Geochemical Anomalies of Frozen Ground due to Hydrocarbon Migration in West Siberian Cryolithozone
Abstract
:1. Introduction
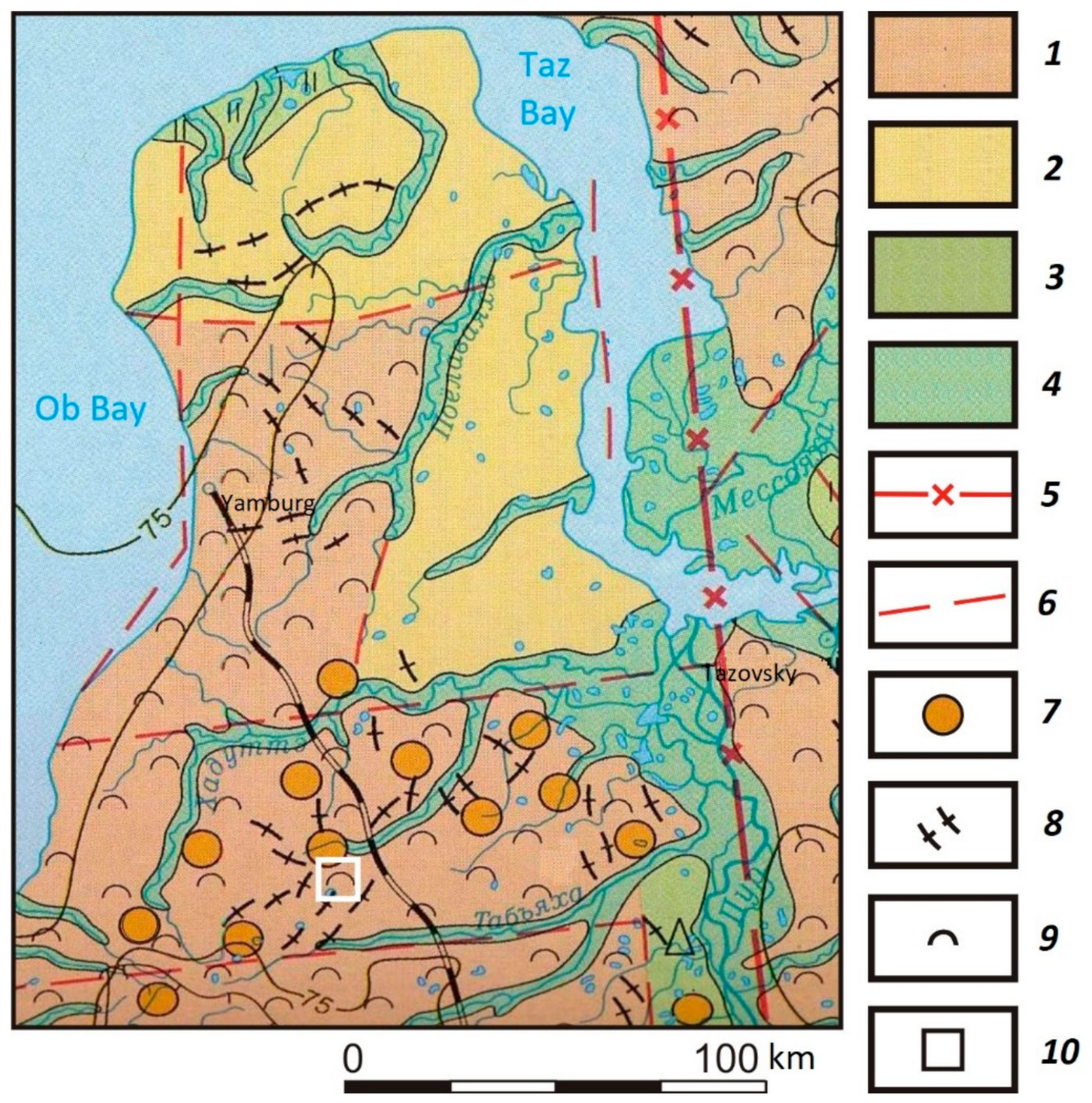
2. Field Region and Methods of Research
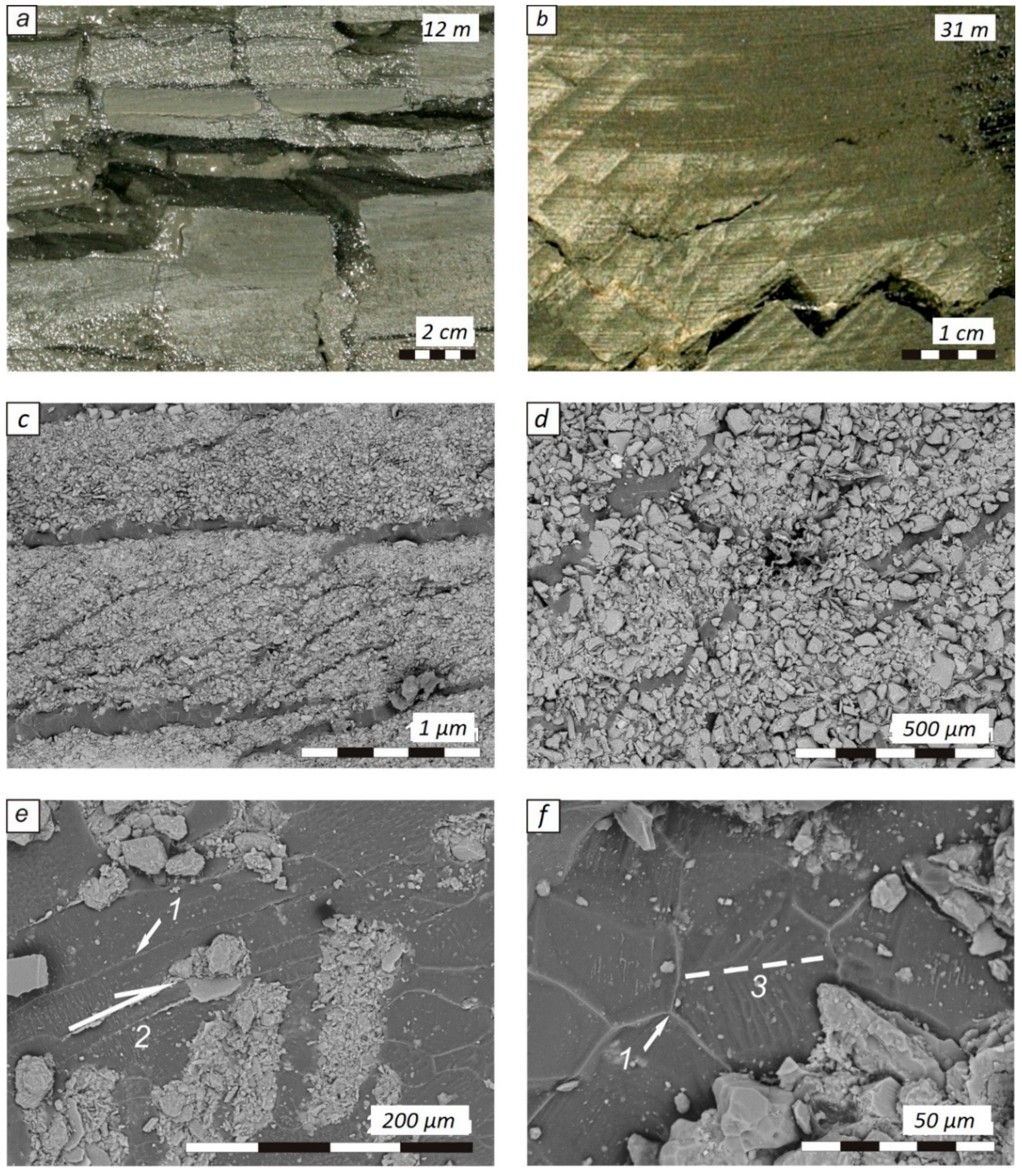
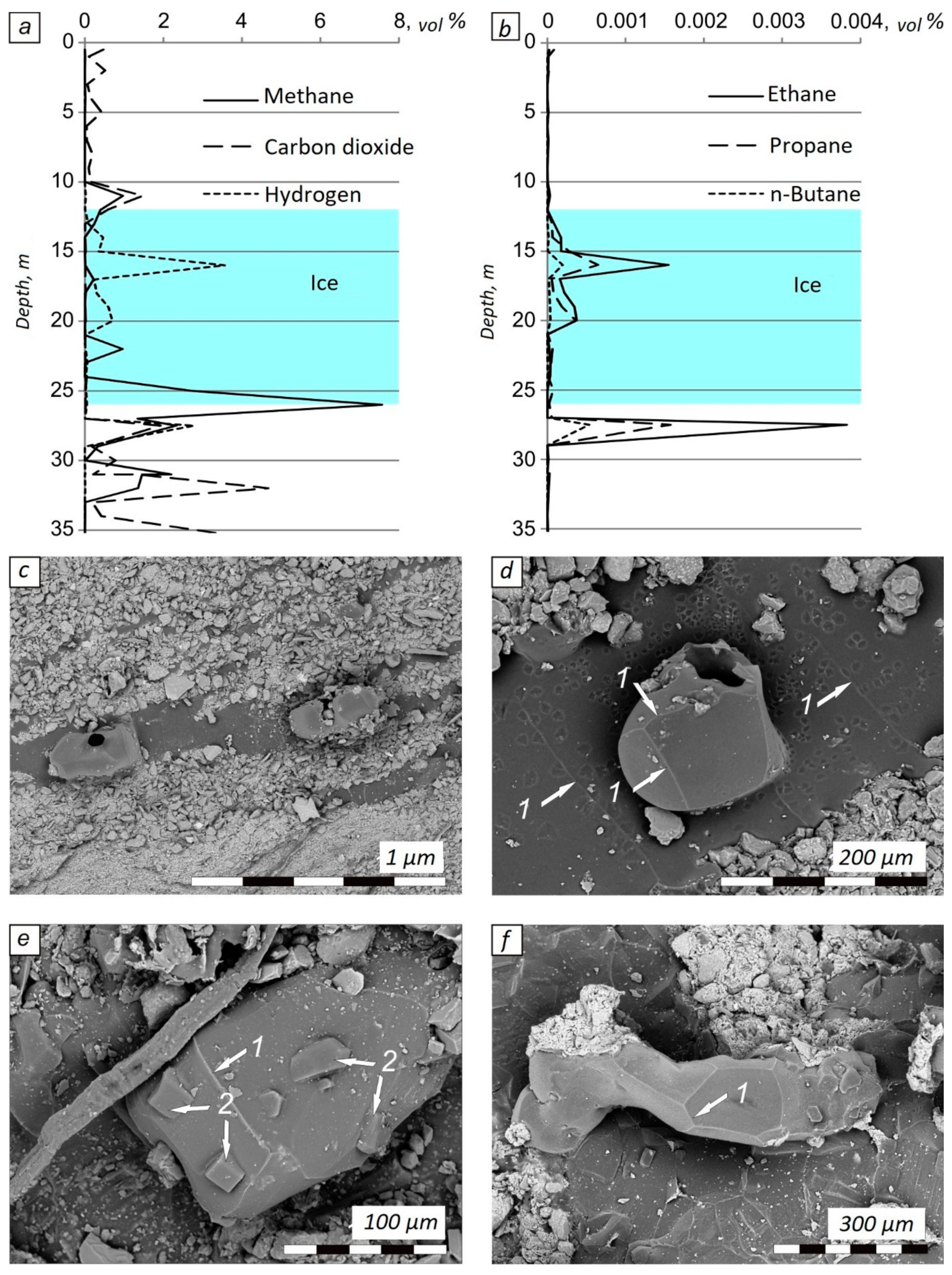
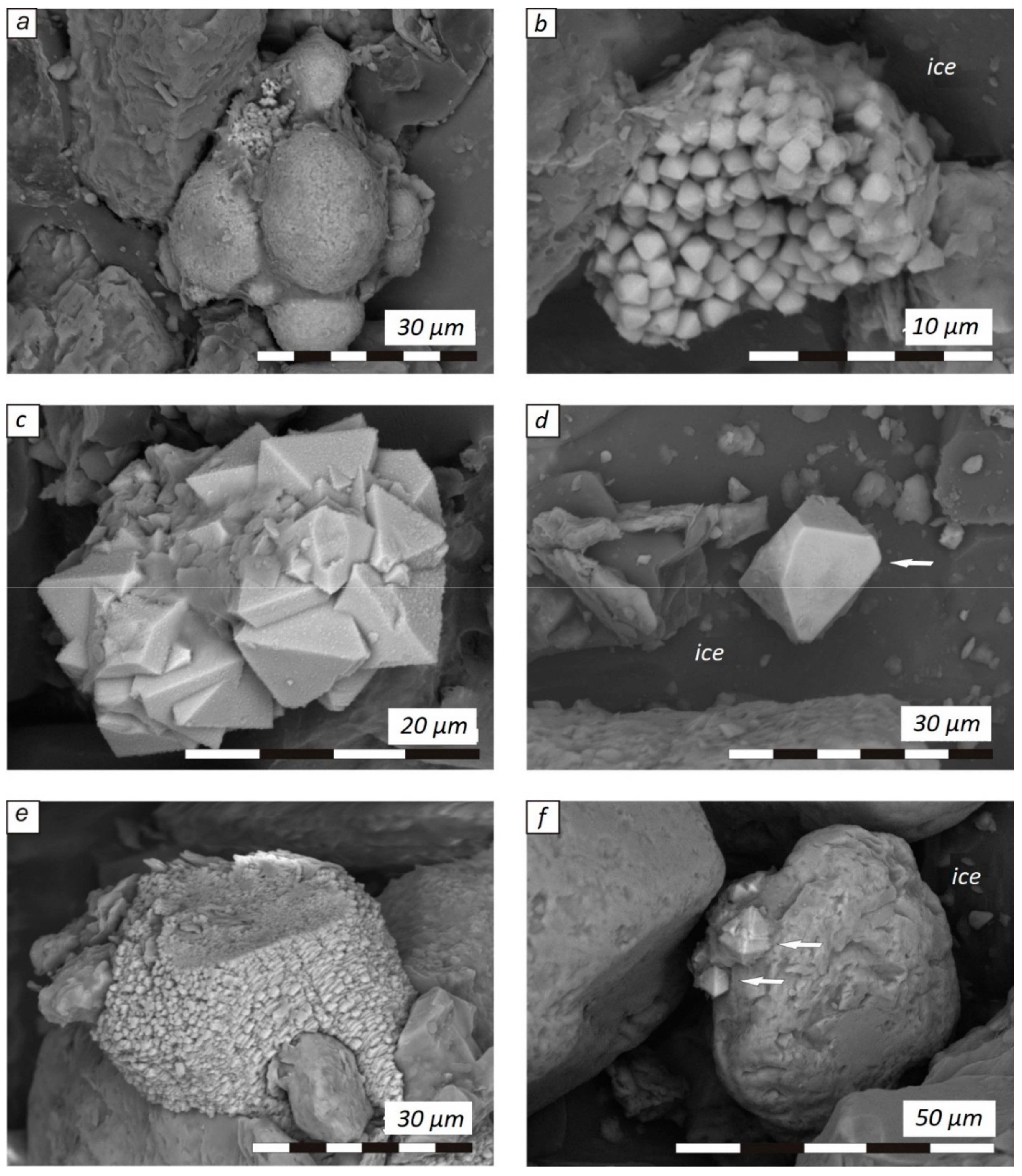
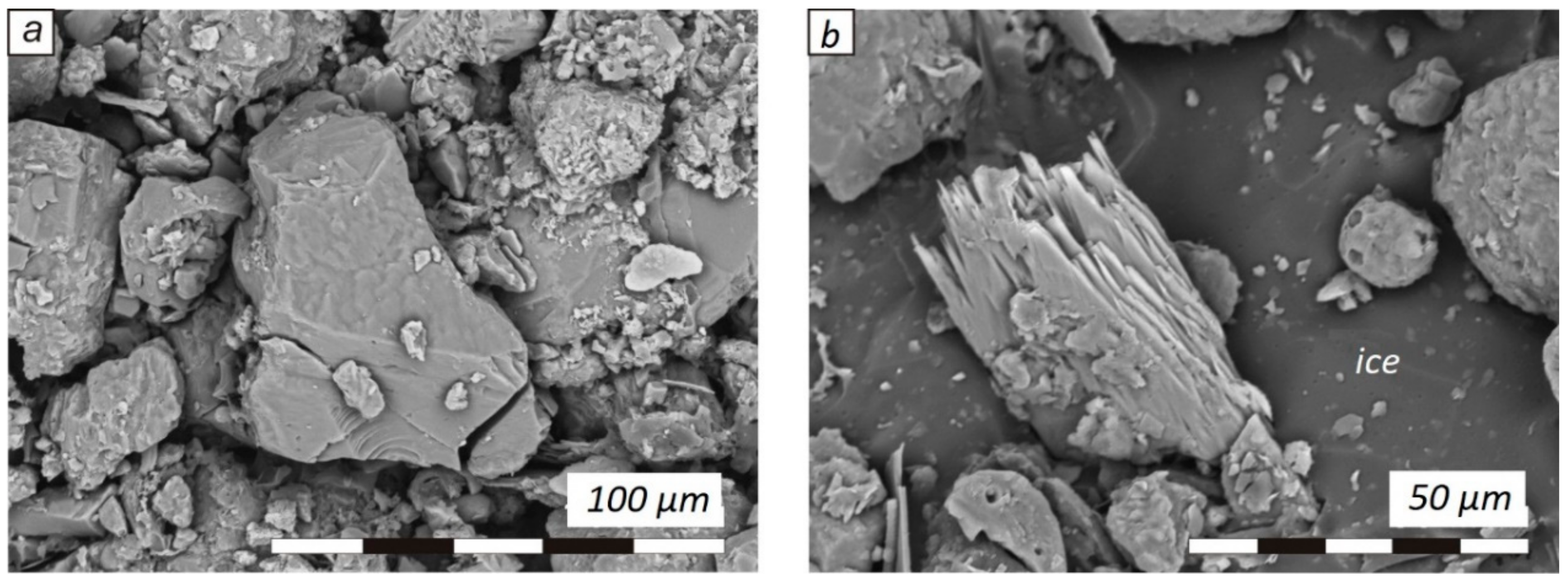
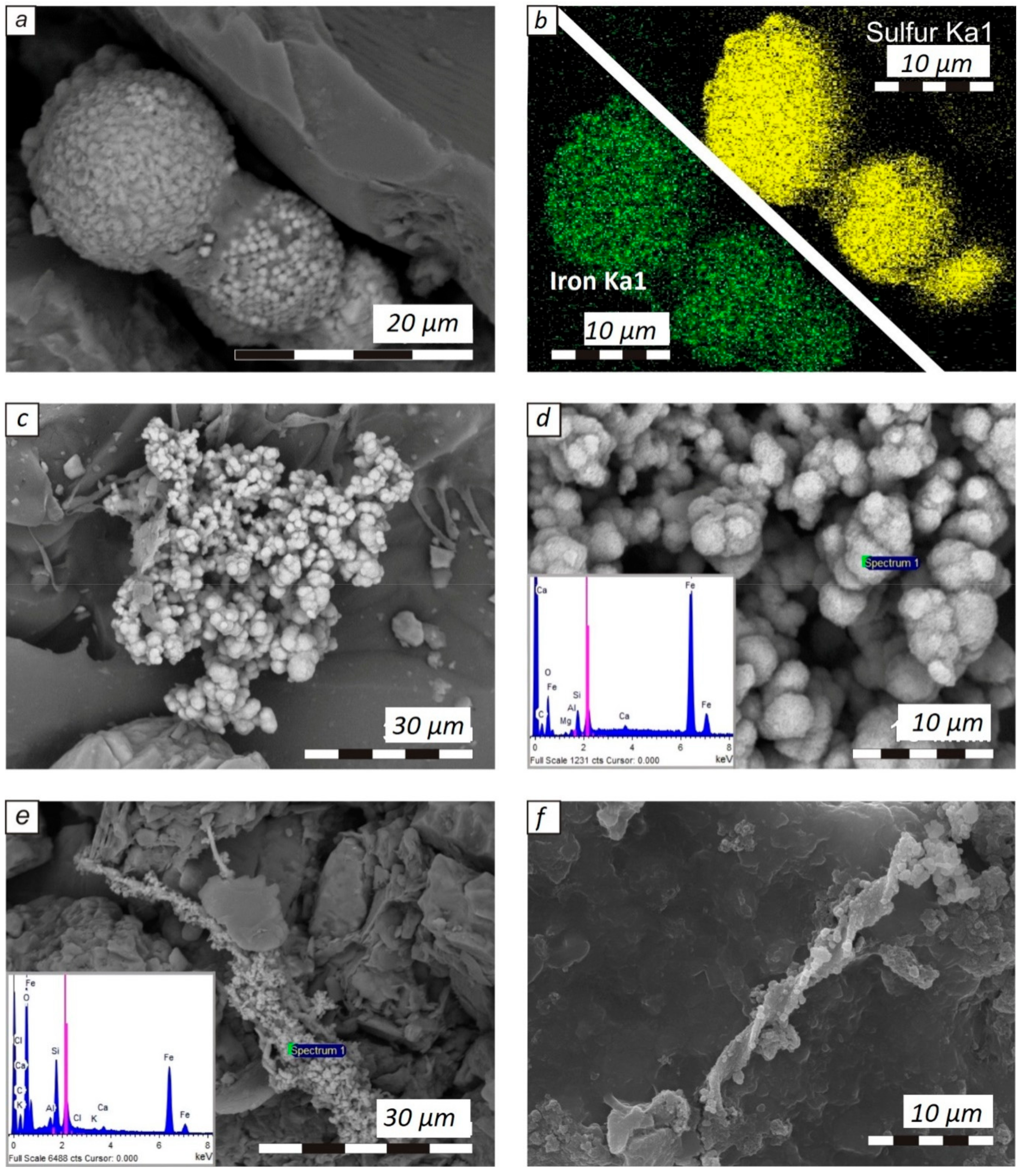
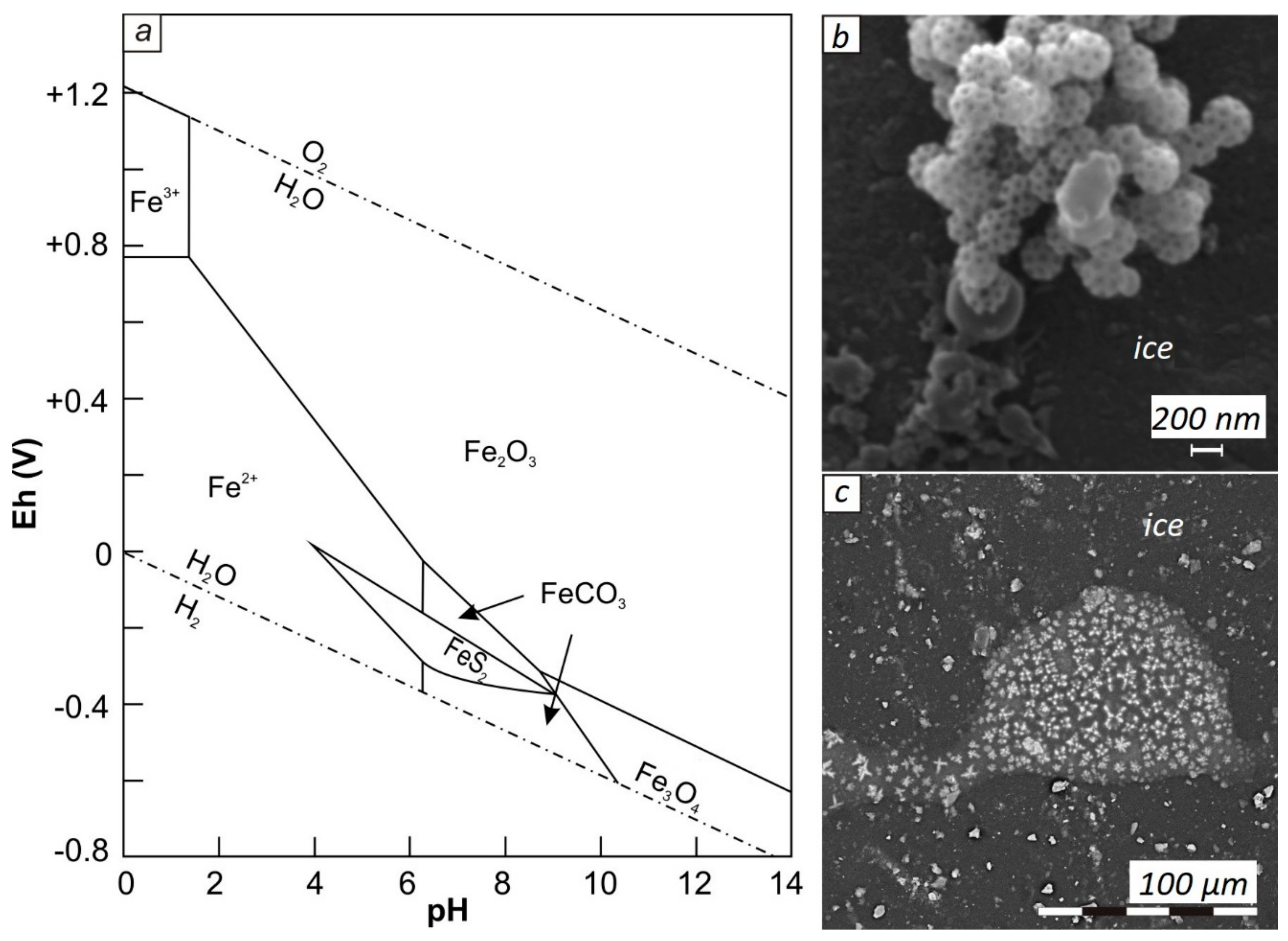
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bischoff, J.; Mangelsdorf, K.; Gattinger, A.; Schloter, M.; Kurchatova, A.N.; Herzschuh, U.; Wagner, D. Response of methanogenic archaea to Late Pleistocene and Holocene climate changes in the Siberian Arctic. Glob. Biogeochem. Cycles 2013, 27, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Schuur, E.A.G.; McGuire, A.D.; Schaedel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hubberten, H.W.; Romanovskii, N.N. The main features of permafrost in the Laptev Sea region, Russia—A review. In Proceedings of the 8th International Conference on Permafrost, Zürich, Switzerland, 21–25 July 2003; pp. 431–436. [Google Scholar]
