Release, Migration, Sorption, and (Re)Precipitation of U during Peraluminous Granite Alteration under Oxidizing Conditions in Central Portugal
Abstract
:1. Introduction
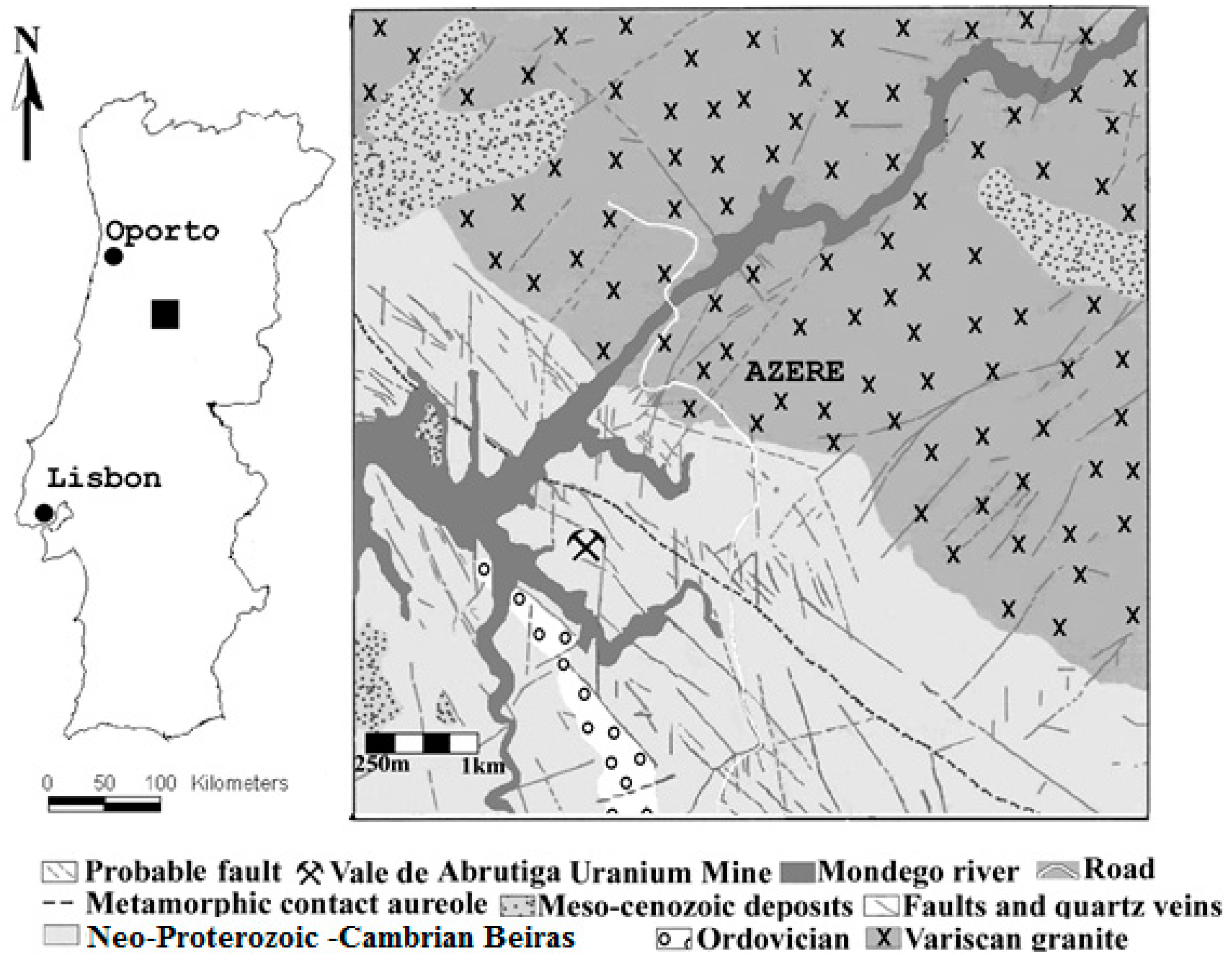
2. Geological Setting
3. Analytical Methods
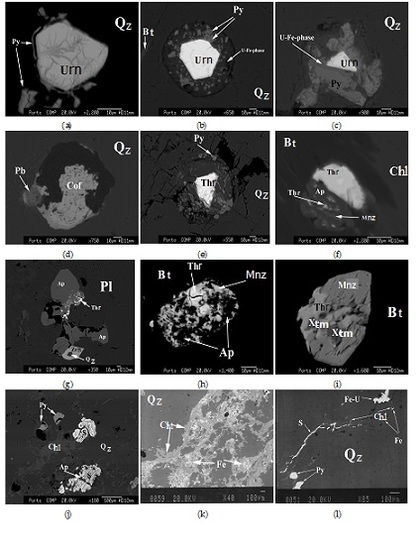
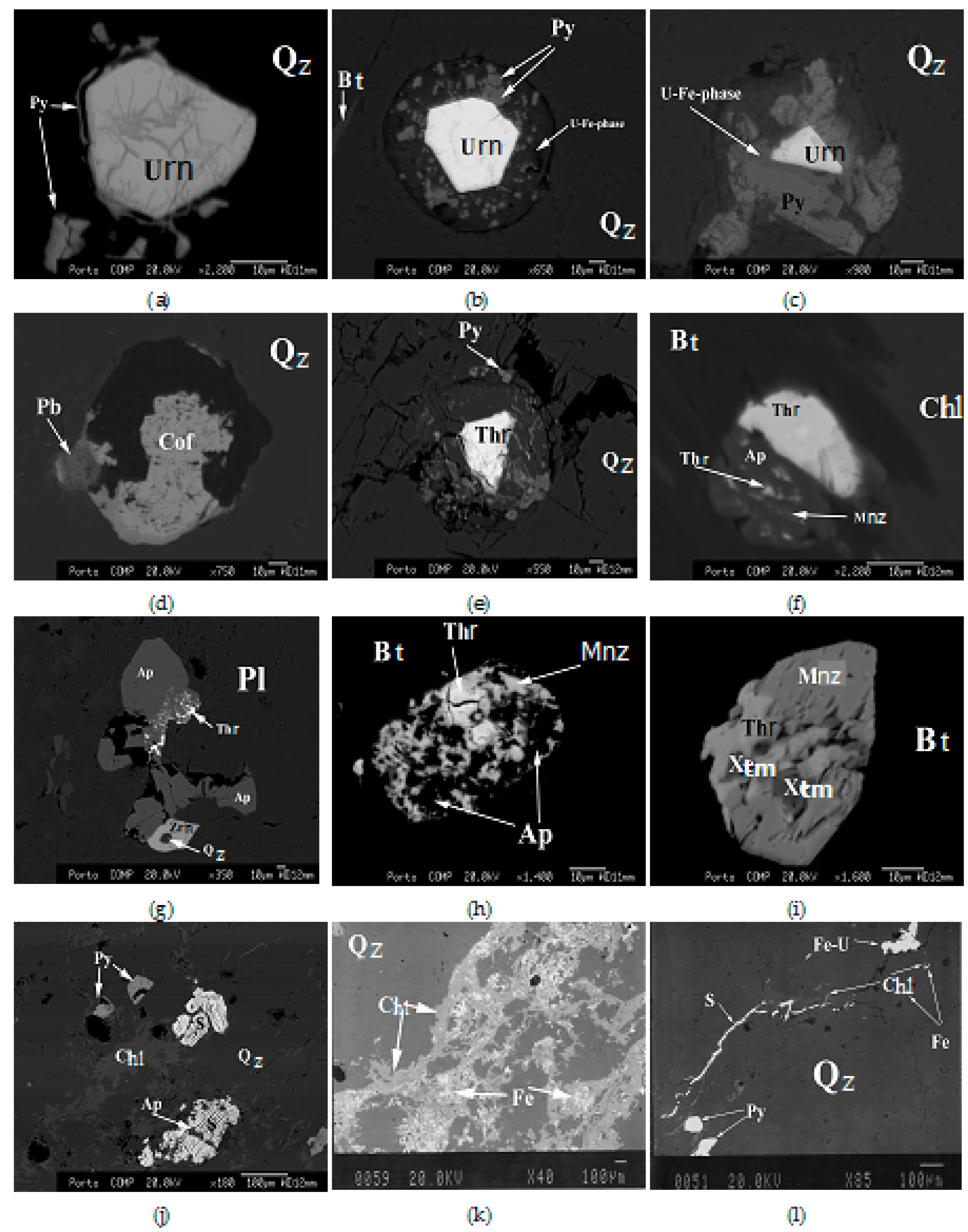
4. Petrographic Relations
4.1. Granite
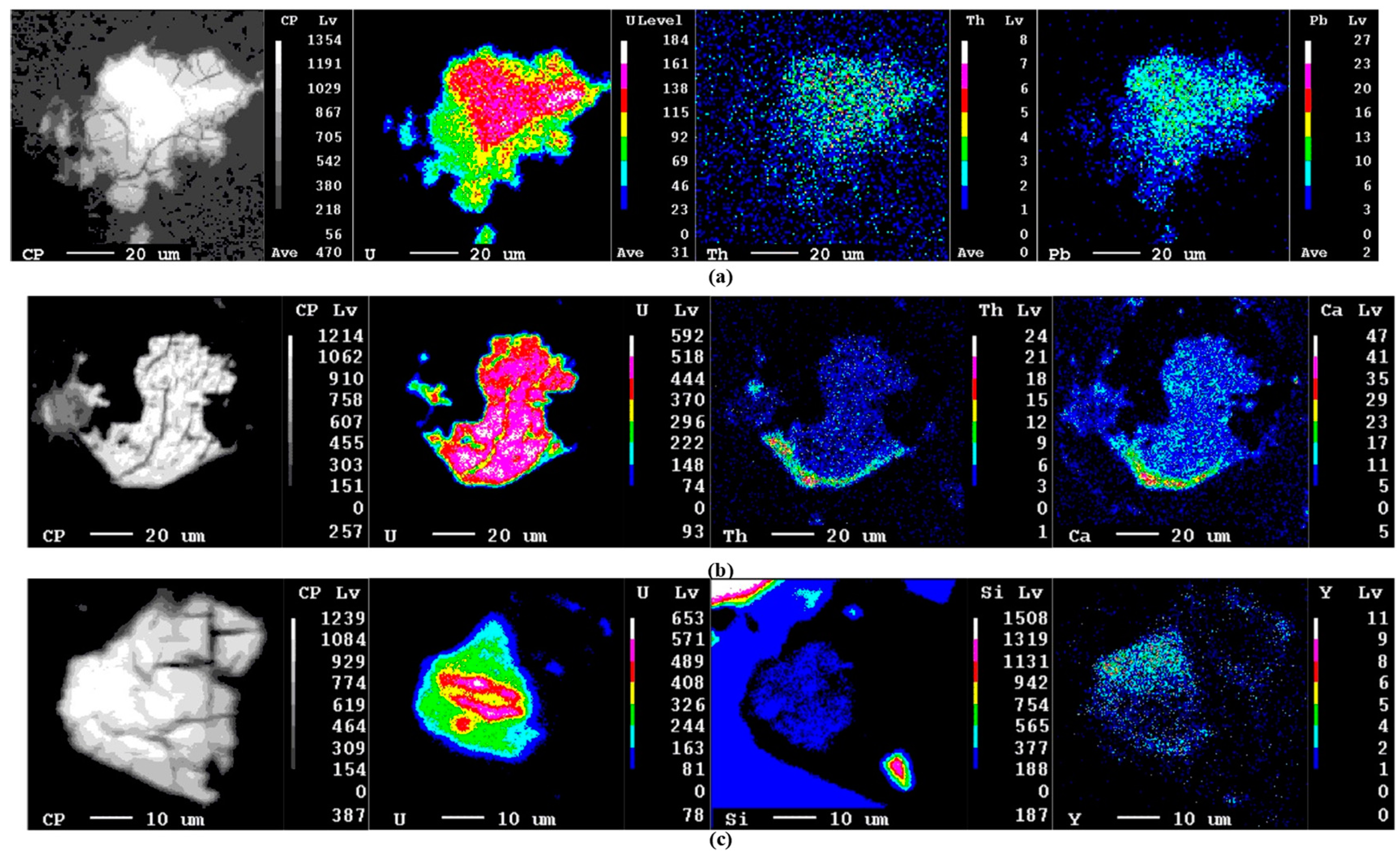
4.2. Mineralized Quartz Veins
5. Chemical Composition of U Minerals and U-bearing Minerals
5.1. Uraninite
5.2. Coffinite
5.3. Thorite
5.4. Zircon
5.5. Xenotime
5.6. Monazite
5.7. Apatite
5.8. Saleeite and Meta-Saleeite
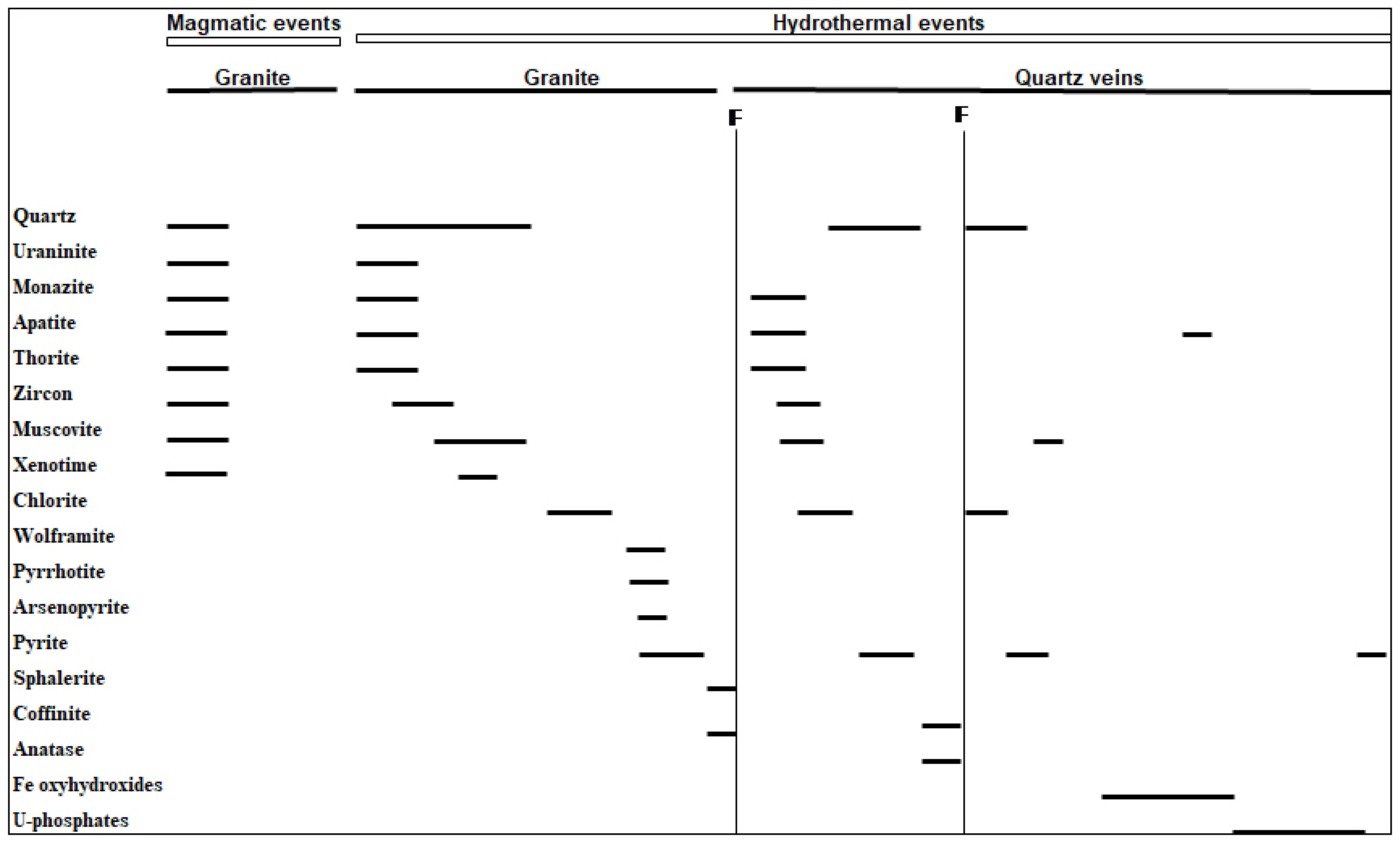
6. Discussion
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischer, M.; Mandarino, J.A. Glossary of Mineral Species; Mineralogical Record: Tucson, AZ, USA, 1995. [Google Scholar]
- Boekhout, F.; Gérard, M.; Kanzari, A.; Michel, A.; Déjeant, A.; Galoisy, L.; Calas, G.; Descostes, M. Uranium migration and retention during weathering of a granitic waste rock pile. Appl. Geochem. 2015, 58, 123–135. [Google Scholar] [CrossRef]
- Burns, P.C.; Finch, R.J. Uranium: Mineralogy, Geochemistry and the Environment; Mineralogical Society of America: Chantilly, VA, USA, 1999; Volume 38, p. 679. [Google Scholar]
- Finch, R.; Murakami, T. Systematics, Paragenesis of U minerals. In Uranium: Mineralogy, Geochemistry and the Environment; Mineralogical Society of America: Chantilly, VA, USA, 1999; Volume 38, pp. 91–180. [Google Scholar]
- Cotelo Neiva, J.M. Jazigos portugueses de minérios de urânio e sua génese. In Engineering Geology and Geological Resources; Cotelo Neiva, J., Ferreira, M.R.P.V., Eds.; Coimbra University Press: Coimbra, Portugal, 2003; Volume 1, pp. 15–76. [Google Scholar]
- Neiva, A.M.R.; Carvalho, P.C.S.; Antunes, I.M.H.R.; Santos, A.C.T.; Cabral Pinto, M.M.S. Spatial and temporal variability of surface water and groundwater before and after the remediation of a Portuguese uranium mine area. Chem. Erde-Geochem. 2015, 75, 345–356. [Google Scholar] [CrossRef]
