Role of Warming in Destabilization of Intrapermafrost Gas Hydrates in the Arctic Shelf: Experimental Modeling
Abstract
:1. Introduction
2. Methods
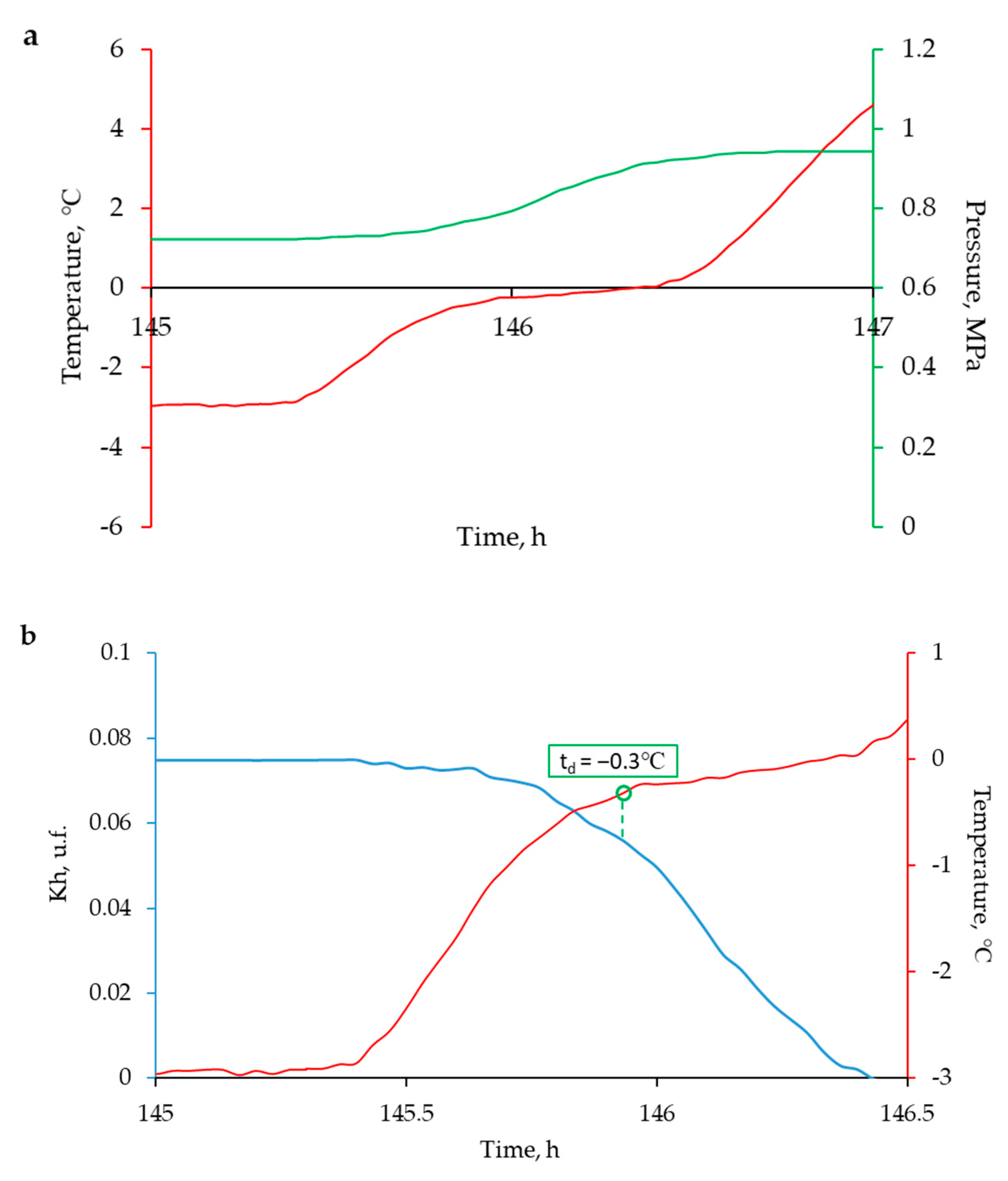
3. Experimental Results
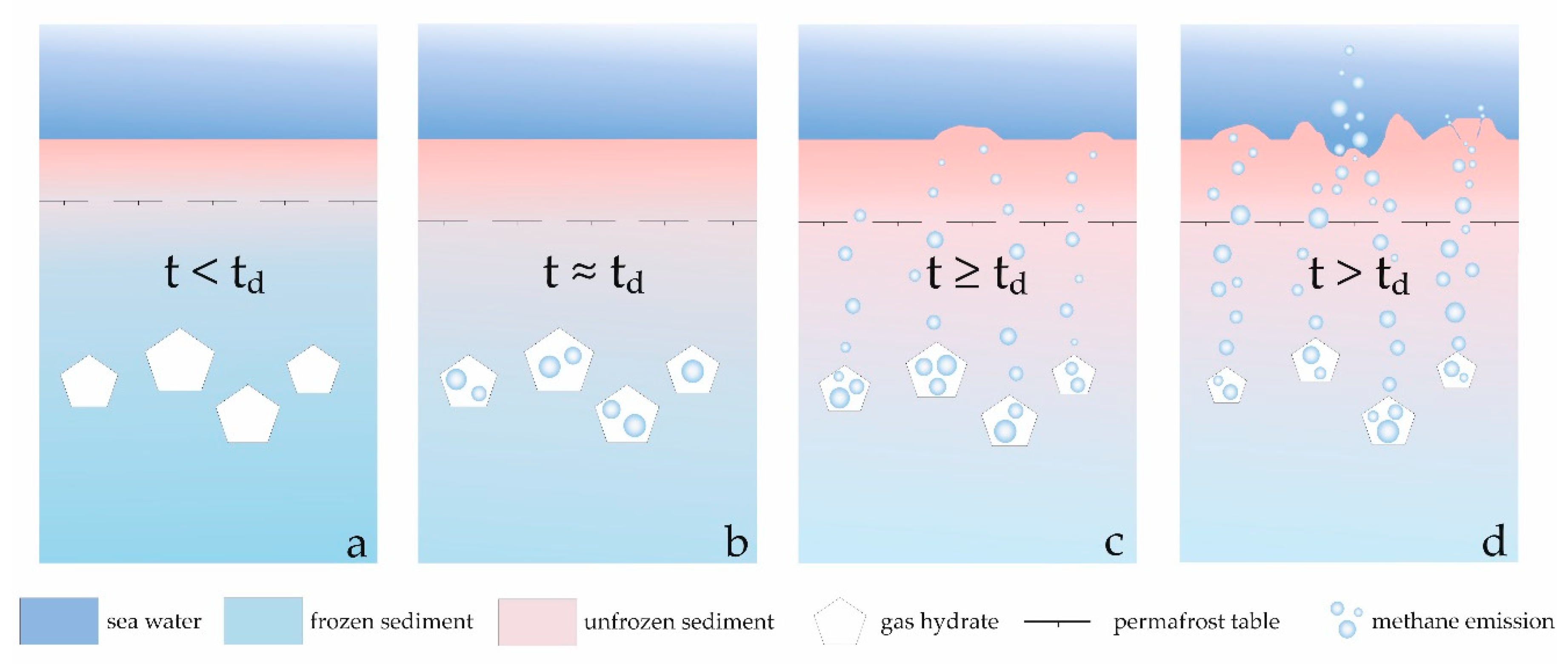
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grigoriev, N.F. Permafrost in the Seaside Zone of Yakutia (Published in Russian); Nauka: Moscow, Russia, 1966; p. 180. [Google Scholar]
- Wood, W.T.; Gettrust, J.F.; Chapman, N.R.; Spence, G.D.; Hyndman, R.D. Decreased Stability of Methane Hydrates in Marine Sediments Owing to Phase-Boundary Roughness. Nature 2002, 420, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, N.E.; Nicolsky, D.Y.; Semiletov, I.P. Current State of Subsea Permafrost on the East Siberian Shelf: Tests of Modeling Results Based on Field Observations. Dokl. Earth Sci. 2010, 429, 1518–1521. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Gustafsson, O.; Sergienko, V.; Lobkovsky, L.; Dudarev, O.; Tumskoy, V.; Grigoriev, M.; Chernykh, D.; Koshurnikov, A.; et al. Current Rates and Mechanisms of Subsea Permafrost Degradation in the East Siberian Arctic Shelf. Nat. Commun. 2017, 8, 15872:1–15872:13. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, V.I.; Salyuk, A.N.; Karnaukh, V.N.; Semiletov, I.P.; Shakhova, N.E. Detection of Areas of Methane Bubble Discharge on the Laptev Sea Shelf in the Eastern Arctic (Published in Russian). Rep. Acad. Sci. 2010, 430, 820–823. [Google Scholar]
- Anisimov, O.A.; Borzenkova, I.I.; Lavrov, S.A.; Strelchenko, Y.G. Modern Dynamics of Underwater Permafrost and Methane Emission on the Shelf of the Seas of the Eastern Arctic (Published in Russian). Ice Snow 2012, 2, 97–105. [Google Scholar]
- Loktev, A.; Bondarev, V.; Kulikov, S.; Rokos, S. Russian Arctic Offshore Permafrost. In Offshore Site Investigation and Geotechnics: Integrated Technologies—Present and Future; Society of Underwater Technology: London, UK, 2012; pp. 1–8. [Google Scholar]
- Sergienko, V.I.; Lobkovskii, L.I.; Semiletov, I.P.; Dudarev, O.V.; Dmitrievskii, N.N.; Shakhova, N.E.; Romanovskii, N.N.; Kosmach, D.A.; Nikol’skii, D.N.; Nikiforov, S.L.; et al. The Degradation of Submarine Permafrost and the Destruction of Hydrates on the Shelf of East Arctic Seas as a Potential Cause of the Methane Catastrophe: Some Results of Integrated Studies in 2011. Dokl. Earth Sci. 2012, 446, 1132–1137. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Kokorev, V.A. Comparative Analysis of Land, Marine, and Satellite Observations of Methane in the Lower Atmosphere in the Russian Arctic under Conditions of Climate Change. Izv. Atmos. Ocean. Phys. 2015, 51, 979–991. [Google Scholar] [CrossRef]