- Leibman, M.O.; Perednya, D.D.; Kizyakov, A.I.; Lein, A.Y.; Savvichev, A.S.; Vanshtein, B.G. Sulfur and carbon isotopes within atmospheric, surface and ground water, snow and ice as indicators of the origin of tabular ground ice in the Russian Arctic. Permafr. Periglac. Process. 2011, 22, 39–48. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, D. Hydrocarbon-induced alteration of soils and sediments. AAPG MEMOIRS 1996, 66, 71–89. [Google Scholar]
- Smith-Rouch, L.S. Petroleum Geology and Resources of the West Siberian Basin, Russia. US Geol. Surv. Bull. 2006, 27, 2201. [Google Scholar]
- Schoell, M.; Picha, F.; Rovenskaya, A.; Nemchenko, N. Genetic characterization of natural gases in NW Siberia. In Proceedings of the American Association of Petroleum Geologists International Conference, Vienna, Austria, 7–10 September 1997; pp. 46–47. [Google Scholar]
- The newest tectonics of flat territory (with elements of structural geomorphology). In Atlas of the Yamal-Nenets Autonomous District; FGUP Omskaya kartograficheskaya fabrika: Omsk, Russia, 2004; pp. 60–61. (In Russian)
- Vasil’chuk, Yu.K.; Budantseva, N.A.; Vasil’chuk, A.C.; Yoshikava, I.; Podborny, Ye.Ye; Chizhova, Ju.N. Isotopic composition of the ice core of the Late Pleistocene bulgunyah at the Pestsovoye deposit in the Evoya River valley in the south of the Taz Peninsula. Kryosphera Zemli 2014, 18, 47–58. (In Russian) [Google Scholar]