- Kanzari, A.; Gerard, M.; Boekhout, F.; Galoisy, L.; Calas, G.; Descostes, M. Impact of incipient weathering on uranium migration in granitic waste rock piles from former U mines (Limousin, France). J. Geochem. Explor. 2017, 183, 114–126. [Google Scholar] [CrossRef]
- Cuney, M.; Friederich, M. Physicochemical and crystal-chemical controls on accessory mineral paragenesis in granitoids: Implications for uranium metallogenesis. Bull. Minerl. 1987, 110, 235–247. [Google Scholar]
- Cabral Pinto, M.M.C.; Silva, M.M.V.G.; Ferreira da Silva, E.A.; Dinis, P.A.; Rocha, F. Transfer processes of potentially toxic elements (PTE) from rocks to soils and the origin of PTE in soils: A case study on the island of Santiago (Cape Verde). J. Geochem. Explor. 2017. [Google Scholar] [CrossRef]
- Plant, J.A.; Simpson, P.R.; Smith, B.; Windley, B.F. Uranium ore deposits-products of the radioactive earth. In Uranium: Mineralogy, Geochemistry and the Environment; Burns, P.C., Finch, R., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1999; Volume 38, pp. 255–319. [Google Scholar]
- Förster, H.-J. The chemical composition of uraninite in Variscan granites of the Erzgebirge, Germany. Mineral. Mag. 1999, 63, 239–252. [Google Scholar] [CrossRef]
- Hecht, L.; Cuney, M. Hydrothermal alteration of monazite in the Precambrian crystalline basement of the Athabasca Basin (Saskatchewan, Canada): Implications for the formation of unconformity-related uranium deposits. Mineral. Depos. 2000, 35, 791–795. [Google Scholar] [CrossRef]
- Förster, H.-J. Composition and origin of intermediate solid solutions in the system thorite–xenotime–zircon–coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- Gaines, R.V.; Skinner, H.C.W.; Foord, E.E.; Mason, B.; Rosenzweig, G.A.; King, V.T.; Dowty, E. Dana’s New Mineralogy, 8th ed.; Wiley and Sons: New York, NY, USA, 1997; p. 1819. [Google Scholar]
- Cabral Pinto, M.M.S.; Silva, M.M.V.G.; Neiva, A.M.R. Geochemistry of U-bearing minerals from the Vale de Abrutiga uranium mine area, Central Portugal. J. Mineral. Geochem. 2008, 185, 183–198. [Google Scholar] [CrossRef]