- Koloskov, E.N.; Firsov, Y.G. The Use of Modern Hydrographic Technologies for the Study of Topography and Bottom Gas Occurrence in the Northern Seas of Russia (Published in Russian). Bull. State Univ. Marit. River Fleet Admiral SB Makarov 2015, 3, 54–62. [Google Scholar]
- James, R.H.; Bousquet, P.; Bussmann, I.; Haeckel, M.; Kipfer, R.; Leifer, I.; Niemann, H.; Ostrovsky, I.; Piskozub, J.; Rehder, G.; et al. Effects of Climate Change on Methane Emissions from Seafloor Sediments in the Arctic Ocean: A Review. Limnol. Oceanogr. 2016, 61, S283–S299. [Google Scholar] [CrossRef]
- Leifer, I.; Chernykh, D.; Shakhova, N.; Semiletov, I. Sonar Gas Flux Estimation by Bubble Insonification: Application to Methane Bubble Flux from Seep Areas in the Outer Laptev Sea. Cryosphere 2017, 11, 1333–1350. [Google Scholar] [CrossRef]
- Thornton, B.F.; Geibel, M.C.; Crill, P.M.; Humborg, C.; Mörth, C.M. Methane Fluxes from the Sea to the Atmosphere across the Siberian Shelf Seas. Geophys. Res. Lett. 2016, 43, 5869–5877. [Google Scholar] [CrossRef]
- Andreassen, K.; Hubbard, A.; Winsborrow, M.; Patton, H.; Vadakkepuliyambatta, S.; Plaza-Faverola, A.; Gudlaugsson, E.; Serov, P.; Deryabin, A.; Mattingsdal, R.; et al. Massive Blow-out Craters Formed by Hydrate-Controlled Methane Expulsion from the Arctic Seafloor. Science 2017, 356, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, K.J.; Kessler, J.D.; Southon, J.R.; Garcia-Tigreros, F.; Schreiner, K.M.; Ruppel, C.D.; Miller, J.B.; Lehman, S.J.; Xu, X. Limited Contribution of Ancient Methane to Surface Waters of the U.S. Beaufort Sea Shelf. Sci. Adv. 2018, 4, eaao4842:1–eaao4842:7. [Google Scholar] [CrossRef] [PubMed]
- Serov, P.; Portnov, A.; Mienert, J.; Semenov, P.; Ilatovskaya, P. Methane Release from Pingo-like Features across the South Kara Sea Shelf, an Area of Thawing Offshore Permafrost. J. Geophys. Res. F Earth Surf. 2015, 120, 1515–1529. [Google Scholar] [CrossRef]
- Vonk, J.E.; Tank, S.E.; Bowden, W.B.; Laurion, I.; Vincent, W.F.; Alekseychik, P.; Amyot, M.; Billet, M.F.; Canario, J.; Cory, R.M.; et al. Reviews and Syntheses: Effects of Permafrost Thaw on Arctic Aquatic Ecosystems. Biogeoscience 2015, 12, 10719–10815. [Google Scholar] [CrossRef]