- Kuzin, I.L. Geomorphology of the West Siberian Plain; State. Polar. Academy: Sankt-Peterburg, Russia, 2005; p. 176. (In Russian) [Google Scholar]
- Dubikov, G.I. Composition and Cryogenic Structure of the Frozen Strata of Western Siberia. GEOS: Moscow, Russia, 2002; p.246. (In Russian) [Google Scholar]
- Kritsuk, L.N. Underground Ice Cryolithozone of Western Siberia; Scientific World: Moscow, Russia, 2010; p. 350. (In Russian) [Google Scholar]
- Bates, T.F.; Comer, J.J. Electron microscopy of clay surfaces. Clays Clay Miner. 1955, 3, 1–25. [Google Scholar] [CrossRef]
- Rogov, V.V.; Kurchatova, A.N. Method of Manufacturing Replica for Analyses of Microstructure of Frozen Rocks in Scanning Electron Microscope. RU Patent 2,528,256 C1, 2013. Available online: http://russianpatents.com (accessed on 13 January 2013).
- Rogov, V.V. Fundamentals of Cryogenesis; GEO: Novosibirsk, Russia, 2009; p. 202. (In Russian) [Google Scholar]
- Zavatskiy, M.D. Study of the Fields of Hydrocarbon Gas Concentrations in Surface Natural Sorbents in Connection with Prospecting and Exploration of Oil and Gas Deposits in Western Siberia. Ph.D. Thesis, TSOGU, Tyumen, Russia, 2009. (In Russian). [Google Scholar]