- Hetherington, C.J.; Harlov, D.E. Metasomatic thorite and uraninite inclusions in xenotime and monazite from granitic pegmatites, Hidra anorthosite massif, southwestern Norway: Mechanics and fluid chemistry. Am. Mineral. 2008, 93, 806–820. [Google Scholar] [CrossRef]
- Abdelouas, A.; Lutze, W.; Nuttall, H.E. Uranium contamination in the subsurface; characterization and remediation. Rev. Mineral. Geochem. 1999, 38, 433–473. [Google Scholar]
- Kotzer, T.G.; Kyser, T.K. U and Pb isotopic and chemical variations in uraninite: Implications for determining the temporal and fluid history of ancient terranes. Am. Mineral. 1993, 78, 1262–1274. [Google Scholar]
- Murphy, W.M.; Shock, E.L. Environmental aqueous geochemistry of actinides. Rev. Mineral. 1999, 38, 221–253. [Google Scholar]
- Neiva, A.M.R.; Carvalho, P.C.S.; Antunes, I.M.H.R.; Cabral Pinto, M.M.S.C.; Santos, A.C.T.; Cunha, P.P.; Costa, M.M. Spatial variability of soils and stream sediments and the remediation effects in a Portuguese uranium mine area. Chem. Erde-Geochem. 2016, 76, 501–518. [Google Scholar] [CrossRef]
- Hsi, C.-K.D.; Langmuir, D. Adsorption of uranyl onto ferric oxyhydroxides: Aplication of the surface complexation site-binding model. Geochim. Cosmochim. Acta 1985, 49, 1931–1941. [Google Scholar]
- Murakami, T.; Ohnuhi, T.; Isobe, H. Mobility of uranium during weathering. Am. Mineral. 1997, 82, 888–899. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Asheley, T.P.M. Tailings dam seepage at the rehabilitated Mary Kathleen uranium mine, Australia. J. Geochem. Explor. 2005, 85, 19–137. [Google Scholar] [CrossRef]
- Matos Dias, J.M. Perspectivas geoeconómicas dos jazigos uraníferos portugueses. Geonovas 1982, 1, 33–39. [Google Scholar]
- Cabral Pinto, M.M.S.; Silva, M.M.V.G. The Vale de Abrutiga uranium phosphates mine, central Portugal. Chem. Erde-Geochem. 2007, 67, 251–252. [Google Scholar] [CrossRef]
- Pagel, M. Acteurs de Distribuition et Concentration de L’uranium et du Thorium Dans Quelques Granites de la Chaine Hercynienne d’Europe. Ph.D. Thesis, L’Institut National Polytechnique de Lorraine, Vandœuvre-lès-Nancy, France, 1981; p. 566.
