured with dark grey humus tongues, moist, silty clay, lumpy-nutty, humus cutans on ped edges, gradual transition, wavy boundary. |
| Btgk | 70–80 | Brown-coloured with dark grey humus tongues, grey-bluish spots, moist, silty clay, tabular, carbonated (white eye), humus cutans on ped edges, gleyic. |
| Calcic Stagnic Greyzemic Epidystric Umbrisols (Siltic) (St, toeslsope) | ||
| O | 0–10 | Peat-like litter of semi-decomposed needles, cones, aboveground dead phytomass; brown, loose, fresh, sharp transition, boundary with middle-sized waves. |
| A | 10–24 | Light grey with dark grey patches, fresh, loose, silt clay loam, small granules, plentiful plant roots, noticeable transition, boundary with mid-sized waves. |
| A’ | 24–50 | Dark grey, moist, silty clay loam/silty clay, granular, packed, with solitary plant roots, clear transition, boundary with small-sized waves. |
| A’/Bg | 50–70 | Brown-coloured with dark grey humus tongues, silty clay, moist, curdled, ferrous, gradual transition, boundary with tongues. |
| Bstk | 70–85 | Brown-coloured with some morphons filled with humus material (speckled) and grey-bluish and ochre spots, moist, silty clay, curdled, carbonated. |
| Horizon | n 2 | Depth, cm | SOC 3, % | pH | CaCO3, % | Exchangeable Cations | Soil Texture | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | KCl | H+ | Ca2+ | Mg2+ | Na+ | K+ | Sand | Clay | Silt | Classes | |||||
| Cmol kg−1 | % | ||||||||||||||
| Endocalcic Stagnic Albic Luvisols (Siltic, Abruptic, Cutanic, Epiloamic) (L1, hilltop) | |||||||||||||||
| A | 1 | 7–13 | 1.9 | 4.1 | 3.1 | 0 | 22.4 | 3.1 | 0.9 | 0.5 | 1.3 | 10.6 | 14.1 | 75.3 | Silt Loam |
| E | 2 | 13–30 | 0.5 | 4.4 ± 0.3 | 3.2 ± 0.1 | 0 | 7.4 ± 0.7 | 3.1 ± 0.0 | 0.9 ± 0.0 | 0.4 | 0.8 ± 0.5 | 6.3 ± 0.9 | 11.6 ± 1.7 | 82.1 ± 0.8 | Silt |
| Eh | 1 | 30–40 | 0.5 | 5.1 | 3.4 | 0 | 6.3 | 10.5 | 3.9 | 0.5 | 0.6 | 3.0 | 25.6 | 71.4 | Silt Loam |
| Bt | 4 | 40–70 | 0.3 ± 0.11 | 6.8 ± 1.1 | 5.2 ± 0.9 | 0.2 ± 0.2 | 3.1 ± 1.7 | 20.6 ± 1.3 | 10 ± 1.0 | 0.6 | 0.9 ± 0.1 | 2.7 ± 1.2 | 50.5 ± 2.0 | 44.3 ± 3.4 | Silty Clay |
| Btk | 1 | 70–80 | 0.2 | 8.2 | 7.3 | 4.6 | 0.3 | 57.9 | 8.4 | 0.6 | 0.9 | 4.2 | 46.7 | 49.1 | |
| S 4 (E/Bt) = 5.1 | |||||||||||||||
| Endocalcic Stagnic Albic Luvisols (Siltic, Abruptic, Cutanic, Epiloamic) (L2, midslope) | |||||||||||||||
| A | 1 | 6–13 | 0.6 | 4.3 | 3.2 | 0 | 19.8 | 7.4 | 1.8 | 0.6 | 0.5 | 7.3 | 18.0 | 74.4 | Silt Loam |
| E | 2 | 13–24 | 0.5 ± 0.1 | 5.0 ± 0.1 | 3.5 ± 0.1 | 0 | 8.1 ± 1.4 | 6.1 | 0.9 | 0.4 | 0.3 | 3.6 ± 0.2 | 13.3 ± 0.0 | 82.9 ± 0.3 | |
| A’ | 2 | 24–42 | 0.6 ± 0.1 | 5.9 ± 0.2 | 4.5 ± 0.2 | 0 | 4.9 ± 0.7 | 14.9 ± 3.2 | 3.5 ± 0.6 | 0.5 ± 0.1 | 0.4 ± 0.1 | 1.7 ± 0.2 | 23.3 ± 5.7 | 75.1 ± 5.5 | Silt Clay Loam |
| Bt | 4 | 42–70 | 0.3 ± 0.1 | 6.5 ± 0.4 | 5.0 ± 0.4 | 2.7 ± 1.3 | 3.2 ± 1.1 | 24.9 ± 6.0 | 4.7 ± 0.6 | 0.6 | 0.7 ± 0.2 | 0.9 ± 0.7 | 50.6 ± 7.4 | 48.4 ± 6.7 | Silty Clay |