- Jones, V.T.; Drozd, R.J. Predictions of oil or gas potential by near surface geochemistry. Am. Assoc. Pet. Geol. Bull. 1983, 67, 932–952. [Google Scholar]
- Milkov, A.V. Worldwide distribution and significance of secondary microbial methane formed during petroleum biodegradation in conventional reservoirs. Org. Geochem. 2011, 42, 184–207. [Google Scholar] [CrossRef]
- Randive, K.R.; Hari, K.R.; Dora, M.L.; Malpe, D.B.; Bhondwe, A.A. Study of Fluid Inclusions: Methods, Techniques and Applications. Geol. Mag. 2014, 29, 19–28. [Google Scholar]
- Kurchatova, A.N.; Mel’nikov, V.P.; Rogov, V.V. Gas-bearing ice crystallites in clayey deposits. Dokl. Earth Sci. 2014, 459, 1510–1513. [Google Scholar] [CrossRef]
- Gogonenkov, G.; Timurziev, A. Srike-slip deformations in the West Siberian basin and their impact of exploration and development of oil and gas reservoirs. Cent. Eur. Geol. 2009, 52, 359–390. [Google Scholar] [CrossRef]
- Gibson, E.K.; Wentworth, S.J.; McKay, D.S. Chemical weathering and diagenesis of a cold desert soil from Wright Valley, Antarctica: An analog of Martian weathering processes. J. Geophys. Res. 1983, 88, 912–928. [Google Scholar] [CrossRef]