- Pinto, M.M.S.C.; Silva, M.M.V.G.; Neiva, A.M.R. Pollution of Water and Stream Sediments associated with the Vale de Abrutiga Uranium mine, Central Portugal. J. Mine Water Environ. 2004, 23, 66–75. [Google Scholar] [CrossRef]
- Silva, M.M.V.G.; Neiva, A.M.R. Geochemistry of Hercynian peraluminous granites and their minerals from Carregal do Sal-Nelas-Lagares da Beira area, Central Portugal. Chem. Erde-Geochem. 2000, 59, 329–349. [Google Scholar]
- Silva, M.M.V.G.; Neiva, A.M.R.; Whitehouse, M.J. Geochemistry of enclaves and host granites from the Nelas area, central Portugal. Lithos 2000, 50, 153–170. [Google Scholar] [CrossRef]
- Poty, B.; Leroy, J.; Cathelineau, M.; Cuney, M.; Friedrich, M.; Lespinasse, M.; Turpin, L. Uranium deposits spatially related to granites in the French part of the Hercynian orogen. In Vein Type Uranium Deposits; IAEA-TC-361; International Atomic Energy Agency: Vienna, Austria, 1986; pp. 215–246. [Google Scholar]
- Smith, D.K., Jr. Uranium mineralogy. In Uranium Geochemistry, Mineralogy, Geology, Exploration and Resources; De Vivo, B., Ippolito, F., Capaldi, G., Simpson, P.R., Eds.; Institute of Mining and Metallurgy: London, UK, 1984; pp. 43–88. [Google Scholar]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Parks, G.A.; Pohl, D.C. Hydrothermal solubility of uraninite. Geochim. Cosmochim. Acta 1988, 52, 863–875. [Google Scholar] [CrossRef]
- Finch, R.J.; Ewing, R.C. The corrosion of uraninite under oxidizing conditions. J. Nucl. Mater. 1992, 190, 133–156. [Google Scholar] [CrossRef]
- Janeczek, J.; Ewing, R.C. Structural formula of uraninite. J. Nucl. Mater. 1992, 190, 128–132. [Google Scholar] [CrossRef]
- Pearcy, E.C.; Prikryl, J.D.; Murphy, W.M.; Leslie, B.W. Alteration of uraninite from the Nopal I deposit, Peña Blanca District, Chihuahua, Mexico, compared to degradation of spent nuclear fuel in the proposed U.S. high-level nuclear waste repository at Yucca Mountain, Nevada. Appl. Geochem. 1994, 9, 713–732. [Google Scholar] [CrossRef]
- Fayek, M.; Janeczek, J.; Ewing, R.C. Mineral chemistry and oxygen isotopic analyses of uraninite, pitchblende and uranium alteration minerals from the Cigar Lake deposit, Saskatchewan, Canada. Appl. Geochem. 1997, 12, 549–565. [Google Scholar] [CrossRef]
- Jensen, K.A.; Ewing, R.C.; Gauthier-Lafaye, F. Uraninite: A 2 Ga spent nuclear fuel from the natural fission reactor at Bangombe´ in Gabon, West Africa. Mater. Res. Soc. Symp. Proc. 1997, 465, 1209–1218. [Google Scholar] [CrossRef]
- Jerden, J.R.; Sinha, A.K. Phosphate based immobilization of uranium in an oxidizing bedrock aquifer. Appl. Geochem. 2003, 18, 823–843. [Google Scholar] [CrossRef]
- Evins, L.Z.; Jensen, A.K.; Ewing, R.C. Uraninite recrystallization and Pb loss in the Oklo and Bangombe natural fission reactors, Gabon. Geochim. Cosmochim. Acta 2005, 69, 1589–1606. [Google Scholar] [CrossRef]
- Bruno, J.; Ewing, R.C. Spent nuclear fuel. Elements 2006, 2, 343–349. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, A.; Ewing, R.C. Fate of trace elements during alteration of uraninite in a hydrothermal vein-type U-deposit from Marshall Pass, Colorado, USA. Geochim. Cosmochim. Acta 2007, 71, 4954–4973. [Google Scholar] [CrossRef]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Mineral. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Speer, J.A. The actinide orthosilicates. Rev. Mineral. 1982, 5, 113–135. [Google Scholar]
- Lumpkin, G.R.; Chakoumakos, B.C. Chemistry and radiation effects of thorite-group minerals from the Harding pegmatite, Taos County, New Mexico. Am. Mineral. 1988, 73, 1405–1419. [Google Scholar]
- Smits, G. (U,Th)-bearing silicates in reefs of the Witwatersrand, South Africa. Can. Mineral. 1989, 27, 643–655. [Google Scholar]
- Janeczek, J.; Ewing, R.C. X-ray powder diffraction study of annealed uraninite. J. Nucl. Mater. 1991, 185, 66–77. [Google Scholar] [CrossRef]
- Rubin, J.N.; Henry, C.H.; Price, J.G. Hydrothermal zircons and zircon overgrowths, Sierra Blanca Peaks, Texas. Am. Mineral. 1989, 74, 865–869. [Google Scholar]
- Harlov, D.E.; Aandersson, U.B.; Förster, H.-J.; Nyström, J.O.; Dulski, P.; Broman, C. Apatite–monazite relations in the Kiirunavaara magnetite–apatite ore, northern Sweden Chem. Geol. 2002, 191, 47–72. [Google Scholar]
- Harlov, D.E.; Wirth, R.; Förster, H.-J. An experimental study of dissolution–reprecipitation in fluorapatite: Fluid infiltration and the formation of monazite. Contrib. Mineral. Petrol. 2005, 150, 268–286. [Google Scholar] [CrossRef]
- Finger, F.; Broska, I.; Roberts, M.P.; Schermaier, A. Replacement of primary monazite by apatite-allanite- epidote coronas in an amphibolite facies granite gneiss from the eastern Alps. Am. Mineral. 1998, 83, 248–258. [Google Scholar] [CrossRef]
- Thomson, B.M.; Smith, C.L.; Busch, R.D.; Siegel, M.D. Removal of metals and radionuclides using apatite and other natural sorbents. J. Environ. Eng. 2003, 129, 492–499. [Google Scholar] [CrossRef]