| Btgk | 1 | 70–80 | 0.2 | 7.9 | 7.1 | 5.8 | 0.4 | 35.9 | 4.8 | 0.6 | 0.8 | 2.4 | 49.2 | 48.4 | |
| S (E/Bt) = 4.5 | |||||||||||||||
| Calcic Stagnic Greyzemic Epidystric Umbrisols (Siltic) (Um, toeslsope) | |||||||||||||||
| A | 2 | 10–24 | 3.0 | 4.5 ± 0.2 | 3.3 ± 0.1 | 0 | 28.0 ± 5.8 | 16.4 ± 1.8 | 3.4 ± 0.2 | 0.8 ± 0.1 | 0.9 | 11.9 ± 3.5 | 27.1 ± 1.2 | 62.6 ± 2.6 | Silt Clay Loam |
| A’ | 3 | 24–50 | 1.6 ± 0.4 | 5.8 ± 0.5 | 4.8 | 0 | 10.3 ± 3.8 | 23.7 ± 3.1 | 2.6 ± 0.8 | 0.7 | 0.6 | 3.6 ± 2.0 | 38.8 ± 10 | 58.4 ± 7.9 | Silty Clay Loam/Silty Clay |
| A’/Bg | 2 | 50–70 | 0.3 ± 0.1 | 7.2 ± 0.6 | 5.6 ± 0.4 | 0 | 2.7 ± 1.9 | 25.4 ± 3.1 | 2.6 ± 0.1 | 0.7 | 0.8 ± 0.2 | 1.2 ± 0.1 | 44.0 ± 1.7 | 53.4 ± 0.4 | Silty Clay |
| Bgk | 2 | 70–85 | 0.2 | 8.2 | 6.3 ± 0.1 | 0.4 | 0.7 | 22.1 ± 1.6 | 2.3 ± 0.5 | 0.7 ± 0.1 | 0.6 | 0.9 ± 0.8 | 38.9 ± 1.6 | 60.3 ± 2.4 | |
| Lab. Number | Horizon | Depth, cm | Carbon, % | Material | δ13CPDB, ‰ | 14C Age Year BP | 14C Age Year BP, 2σ | |
|---|---|---|---|---|---|---|---|---|
| Median | ||||||||
| Endocalcic Stagnic Albic Luvisols (Abruptic, Cutanic, Epiloamic, Siltic), hilltop | ||||||||
| NSK-G1 | E | 9–10 | 1.14 | Phytolith | −32.1 | 3663 ± 53 | 2151–1902 | 2026 |
| NSK-G8 | 47.34 | HAII | −29.6 | 3781 ± 68 | 2410–2031 | 2220 | ||
| NSK-G3 | Eh | 29–30 | 1.19 | Phytolith | −30.0 | 6301 ± 26 | 5321–5220 | 5270 |
| NSK-G6 | 41.34 | HAII | −31.3 | 5700 ± 25 | 4600–4462 | 4531 | ||
| Endocalcic Stagnic Albic Luvisols (Abruptic, Cutanic, Epiloamic, Siltic), midslope | ||||||||
| NSK-G4 | E | 19–20 | 0.89 | Phytolith | −28.6 | 5154 ± 32 | 4005–3936 | 3970 |
| NSK-G9 | 30.53 | HAII | −31.2 | 4051 ± 23 | 2632–2544 | 2588 | ||
| NSK-G2 | A’ | 34–35 | 1.43 | Phytolith | −30.4 | 6883 ± 32 | 5843–5711 | 5777 |
| NSK-G7 | 47.97 | HAII | −26.7 | 5775 ± 25 | 4696–4548 | 4622 | ||
| Calcic Stagnic Greyzemic Epidystric Umbrisols (Siltic), toeslope | ||||||||
| NSK-G5 | A’ | 59–60 | 3.35 | Phytolith | −29.4 | 7475 ± 27 | 6466–6404 | 6435 |
| NSK-G10 | 37.70 | HAII | −29.3 | 5311 ± 25 | 4185–4051 | 4118 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilov, D.A.; Loiko, S.V.; Klimova, N.V. Holocene Soil Evolution in South Siberia Based on Phytolith Records and Genetic Soil Analysis (Russia). Geosciences 2018, 8, 402. https://doi.org/10.3390/geosciences8110402
Gavrilov DA, Loiko SV, Klimova NV. Holocene Soil Evolution in South Siberia Based on Phytolith Records and Genetic Soil Analysis (Russia). Geosciences. 2018; 8(11):402. https://doi.org/10.3390/geosciences8110402
Chicago/Turabian StyleGavrilov, Denis A., Sergey V. Loiko, and Nina V. Klimova. 2018. "Holocene Soil Evolution in South Siberia Based on Phytolith Records and Genetic Soil Analysis (Russia)" Geosciences 8, no. 11: 402. https://doi.org/10.3390/geosciences8110402