- Lobkovskiy, L.I.; Nikiforov, S.L.; Dmitrevskiy, N.N.; Libina, N.V.; Semiletov, I.P.; Ananiev, R.A.; Meluzov, A.A.; Roslyakov, A.G. Gas Extraction and Degradation of the Submarine Permafrost Rocks on the Laptev Sea Shelf. Oceanology 2015, 55, 283–290. [Google Scholar] [CrossRef]
- Ostanin, I.; Anka, Z.; di Primio, R. Role of Faults in Hydrocarbon Leakage in the Hammerfest Basin, SW Barents Sea: Insights from Seismic Data and Numerical Modelling. Geosciences 2017, 7, 28. [Google Scholar] [CrossRef]
- Baranov, B.V.; Lobkovsky, L.I.; Dozorova, K.A.; Tsukanov, N.V. The Fault System Controlling Methane Seeps on the Shelf of the Laptev Sea. Dokl. Earth Sci. 2019, 486, 571–574. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Salyuk, A.; Yusupov, V.; Kosmach, D.; Gustafsson, Ö. Extensive Methane Venting to the Atmosphere from Sediments of the East Siberian Arctic Shelf. Science 2010, 327, 1246–1250. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Hubberten, H.W.; Gavrilov, A.V.; Eliseeva, A.A.; Tipenko, G.S. Offshore Permafrost and Gas Hydrate Stability Zone on the Shelf of East Siberian Seas. Geo-Marine Lett. 2005, 25, 167–182. [Google Scholar] [CrossRef]
- Burwicz, E.; Rüpke, L.; Wallmann, K. Estimation of the Global Amount of Submarine Gas Hydrates Formed via Microbial Methane Formation Based on Numerical Reaction-Transport Modeling and a Novel Parameterization of Holocene Sedimentation. Geochim. Cosmochim. Acta 2011, 75, 4562–4576. [Google Scholar] [CrossRef]
- Perlstein, G.Z.; Sergeev, D.O.; Tipenko, G.S.; Tumskoy, V.E.; Khimenkov, A.N.; Vlasov, A.N.; Merzlyakov, V.P.; Stanilovskaya, Y.V. Hydrocarbon Gases and Cryolithozone of the Arctic Shelf (Published in Russian). Arct. Ecol. Econ. 2015, 2, 35–44. [Google Scholar]
- Ruppel, C.D.; Kessler, J.D. The Interaction of Climate Change and Methane Hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Chuvilin, E. Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf. Geosciences 2019, 9, 251. [Google Scholar] [CrossRef]
- Sloan, E.D. Clathrate Hydrates of Natural Gases, Second Edition, Revised and Expanded; CRC Press: New York, NY, USA, 1998; p. 705. ISBN 9780824799373. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Max, M. Natural Gas Hydrate In Oceanic and Permafrost Environments; Kluwer Academic Publishers: Washington, WA, USA, 2000; p. 419. ISBN 978-1-4020-1362-1. [Google Scholar] [CrossRef]
- Solov’ev, V.A.; Ginsburg, G.D. Submarine Gas Hydrates (Published in Russian); VNII Oceanologii: St Petersburg, Russia, 1994; p. 194. ISBN 5-7173-0290-8. [Google Scholar]