- Wark, D.A.; Miller, C.F. Accessory mineral behavior during differentiation of a granite suite: Monazite, xenotime and zircon in the Sweetwater Wash pluton, southeastern California, U.S.A. Chem. Geol. 1993, 110, 49–67. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M. Crystal chemistry of the monazite and xenotime structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Broska, I.; Williams, C.T.; Geza Nagy, M.J. Alteration and breakdown of xenotime-(Y) and monazite- (Ce) in granitic rocks of the Western Carpathians, Slovakia. Lithos 2005, 82, 71–83. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Van Bronswijk, W.; Kurtz, R. Transport of uranium, thorium, and lead in metamict zircon under low-temperature hydrothermal conditions. Chem. Geol. 2002, 191, 141–154. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Kurtz, R.; Van Bronswijk, W.; Schleicher, H. Experimental hydrotermal alteration of partially metamict zircon. Amer. Mineral. 2003, 88, 1496–1513. [Google Scholar] [CrossRef]
- Horie, K.; Hidakab, H.; Gauthier-Lafaye, F. Elemental distribution in apatite, titanite and zircon during hydrothermal alteration: Durability of immobilization mineral phases for actinides. Phys. Chem. Earth 2008, 33, 962–968. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals—Orthosilicaes, 2nd ed.; The Geological Society: London, UK, 1997; Volume 1, p. 919. [Google Scholar]
- Bailey, S.W. Summary of recommendations of AIPEA nomenclature committee. Clay Miner. 1980, 15, 85–93. [Google Scholar] [CrossRef]
- Janeczek, J.; Ewing, R.C. Dissolution and alteration of uraninite under reducing conditions. J. Nucl. Mater. 1992, 190, 157–173. [Google Scholar] [CrossRef]
- Ordoñez-Regis, E.; Romero Guzmán, E.T.; Ordoñez Regil, E.N. Surface modification in natural fluorapatite after uranyl solution treatment. J. Radioanal. Nucl. Chem. 1999, 240, 541–554. [Google Scholar] [CrossRef]
- Arey, J.S.; Seaman, J.; Bertch, P.M. Immobilization of Uranium in Contaminated Sediments by Hydroxyapatite Addition. Environ. Sci. Technol. 1999, 33, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.J.; Miller, C.F.; D’Andrea, J.L.; Ayers, J.C.; Harrison, T.M.; Coath, C.D. Low temperature replacement of monazite in the Ireteba granite, Southern Nevada: Geochronological implications. Chem. Geol. 2000, 172, 95–112. [Google Scholar] [CrossRef]
- Jeanjean, J.; Rouchaud, J.C.; Tran, L.; Fedoroff, M. Sorption of uranium and other heavy metals on hydroxyapatite. J. Radioanal. Nucl. Chem. 2005, 201, 529–539. [Google Scholar] [CrossRef]
- Conca, J.L.; Wright, J. An Apatite II permeable reactive barrier to remediate groundwater containing Zn, Pb and Cd. Appl. Geochem. 2006, 21, 1288–1300. [Google Scholar] [CrossRef]
- Simon, F.G.; Biermann, V.; Peplinski, B. Uranium removal from groundwater using hydroxyapatite. Appl. Geochem. 2008, 23, 2137–2145. [Google Scholar] [CrossRef]
- Wellman, D.M.; Glovack, J.N.; Parker, K.; Richards, E.L.; Pierce, E.M. Sequestration and retention of uranium(VI) in the presence of hydroxylapatite under dynamic geochemical conditions. Environ. Chem. 2008, 5, 40–50. [Google Scholar] [CrossRef]

| Uraninite | Coffinite | Thorite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unaltered Granite | Altered Granite | Unaltered Granite | Altered Granite | ||||||||
| Mean | σ | Mean | σ | Mean | σ | Mean | σ | Mean | σ | ||
| Al2O3 | 0.04 | 0.07 | 0.07 | 0.11 | 0.05 | 0.13 | P2O5 | 3.01 | 2.26 | 3.60 | 2.49 |
| SiO2 | 0.14 | 0.16 | 0.51 | 0.40 | 16.00 | 1.64 | SiO2 | 14.49 | 3.13 | 14.74 | 3.02 |
| P2O5 | 0.03 | 0.03 | 0.01 | 0.01 | 0.04 | 0.08 | TiO2 | 0.15 | 0.13 | 0.10 | 0.09 |
| CaO | 0.09 | 0.10 | 0.03 | 0.09 | 0.90 | 0.83 | ZrO2 | 0.41 | 0.25 | 0.22 | 0.39 |
| ZrO2 | 0.04 | 0.02 | 0.02 | 0.03 | 0.06 | 0.19 | HfO2 | 0.04 | 0.02 | 0.03 | 0.02 |
| HfO2 | 0.02 | 0.05 | — | — | — | 0.01 | ThO2 | 54.00 | 4.66 | 54.5 | 4.55 |
| TiO2 | — | — | — | — | — | — | UO2 | 3.56 | 3.06 | 4.41 | 3.84 |
| Fe2O3 | 0.52 | 0.62 | 0.30 | 0.41 | 0.29 | 0.47 | Al2O3 | 0.37 | 0.25 | 0.34 | 0.27 |
| Y2O3 | 0.25 | 0.14 | 0.40 | 0.10 | 0.18 | 0.25 | Y2O3 | 1.29 | 1.66 | 0.28 | 0.28 |
| La2O3 | 0.01 | 0.02 | — | 0.01 | 0.01 | 0.03 | La2O3 | 0.23 | 0.25 | 0.14 | 0.14 |
| Ce2O3 | 0.08 | 0.06 | 0.06 | 0.04 | 0.09 | 0.09 | Ce2O3 | 0.83 | 1.01 | 0.19 | 0.08 |
| Pr2O3 | — | — | — | 0.01 | 0.01 | 0.02 | Pr2O3 | 0.18 | 0.17 | 0.05 | 0.05 |
| Nd2O3 | 0.02 | 0.03 | 0.05 | 0.03 | — | — | Nd2O3 | 0.57 | 0.71 | 0.22 | 0.16 |
| Sm2O3 | 0.01 | 0.01 | 0.01 | 0.02 | — | — | Sm2O3 | 0.05 | 0.05 | 0.04 | 0.04 |
| Gd2O3 | — | — | 0.03 | 0.01 | — | 0.01 | Gd2O3 | 0.15 | 0.25 | 0.11 | 0.13 |
| Dy2O3 | 0.04 | 0.01 | 0.0 | 0.03 | — | 0.01 | Dy2O3 | 0.55 | 0.19 | 0.58 | 0.07 |
| Ho2O3 | 0.03 | — | 0.04 | 0.02 | 0.01 | 0.01 | Ho2O3 | 0.03 | 0.04 | 0.01 | 0.01 |
| Er2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | Er2O3 | 0.04 | 0.06 | 0.02 | 0.01 |
| Yb2O3 | — | — | 0.02 | 0.02 | — | — | Yb2O3 | 0.09 | 0.09 | 0.03 | — |
| Lu2O3 | 0.01 | 0.01 | — | — | — | 0.01 | Lu2O3 | 0.04 | 0.05 | 0.03 | — |
| PbO | 3.82 | 0.17 | 3.67 | 0.05 | 0.63 | 0.75 | FeO | 1.14 | 0.76 | 1.45 | 1.43 |
| ThO2 | 1.57 | 0.55 | 1.51 | 0.83 | 0.61 | 0.96 | CaO | 1.92 | 0.56 | 2.07 | 0.92 |
| UO2 | 91.98 | 1.76 | 89.57 | 1.31 | 76.56 | 5.17 | PbO | 0.79 | 1.03 | 1.42 | 1.23 |
| F | 0.12 | 0.22 | 0.42 | 0.11 | 0.34 | 0.06 | F | 0.40 | 0.30 | 0.37 | 0.55 |
| Total | 98.84 | 1.40 | 96.82 | 1.30 | 95.67 | 3.86 | Total | 84.35 | 4.09 | 87.96 | 3.008 |
| Al | 0.002 | 0.004 | 0.004 | 0.006 | 0.003 | 0.008 | P | 0.147 | 0.097 | 0.202 | 0.124 |
| Si | 0.007 | 0.007 | 0.023 | 0.018 | 0.933 | 0.071 | Si | 0.839 | 0.139 | 0.787 | 0.193 |
| P | 0.001 | 0.001 | — | — | 0.002 | 0.004 | Ti | 0.007 | 0.006 | 0.007 | 0.004 |