- Konishchev, V.N.; Rogov, V.V. Investigations of cryogenic weathering of Europe and Northern Asia. Permafr. Periglac. Process. 1993, 4, 49–64. [Google Scholar] [CrossRef]
- Kurchatova, A.N.; Slagoda, E.A.; Obzhirov, A.I.; Shakirov, R.B.; Rogov, V.V. Microstructure of diatomaceous muds of hydrate-saturated deposits of the Okhotsk Sea/Arctic, Subarctic: Mosaic, contrast, In Cryosphere Variability/Abstracts of the International Conference; Epokha: Tyumen, Russia, Russia 2015. (In Russian) [Google Scholar]
- Kurchatova, A.N.; Rogov, V.V. Authigenic carbonates in sediments of the Ice Complex of the Coastal Plains of the Eastern Arctic. Kriosf. Zemli. Earth’s Cryosphere 2013, 17, 60–69. (In Russian) [Google Scholar]
- Hoehler, T.M.; Alperin, M.J.; Albert, D.B.; Martens, C.S. Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Glob. Biogeochem. Cycles 1994, 8, 451–463. [Google Scholar] [CrossRef]
- Hinrichs, K.-U.; Hayes, J.M.; Sylva, S.P.; Brewer, P.G.; DeLong, R.F. Methane-consuming archaebacteria in marine sediments. Nature 1999, 398, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.W.; Fujita, Y.; Delwiche, M.E.; Blackwelder, D.B.; Sheridan, P.P.; Uchida, T.; Colwell1, F.S. Microbial Communities from Methane Hydrate-Bearing Deep Marine Sediments in a Forearc Basin. Appl. Environ. Microbiol. 