- Max, M.D.; Johnson, A.H.; Dillon, W.P. Natural Gas Hydrate—Arctic Ocean Deepwater Resource Potential; Springer: Dordrecht, The Netherlands, 2013; p. 113. ISBN 9783319025070. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences 2018, 8, 431. [Google Scholar] [CrossRef]
- Chuvilin, E.; Ekimova, V.; Bukhanov, B.; Grebenkin, S.; Shakhova, N.; Semiletov, I. Role of Salt Migration in Destabilization of Intra Permafrost Hydrates in the Arctic Shelf: Experimental Modeling. Geosciences 2019, 9, 188. [Google Scholar] [CrossRef]
- Loktev, A.; Tokarev, M.; Chuvilin, E. Problems and Technologies of Offshore Permafrost Investigation. Proced. Eng. 2017, 189, 459–465. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Tymskoy, V.; Shakhova, N.; Dudarev, O.; Semiletov, I. Thermal Conductivity of Bottom Sediments in the Area of Buor-Haya Bay (Laptev Sea Shelf) (Published in Russian). Earth’s Cryosph. 2013, XVII, 24–36. [Google Scholar]
- Romanovskii, N.N.; Gavrilov, A.V.; Tumskoy, V.E.; Kholodov, A.L.; Siegert, C.; Hubberten, H.W.; Sher, A.V. Environmental Evolution in the Laptev Sea Region during Late Pleistocene and Holocene. Polarforschung 2000, 68, 237–245. [Google Scholar]
- Romanovskii, N.N.; Hubberten, H.W. Results of Permafrost Modelling of the Lowlands and Shelf of the Laptev Sea Region, Russia. Permafr. Periglac. Process. 2001, 12, 191–202. [Google Scholar] [CrossRef]
- Nicolsky, D.; Shakhova, N. Modeling Sub-Sea Permafrost in the East Siberian Arctic Shelf: The Dmitry Laptev Strait. Environ. Res. Lett. 2010, 5, 015006. [Google Scholar] [CrossRef]
- Nicolsky, D.J.; Romanovsky, V.E.; Romanovskii, N.N.; Kholodov, A.L.; Shakhova, N.E.; Semiletov, I.P. Modeling Sub-Sea Permafrost in the East Siberian Arctic Shelf: The Laptev Sea Region. J. Geophys. Res. Earth Surf. 2012, 117, F03028. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Tumskoi, V.E. Retrospective Approach to the Estimation of the Contemporary Extension and Structure of the Shelf Cryolithozone in East Arctic (Published in Russian). Earth’s Cryosph. 2011, 15, 3–14. [Google Scholar]
- Overduin, P.P.; Schneider von Deimling, T.; Miesner, F.; Grigoriev, M.N.; Ruppel, C.; Vasiliev, A.; Lantuit, H.; Juhls, B.; Westermann, S. Submarine Permafrost Map in the Arctic Modeled Using 1-D Transient Heat Flux (SuPerMAP). J. Geophys. Res. Ocean. 2019. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Eliseeva, A.A.; Gavrilov, A.V.; Tipenko, G.S.; Hubberten, X. The Long-Term Dynamics of Frozen Strata and the Zone of Gas Hydrate Stability in the Rift Structures of the Arctic Shelf of Eastern Siberia (Report 2) (Published in Russian). Earth’s Cryosph. 2006, 10, 29–38. [Google Scholar]