| Ca | 0.004 | 0.005 | 0.001 | 0.005 | 0.055 | 0.050 | Zr | 0.012 | 0.007 | 0.037 | 0.013 |
| Zr | 0.001 | 0.001 | — | 0.001 | 0.002 | 0.006 | Hf | — | 0.000 | 0.001 | — |
| Hf | — | 0.001 | — | — | — | — | Th | 0.710 | 0.129 | 0.660 | 0.082 |
| Ti | — | — | — | — | — | — | U | 0.046 | 0.045 | 0.033 | 0.050 |
| Fe | 0.020 | 0.023 | 0.011 | 0.016 | 0.014 | 0.024 | Al | 0.025 | 0.019 | 0.031 | 0.017 |
| Y | 0.006 | 0.004 | 0.010 | 0.002 | 0.006 | 0.008 | Y | 0.040 | 0.053 | 0.032 | 0.009 |
| La | — | — | — | — | — | 0.001 | La | 0.005 | 0.005 | 0.006 | 0.003 |
| Ce | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | Ce | 0.018 | 0.019 | 0.019 | 0.002 |
| Pr | — | — | — | — | — | — | Pr | 0.004 | 0.003 | 0.003 | 0.001 |
| Nd | — | 0.001 | 0.001 | 0.001 | — | — | Nd | 0.012 | 0.013 | 0.014 | 0.003 |
| Sm | — | — | — | — | — | — | Sm | 0.001 | 0.001 | 0.00 | 0.00 |
| Gd | — | — | 0.001 | — | — | — | Gd | 0.003 | 0.004 | 0.01 | 0.00 |
| Dy | 0.001 | — | 0.001 | — | — | — | Dy | 0.010 | 0.005 | 0.01 | 0.00 |
| Ho | — | — | 0.001 | — | — | — | Ho | 0.001 | — | 0.00 | — |
| Er | — | — | — | — | — | — | Er | 0.001 | 0.001 | 0.00 | — |
| Yb | — | — | — | — | — | — | Yb | 0.002 | 0.002 | 0.00 | — |
| Lu | — | — | — | — | — | — | Lu | 0.001 | — | 0.00 | — |
| Pb | 0.047 | 0.002 | 0.045 | 0.001 | 0.010 | 0.012 | Fe | 0.055 | 0.038 | 0.056 | 0.064 |
| Th | 0.016 | 0.006 | 0.016 | 0.009 | 0.008 | 0.013 | Ca | 0.119 | 0.033 | 0.150 | 0.051 |
| U | 0.927 | 0.022 | 0.903 | 0.014 | 0.995 | 0.075 | Pb | 0.012 | 0.015 | 0.012 | 0.019 |
| F | 0.017 | 0.030 | 0.060 | 0.016 | 0.060 | 0.023 | F | 0.074 | 0.080 | 0.07 | 0.09 |
| Total | 1.05 | 0.02 | 1.08 | 0.02 | 2.07 | 0.02 | Total | 2.142 | 0.0774 | 2.1433 | 0.1 |
| n = 13 | n = 13 | n = 14 | n = 15 | n = 17 | |||||||
| Zircon | Xenotime | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unaltered Granite | Altered Granite | Quartz Veins | Unaltered Granite | Altered Granite | Quartz Veins | ||||||||
| Mean | σ | Mean | σ | Mean | σ | Mean | σ | Mean | σ | Mean | σ | ||
| SiO2 | 31.50 | 0.89 | 30.56 | 1.14 | 34.15 | 1.78 | P2O5 | 33.68 | 0.56 | 34.02 | 0.38 | 32.85 | 0.75 |
| ZrO2 | 65.24 | 2.78 | 61.29 | 3.21 | 61.74 | 1.54 | SiO2 | 0.79 | 0.20 | 0.71 | 0.16 | n.d. | n.d. |
| HfO2 | 1.42 | 0.23 | 1.31 | 0.37 | 1.31 | 0.21 | TiO2 | — | — | — | — | n.d. | n.d. |
| TiO2 | 0.01 | 0.02 | 0.03 | 0.02 | 0.05 | 0.07 | ZrO2 | — | — | — | — | 0.06 | 0.16 |
| Al2O3 | 0.04 | 0.17 | 0.31 | 0.43 | 0.24 | 0.27 | HfO | 0.24 | 0.02 | 0.26 | 0.02 | — | — |
| Fe2O3 | 0.20 | 0.16 | 0.30 | 0.19 | 0.31 | 0.29 | ThO2 | 0.15 | 0.10 | 0.13 | 0.09 | 0.44 | 0.21 |
| MgO | 0.02 | 0.07 | 0.07 | 0.13 | 0.14 | 0.04 | UO2 | 2.35 | 0.49 | 1.57 | 0.39 | 0.45 | 0.37 |
| MnO | 0.02 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 | Y2O3 | 38.90 | 0.29 | 39.29 | 0.60 | 43.49 | 1.01 |
| WO3 | 0.05 | 0.10 | 0.11 | 0.10 | 0.07 | 0.05 | La2O3 | — | — | — | — | — | — |
| K2O | 0.01 | 0.02 | 0.02 | 0.02 | 0.12 | 0.12 | Ce2O3 | 0.06 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 |
| Y2O3 | 0.12 | 0.23 | 0.55 | 0.30 | 0.32 | 0.42 | Pr2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.07 |
| La2O3 | 0.10 | 0.09 | 0.14 | 0.08 | 0.04 | 0.03 | Nd2O3 | 0.37 | 0.05 | 0.35 | 0.04 | 0.12 | 0.03 |
| Ce2O3 | 0.07 | 0.13 | 0.14 | 0.14 | 0.05 | 0.03 | Sm2O3 | 0.63 | 0.05 | 0.64 | 0.11 | 0.53 | 0.09 |
| CaO | 0.07 | 0.24 | 0.36 | 0.51 | 0.05 | 0.13 | Eu2O3 | n.d. | n.d. | n.d. | n.d. | 0.29 | 0.10 |
| PbO | 0.07 | 0.11 | 0.23 | 0.16 | 0.15 | 0.11 | Gd2O3 | 1.91 | 0.15 | 2.00 | 0.35 | 3.27 | 0.58 |
| UO2 | 0.05 | 0.11 | 0.22 | 0.15 | 0.13 | 0.21 | Tb2O3 | n.d. | n.d. | n.d. | n.d. | 0.85 | 0.07 |
| ThO2 | 0.05 | 0.07 | 0.08 | 0.09 | 0.06 | 0.06 | Dy2O3 | 5.47 | 0.35 | 5.51 | 0.55 | 6.54 | 0.13 |
| P2O5 | 0.12 | 0.14 | 0.40 | 0.21 | 0.17 | 0.17 | Ho2O3 | 1.07 | 0.03 | 1.11 | 0.04 | 2.74 | 0.22 |
| Total | 99.16 | 2.89 | 96.17 | 3.31 | 99.16 | 2.38 | Er2O3 | 2.34 | 0.28 | 2.36 | 0.43 | 3.61 | 0.17 |
| Tm2O3 | n.d. | n.d. | n.d. | n.d. | 0.50 | 0.06 | |||||||
| Si | 3.927 | 0.056 | 3.922 | 0.068 | 4.185 | 0.110 | Yb2O3 | 3.14 | 0.25 | 3.10 | 0.44 | 2.74 | 0.26 |
| P | 0.013 | 0.014 | 0.043 | 0.022 | — | — | Lu2O3 | 0.67 | 0.03 | 0.64 | 0.06 | 0.93 | 0.08 |
| Al iv | 0.006 | 0.013 | 0.047 | 0.033 | 0.035 | 0.038 | FeO | 0.13 | 0.04 | 0.11 | 0.07 | n.d. | n.d. |
| Σ | 3.945 | 0.053 | 4.01 | 0.05 | 4.220 | 0.112 | CaO | 0.28 | 0.06 | 0.24 | 0.05 | n.d. | n.d. |
| Al vi | — | 0.020 | — | 0.067 | — | — | PbO | 0.15 | 0.06 | 0.15 | 0.07 | n.d. | n.d. |
| Zr | 3.966 | 0.074 | 3.835 | 0.085 | 3.690 | 0.106 | F | 0.02 | 0.02 | 0.03 | 0.04 | n.d. | n.d. |
| Hf | 0.051 | 0.008 | 0.048 | 0.014 | 0.046 | 0.010 | Total | 92.36 | 0.85 | 92.23 | 0.80 | 99.48 | 0.66 |
| Fe3+ | 0.019 | 0.017 | 0.029 | 0.020 | 0.028 | 0.028 | |||||||
| Mg | 0.004 | 0.013 | 0.013 | 0.024 | 0.026 | 0.012 | P | 4.021 | 0.044 | 4.044 | 0.03 | 3.845 | 0.041 |
| Ti | 0.001 | 0.002 | 0.003 | 0.002 | 0.001 | 0.001 | Si | 0.111 | 0.027 | 0.100 | 0.02 | n.d. | n.d. |
| Pb | 0.002 | 0.004 | 0.008 | 0.006 | 0.005 | 0.003 | Ti | — | — | — | — | n.d. | n.d. |
| U | 0.001 | 0.003 | 0.006 | 0.005 | 0.004 | 0.005 | Zr | — | — | — | — | 0.004 | 0.010 |
| Th | 0.001 | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | Hf | 0.011 | 0.001 | 0.012 | 0.001 | — | — |