2002, 68, 3759–3770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Knittel, K.; Lösekann, T.; Boetius, A.; Kort, R.; Amann, R. Diversity and distribution of methanotrophic archaea at cold seeps. Appl. Environ. Microbiol. 2005, 71, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Sivan, O.; Adler, M.; Pearson, A.; Gelman, F.; Bar-Or, I.; John, S.G.; Eckert, W. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr. 2011, 56, 1536–1544. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Rasigraf, O.; Sapart, C.J.; Jilbert, T.; Jetten, M.S.M.; Röckmann, T.; van der Veen, C.; Bândă, N.; Kartal, B.; Ettwig, K.F.; et al. Iron-Mediated Anaerobic Oxidation of Methane in Brackish Coastal Sediments. Environ. Sci. Technol. 2015, 49, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, A.I. Thermophilic Iron-Reducing Prokaryotes. Ph.D. Thesis, Institute of Microbiology of the Russian Academy of Sciences, Moscow, Russia, 2008. (In Russian). [Google Scholar]
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, A.S. Geomicrobiology of Sulfuric Acid Speleogenesis: Microbial Diversity, Nutrient Cycling, and Controls on Cave Formation. Master’s Thesis, The University of Texas, Austin, TX, USA, 2004. [Google Scholar]
- Eby, G.N. Principles of Environmental Geochemistry; Brooks/Cole: Boston, MA, USA, 2004. [Google Scholar]
- Garrels, R.M.; Krayst, C.L. Solutions, Minerals, Equilibrium; Harper & Row: New York, NY, USA, 1965. [Google Scholar]
- Imhoff, J.F. The family Ectothiorhodospiraceae. In Prokaryotes; Springer: New York, NY, USA, 2006; pp. 874–886. [Google Scholar]
- Frankel, R.B.; Bazylinski, D.A. Biologically Induced Mineralization by Bacteria. Rev. Miner. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
| Fields | Phase | Tph, °C | Depth, m | C2–C4, % | d13CCH4, ‰ | dDCH4,‰ |
|---|---|---|---|---|---|---|
| Cenomanian Complex | ||||||
| Noth-Urengoy, Pestsovoe | Gas | 30–37 | 1043–1350 | 0–0.56 | −50.86– −56.11 | −204.5– −218.7 |
| Lower Cretaceous Complex | ||||||
| Pestsovoe | Oil—gas— condensate | 62.5–93 | 1836–3380 | 1.32–12.16 | −38.00– −41.31 | −207.2– −230.7 |
| Noth-Urengoy | Gas—condensate, oil—gas—condensate | 66.0–82.6 | 2546–3180 | 1.39–15.4 | −35.09– −38.78 | −226.8– −237.5 |
| Jurassic Complex | ||||||
| Etypurskoe (J3) | Oil—gas—condensate | 83–101 | 2907–3058 | 10.50–26.10 | −40.70– −42.88 | −257.7– −258.0 |