- Delisle, G. Temporal Variability of Subsea Permafrost and Gas Hydrate Occurrences as Function of Climate Change in the Laptev Sea, Siberia. Polarforschung 2000, 68, 221–225. [Google Scholar]
- Mazarovich, A.O. Real and Potential Geological Hazards on the Ocean Floor, Slopes, and Shelf. Her. Russ. Acad. Sci. 2012, 82, 320–325. [Google Scholar] [CrossRef]
- Sahling, H.; Römer, M.; Pape, T.; Bergès, B.; dos Santos Fereirra, C.; Boelmann, J.; Geprägs, P.; Tomczyk, M.; Nowald, N.; Dimmler, W.; et al. Gas Emissions at the Continental Margin West of Svalbard: Mapping, Sampling, and Quantification. Biogeosciences 2014, 11, 6029–6046. [Google Scholar] [CrossRef]
- Bondur, V.G.; Kuznetsova, T.V.; Vorobyev, V.E.; Zamshin, V. Detection of Gas Shows on the Russian Shelf Based on Satellite Imagery Data (Published in Russian). Georesour. Geoenergy Geopolit. 2014, 1, 1–23. [Google Scholar]
- Paull, C.K.; Ussler, W.; Dallimore, S.R.; Blasco, S.M.; Lorenson, T.D.; Melling, H.; Medioli, B.E.; Nixon, F.M.; McLaughlin, F.A. Origin of Pingo-like Features on the Beaufort Sea Shelf and Their Possible Relationship to Decomposing Methane Gas Hydrates. Geophys. Res. Lett. 2007, 34, L01603:1–L01603:5. [Google Scholar] [CrossRef]
- Biastoch, A.; Treude, T.; Rpke, L.H.; Riebesell, U.; Roth, C.; Burwicz, E.B.; Park, W.; Latif, M.; Böning, C.W.; Madec, G.; et al. Rising Arctic Ocean Temperatures Cause Gas Hydrate Destabilization and Ocean Acidification. Geophys. Res. Lett. 2011, 38, 8. [Google Scholar] [CrossRef]
- Mau, S.; Romer, M.; Torres, M.E.; Bussmann, I.; Pape, T.; Damm, E.; Geprags, P.; Wintersteller, P.; Hsu, C.W.; Loher, M.; et al. Widespread Methane Seepage along the Continental Margin off Svalbard—From Bjornoya to Kongsfjorden. Sci. Rep. 2017, 7, 42997:1–42997:13. [Google Scholar] [CrossRef]
- Westbrook, G.K.; Thatcher, K.E.; Rohling, E.J.; Piotrowski, A.M.; Pälike, H.; Osborne, A.H.; Nisbet, E.G.; Minshull, T.A.; Lanoisellé, M.; James, R.H.; et al. Escape of Methane Gas from the Seabed along the West Spitsbergen Continental Margin. Geophys. Res. Lett. 2009, 36, L15608. [Google Scholar] [CrossRef]
- Majorowicz, J.A.; Hannigan, P.K. Natural Gas Hydrates in the Offshore Beaufort-Mackenzie Basin—Study of a Feasible Energy Source II. Nat. Resour. Res. 2000, 9, 201–214. [Google Scholar] [CrossRef]
- Bogoyavlensky, V.; Kishankov, A.; Yanchevskaya, A.; Bogoyavlensky, I. Forecast of Gas Hydrates Distribution Zones in the Arctic Ocean and Adjacent Offshore Areas. Geosciences 2018, 8, 453. [Google Scholar] [CrossRef]