| Y | 0.008 | 0.015 | 0.038 | 0.020 | 0.029 | 0.017 | Th | 0.005 | 0.003 | 0.004 | 0.003 | 0.014 | 0.007 |
| La | 0.004 | 0.004 | 0.005 | 0.003 | 0.002 | 0.001 | U | 0.074 | 0.015 | 0.049 | 0.012 | 0.014 | 0.011 |
| Ce | 0.003 | 0.003 | 0.003 | 0.003 | 0.001 | 0.001 | Y | 2.920 | 0.020 | 2.935 | 0.027 | 3.200 | 0.059 |
| Ca | 0.009 | 0.036 | 0.049 | 0.075 | 0.007 | 0.015 | La | — | — | — | — | — | — |
| Σ | 4.07 | 0.06 | 4.04 | 0.07 | 3.830 | 0.113 | Ce | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 |
| N | n = 72 | n = 20 | n = 55 | Pr | — | — | — | 0.001 | 0.003 | 0.004 | |||
| Nd | 0.018 | 0.002 | 0.018 | 0.002 | 0.006 | 0.001 | |||||||
| Sm | 0.030 | 0.003 | 0.031 | 0.01 | 0.025 | 0.005 | |||||||
| Eu | n.d. | n.d. | n.d. | n.d. | 0.014 | 0.005 | |||||||
| Gd | 0.089 | 0.008 | 0.093 | 0.017 | 0.150 | 0.028 | |||||||
| Tb | n.d. | n.d. | n.d. | n.d. | 0.038 | 0.003 | |||||||
| Dy | 0.248 | 0.018 | 0.249 | 0.026 | 0.291 | 0.008 | |||||||
| Ho | 0.048 | 0.001 | 0.050 | 0.002 | 0.120 | 0.011 | |||||||
| Er | 0.140 | 0.016 | 0.141 | 0.025 | 0.212 | 0.008 | |||||||
| Tm | n.d. | n.d. | n.d. | n.d. | 0.022 | 0.003 | |||||||
| Yb | 0.135 | 0.010 | 0.133 | 0.018 | 0.116 | 0.011 | |||||||
| Lu | 0.029 | 0.001 | 0.027 | 0.002 | 0.039 | 0.003 | |||||||
| Fe | 0.016 | 0.005 | 0.013 | 0.009 | n.d. | n.d. | |||||||
| Ca | 0.042 | 0.009 | 0.035 | 0.008 | n.d. | n.d. | |||||||
| Pb | 0.006 | 0.002 | 0.006 | 0.003 | n.d. | n.d. | |||||||
| F | 0.008 | 0.009 | 0.013 | 0.016 | n.d. | n.d. | |||||||
| Total | 7.96 | 0.025 | 7.95 | 0.008 | 8.09 | 0.027 | |||||||
| n = 7 | n = 9 | n = 6 | |||||||||||
| Monazite | Apatite | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Granite | Quartz Veins | Granite | Quartz Veins | |||||||||||
| Unaltered Granite | Altered Granite | Unaltered Granite | Altered Granite | |||||||||||
| Mean | σ | Mean | σ | Mean | σ | Mean | σ | Mean | σ | Mean | σ | Crystal A | ||
| P2O5 | 29.44 | 0.73 | 30.06 | 0.84 | 28.54 | 1.41 | FeO | 0.51 | 0.25 | 0.66 | 0.50 | 0.40 | 0.33 | 0.55 |
| SiO2 | 0.20 | 0.13 | 0.37 | 0.32 | 0.41 | 0.50 | MnO | 0.55 | 0.15 | 0.08 | 0.09 | 0.03 | 0.03 | n.d. |
| Ce2O3 | 25.33 | 1.47 | 25.84 | 1.31 | 29.99 | 1.18 | MgO | 0.03 | 0.02 | 0.21 | 0.22 | 0.09 | 0.04 | 0.19 |
| La2O3 | 10.45 | 1.05 | 11.08 | 0.89 | 13.47 | 1.92 | CaO | 52.28 | 1.73 | 49.25 | 2.07 | 53.19 | 0.59 | 53.98 |
| Nd2O3 | 10.84 | 0.58 | 10.85 | 0.86 | 13.21 | 1.53 | SrO | 0.03 | 0.03 | 0.04 | 0.02 | n.d. | n.d. | n.d. |
| Pr2O3 | 2.57 | 0.51 | 2.63 | 0.20 | 2.64 | 0.77 | Na2O | 0.11 | 0.04 | 0.05 | 0.04 | 0.01 | — | 0.01 |
| Sm2O3 | 1.68 | 1.18 | — | — | 1.65 | 1.07 | K2O | 0.04 | 0.04 | 0.21 | 0.13 | — | — | — |
| Gd2O3 | 2.86 | 0.32 | 3.04 | 0.29 | n.d. | n.d. | P2O5 | 41.17 | 0.48 | 36.82 | 2.69 | 41.39 | 1.34 | 38.47 |
| Dy2O3 | 0.91 | 0.21 | 0.67 | 0.14 | n.d. | n.d. | SiO2 | 0.16 | 0.20 | 1.56 | 1.00 | — | — | — |
| Ho2O3 | — | — | — | — | n.d. | n.d. | TiO2 | — | 0.01 | 0.02 | 0.03 | 0.01 | 0.02 | n.d. |
| Er2O3 | — | — | — | 0.01 | n.d. | n.d. | Al2O3 | 0.05 | 0.09 | 0.56 | 0.29 | 0.04 | 0.07 | 0.04 |
| Yb2O3 | — | 0.01 | — | — | n.d. | n.d. | BaO | — | — | — | — | n.d. | n.d. | n.d. |
| Lu2O3 | 0.07 | 0.03 | 0.05 | 0.02 | n.d. | n.d. | PbO | 0.01 | 0.03 | — | — | — | — | 0.01 |
| Fe2O3 | 0.07 | 0.12 | 0.08 | 0.15 | 0.46 | 1.09 | UO2 | 0.01 | 0.01 | — | 0.01 | 0.13 | 0.09 | 1.09 |
| CaO | 1.73 | 0.30 | 1.42 | 0.31 | 0.24 | 0.17 | ThO2 | 0.01 | 0.01 | 0.56 | 0.48 | — | — | — |
| PbO | 0.18 | 0.13 | 0.17 | 0.08 | 0.03 | 0.07 | Y2O3 | 0.29 | 0.07 | 0.47 | 0.23 | — | — | — |
| Al2O3 | 0.05 | 0.11 | 0.14 | 0.16 | 0.13 | 0.32 | La2O3 | 0.04 | 0.06 | 0.31 | 0.25 | — | — | 0.02 |
| Y2O3 | 2.14 | 0.75 | 2.38 | 0.52 | 0.57 | 0.29 | Ce2O3 | 0.11 | 0.06 | 0.52 | 0.30 | — | — | 0.02 |
| ThO2 | 7.91 | 1.01 | 6.84 | 0.73 | 1.14 | 0.98 | F | 2.24 | 0.30 | 2.08 | 0.47 | 2.76 | 0.13 | 2.62 |
| U3O8 | 1.26 | 0.53 | 0.77 | 0.56 | 0.19 | 0.10 | Cl | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| ZrO2 | 0.01 | 0.01 | 0.02 | 0.02 | n.d. | n.d. | Total | 97.67 | 1.97 | 93.42 | 2.75 | 98.06 | 1.49 | 97.01 |
| HfO | 0.02 | 0.01 | 0.01 | 0.01 | n.d. | n.d. | O≡F | 0.95 | 0.88 | 1.16 | 1.10 | |||
| MgO | n.d. | n.d. | n.d. | n.d. | 0.01 | 0.02 | O≡Cl | — | — | — | n.d. | |||
| F | 0.27 | 0.17 | 0.20 | 0.12 | 0.43 | 0.18 | Total | 96.72 | 92.55 | 96.90 | 95.91 | |||
| Total | 97.99 | 1.75 | 96.62 | 3.42 | 93.11 | 2.11 | ||||||||
| O≡F | 0.12 | 0.07 | 0.08 | 0.05 | 0.18 | P | 5.883 | 0.063 | 5.628 | 0.212 | 5.859 | 0.093 | 5.629 | |
| Total | 97.88 | 1.75 | 96.54 | 3.45 | 92.93 | Si | 0.028 | 0.034 | 0.282 | 0.184 | — | — | — | |
| Σ | 5.91 | 0.06 | 5.91 | 5.86 | 0.09 | 5.63 | ||||||||
| P | 3.952 | 0.037 | 4.012 | 0.040 | 4.029 | 0.106 | ||||||||
| Si | 0.032 | 0.021 | 0.059 | 0.053 | 0.068 | 0.081 | Al | 0.009 | 0.017 | 0.120 | 0.061 | 0.008 | 0.014 | 0.007 |
| P + Si | 3.98 | 4.07 | 4.10 | Mg | 0.008 | 0.006 | 0.056 | 0.060 | 0.022 | 0.011 | 0.049 | |||
| Ce | 1.470 | 0.098 | 1.492 | 0.072 | 1.831 | 0.080 | Fe | 0.072 | 0.035 | 0.099 | 0.076 | 0.056 | 0.046 | 0.080 |
| La | 0.611 | 0.069 | 0.645 | 0.058 | 0.828 | 0.115 | Mn | 0.078 | 0.021 | 0.012 | 0.013 | 0.004 | 0.004 | n.d. |
| Nd | 0.614 | 0.032 | 0.611 | 0.036 | 0.787 | 0.104 | Na | 0.037 | 0.012 | 0.018 | 0.015 | 0.003 | 0.001 | 0.003 |
| Pr | 0.148 | 0.030 | 0.151 | 0.009 | 0.160 | 0.047 | K | 0.008 | 0.008 | 0.049 | 0.029 | — | — | — |