| Pestsovoe (J3, J2) | Oil—gas—condensate | 112 | 3800–4000 | 16.5–17.5 | ||
| Fields | Well | Depth, m | Gas Composition, % | d13CCH4, ‰ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | C4H10 | CO2 | N2 | He | Ar | H2 | ||||
| Noth-Urengoy | 74 | 1208–1212 | 99.20 | 0.004 | 0.001 | - | - | 0.80 | 0.014 | 0.007 | 0.001 | −53.56 |
| Pestsovoe | 3 | 1260–1268 | 98.10 | 0.340 | 0.160 | 0.054 | 0.09 | 1.23 | 0.017 | 0.010 | - | −52.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurchatova, A.; Rogov, V.; Taratunina, N. Geochemical Anomalies of Frozen Ground due to Hydrocarbon Migration in West Siberian Cryolithozone. Geosciences 2018, 8, 430. https://doi.org/10.3390/geosciences8120430
Kurchatova A, Rogov V, Taratunina N. Geochemical Anomalies of Frozen Ground due to Hydrocarbon Migration in West Siberian Cryolithozone. Geosciences. 2018; 8(12):430. https://doi.org/10.3390/geosciences8120430
Chicago/Turabian StyleKurchatova, Anna, Victor Rogov, and Natalia Taratunina. 2018. "Geochemical Anomalies of Frozen Ground due to Hydrocarbon Migration in West Siberian Cryolithozone" Geosciences 8, no. 12: 430. https://doi.org/10.3390/geosciences8120430