| Sm | 0.092 | 0.065 | — | — | 0.095 | 0.061 | Ca | 9.453 | 0.182 | 9.525 | 0.431 | 9.529 | 0.193 | 9.996 |
| Gd | 0.150 | 0.068 | 0.159 | 0.013 | n.d. | n.d. | Sr | — | — | — | — | n.d. | n.d. | n.d. |
| Dy | 0.046 | 0.021 | 0.034 | 0.006 | n.d. | n.d. | Ba | — | — | — | — | n.d. | n.d. | n.d. |
| Ho | — | — | — | — | n.d. | n.d. | Pb | 0.001 | 0.001 | — | — | — | — | — |
| Er | — | — | — | — | n.d. | n.d. | U | — | — | — | — | 0.005 | 0.010 | 0.042 |
| Yb | — | — | — | — | n.d. | n.d. | Th | — | — | 0.023 | 0.020 | — | — | — |
| Lu | 0.003 | 0.002 | 0.002 | 0.001 | n.d. | n.d. | Σ | 9.67 | 0.23 | 9.90 | 0.50 | 9.63 | 0.23 | 10.18 |
| Fe | 0.008 | 0.014 | 0.009 | 0.018 | 0.058 | 0.135 | ||||||||
| Ca | 0.294 | 0.051 | 0.240 | 0.048 | 0.043 | 0.030 | F | 1.198 | 0.157 | 1.187 | 0.292 | 1.457 | 0.139 | 1.43 |
| Pb | 0.008 | 0.006 | 0.007 | 0.003 | 0.001 | 0.003 | Cl | 0.007 | 0.006 | 0.003 | 0.004 | 0.002 | 0.002 | 0.002 |
| Al | 0.009 | 0.020 | 0.026 | 0.029 | 0.026 | 0.061 | OH | 0.795 | 0.158 | 0.810 | 0.293 | 0.541 | 0.072 | 0.568 |
| Y | 0.181 | 0.062 | 0.200 | 0.040 | 0.051 | 0.026 | n = 27 | n = 14 | n = 16 | |||||
| Th | 0.285 | 0.039 | 0.245 | 0.024 | 0.043 | 0.037 | ||||||||
| U | 0.043 | 0.018 | 0.026 | 0.018 | 0.007 | 0.004 | ||||||||
| Zr | 0.001 | 0.001 | 0.001 | 0.001 | n.d. | n.d. | ||||||||
| Hf | 0.001 | — | 0.001 | — | n.d. | n.d. | ||||||||
| Mg | n.d. | n.d. | n.d. | n.d. | 0.002 | 0.006 | ||||||||
| F | 0.135 | 0.088 | 0.097 | 0.066 | 0.094 | 0.021 | ||||||||
| Total | 4.10 | 3.95 | 3.93 | |||||||||||
| n = 18 | n = 12 | n = 23 | ||||||||||||
| U-Phosphates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mineralized Quartz Veins | ||||||||
| Associated with Apatite | ||||||||
| Meta-Saleeite | Saleeite | Meta-Saleeite | Saleeite | |||||
| Mean | σ | Mean | σ | Mean | σ | Mean | σ | |
| FeO | 0.72 | 0.38 | 0.77 | 0.75 | 0.82 | 0.53 | 0.95 | 0.71 |
| MnO | 0.01 | 0.01 | — | — | — | — | 0.04 | 0.06 |
| MgO | 4.27 | 0.24 | 4.32 | 0.20 | 4.22 | 0.29 | 4.16 | 0.32 |
| TiO2 | 0.03 | 0.03 | 0.05 | 0.05 | 0.05 | 0.04 | 0.03 | 0.02 |
| CaO | 0.69 | 0.26 | 0.80 | 0.24 | 0.07 | 0.05 | 0.13 | 0.08 |
| ZnO | n.d. | n.d. | n.d. | n.d. | 0.18 | 0.03 | 0.23 | 0.03 |
| Y2O3 | n.d. | n.d. | n.d. | n.d. | 0.01 | 0.01 | 0.02 | 0.04 |
| PbO | 0.05 | 0.08 | 0.01 | 0.01 | 0.10 | 0.13 | 0.02 | 0.03 |
| ThO2 | 0.01 | 0.01 | 0.01 | 0.01 | — | 0.01 | 0.03 | 0.05 |
| UO2 | 60.39 | 2.04 | 58.74 | 2.32 | 61.48 | 0.87 | 59.88 | 1.24 |
| La2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | — | — |
| Ce2O3 | 0.03 | 0.04 | — | — | 0.02 | 0.03 | 0.03 | 0.03 |
| Na2O | n.d. | n.d. | n.d. | n.d. | 0.08 | 0.17 | 0.04 | 0.04 |
| K2O | n.d. | n.d. | n.d. | n.d. | 0.06 | 0.07 | 0.01 | 0.02 |
| SiO2 | 0.06 | 0.08 | 0.01 | 0.02 | 0.47 | 0.37 | 0.23 | 0.26 |
| Al2O3 | 0.15 | 0.07 | 0.24 | 0.14 | 0.04 | 0.05 | 0.13 | 0.10 |
| P2O5 | 16.91 | 0.88 | 15.53 | 0.51 | 15.92 | 0.23 | 15.14 | 0.63 |
| F | 0.46 | 0.14 | 0.48 | 0.09 | n.d. | n.d. | n.d. | n.d. |
| Total | 83.79 | 1.01 | 80.95 | 0.59 | 83.42 | 0.94 | 81.07 | 1.85 |
| Fe | 0.084 | 0.046 | 0.097 | 0.098 | 0.099 | 0.063 | 0.124 | 0.086 |
| Mn | 0.001 | 0.001 | — | — | — | — | 0.005 | 0.006 |
| Mg | 0.885 | 0.071 | 0.979 | 0.054 | 0.901 | 0.071 | 0.967 | 0.080 |
| Ti | 0.003 | 0.004 | 0.005 | 0.005 | 0.006 | 0.004 | 0.004 | 0.002 |
| Ca | 0.102 | 0.035 | 0.130 | 0.043 | 0.010 | 0.008 | 0.021 | 0.013 |
| Zn | n.d. | n.d. | n.d. | n.d. | 0.039 | 0.020 | 0.054 | 0.028 |
| Y | n.d. | n.d. | n.d. | n.d. | 0.001 | 0.001 | 0.002 | 0.003 |
| Pb | 0.002 | 0.003 | — | — | 0.004 | 0.005 | 0.001 | 0.001 |
| Th | — | — | — | — | — | — | 0.001 | 0.002 |
| U | 1.869 | 0.161 | 1.988 | 0.129 | 1.961 | 0.061 | 2.080 | 0.058 |
| La | — | — | — | — | 0.002 | 0.002 | — | — |
| Ce | 0.001 | 0.002 | — | — | 0.001 | 0.001 | 0.002 | 0.002 |
| Na | n.d. | n.d. | n.d. | n.d. | 0.022 | 0.047 | 0.012 | 0.011 |
| K | n.d. | n.d. | n.d. | n.d. | 0.010 | 0.014 | 0.002 | 0.004 |
| Si | 0.008 | 0.011 | 0.001 | 0.003 | 0.068 | 0.050 | 0.036 | 0.039 |
| Al | 0.025 | 0.011 | 0.043 | 0.025 | 0.007 | 0.008 | 0.024 | 0.018 |
| P | 1.992 | 0.011 | 1.999 | 0.003 | 1.932 | 0.050 | 2.000 | 0.039 |
| H2O | 8 | 10 | 8 | 10 | ||||
| P + Si | 2.00 | 2.00 | 2.00 | 2.00 | ||||
| Σ | 1.07 | 1.21 | 1.01 | 1.12 | ||||
| U | 1.87 | 1.99 | 1.96 | 2.08 | ||||
| n = 10 | n = 8 | n = 12 | n = 12 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral Pinto, M.M.S.; Silva, M.M.V.G.; Neiva, A.M.R.; Guimarães, F.; Silva, P.B. Release, Migration, Sorption, and (Re)Precipitation of U during Peraluminous Granite Alteration under Oxidizing Conditions in Central Portugal. Geosciences 2018, 8, 95. https://doi.org/10.3390/geosciences8030095
Cabral Pinto MMS, Silva MMVG, Neiva AMR, Guimarães F, Silva PB. Release, Migration, Sorption, and (Re)Precipitation of U during Peraluminous Granite Alteration under Oxidizing Conditions in Central Portugal. Geosciences. 2018; 8(3):95. https://doi.org/10.3390/geosciences8030095
Chicago/Turabian StyleCabral Pinto, Marina M. S., Maria M. V. G. Silva, Ana M. R. Neiva, Fernanda Guimarães, and Paulo B. Silva. 2018. "Release, Migration, Sorption, and (Re)Precipitation of U during Peraluminous Granite Alteration under Oxidizing Conditions in Central Portugal" Geosciences 8, no. 3: 95. https://doi.org/10.3390/geosciences8030095