- Moridis, G.J.; Collett, T.S.; Dallimore, S.R.; Satoh, T.; Hancock, S.; Weatherill, B. Numerical Studies of Gas Production from Several CH4 Hydrate Zones at the Mallik Site, Mackenzie Delta, Canada. J. Pet. Sci. Eng. 2004, 43, 219–238. [Google Scholar] [CrossRef]
- Overduin, P.; Liebner, S.; Knoblauch, C.; Kneier, F.; Günther, F.; Schirrmeister, L.; Wetterich, S. Subsea Permafrost Degradation and Inferred Methane Release in Shallow Coastal Water of the Central Laptev Sea. In Proceeding of PERGAMON Final Symposium, Kiel, Germany, 4–7 November 2013. [Google Scholar]
- Chuvilin, E.; Davletshina, D. Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling. Geosciences 2018, 8, 467. [Google Scholar] [CrossRef]
- GOST 12536-2014 Soils. Laboratory Determination of the Grain (Grain-Size) and Microaggregate Composition (Publsihed in Russian); Standartinform: Moscow, Russia, 2014. [Google Scholar]
- Chuvilin, E.; Bukhanov, B. Effect of Hydrate Formation Conditions on Thermal Conductivity of Gas-Saturated Sediments. Energy Fuels 2017, 31, 5246–5254. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Semenov, A.P.; Bogoyavlensky, V.I.; Medvedev, V.I.; Bogoyavlensky, I.V. Experimental Modeling of Methane Release from Intrapermafrost Relic Gas Hydrates When Sediment Temperature Change. Cold Reg. Sci. Technol. 2018, 149, 46–50. [Google Scholar] [CrossRef]
- Denisov, S.N.; Arzhanov, M.M.; Eliseev, A.V.; Mokhov, I. Assessing the Response of the Subanalytic Methane. Rep. Acad. Sci. 2011, 441, 685. [Google Scholar]
- Romanovskii, N.N.; Hubberten, H.W.; Gavrilov, A.V.; Eliseeva, A.A.; Tipenko, G.S.; Kholodov, A.L.; Romanovsky, V.E. Permafrost and Gas Hydrate Stability Zone Evolution on the Eastern Part of the Eurasia Arctic Sea Shelf in the Middle Pleistocene-Holocene (Published in Russian). Earth’s Cryosph. 2003, 7, 51–64. [Google Scholar]
- Tipenko, G.S.; Romanovsky, N.N.; Kholodov, A.L. Modeling the Dynamics of the Submarine Cryolithozone and the Zone of Stability of Gas Hydrates: A Mathematical Solution, Numerical Implementation and the Results of Test Calculations (Published in Russian). Earth’s Cryosph. 1999, 3, 71–78. [Google Scholar]
- Malakhova, V.V. Mathematical Modeling of the Long-Term Dynamics of the Submarine Permafrost of the Arctic Shelf (Published in Russian). Interexpo Geo-Siberia 2014, 4, 1:1–1:5. [Google Scholar]
- Malakhova, V.V.; Golubeva, E.; Eliseev, A.V.; Platov, G. Arctic Ocean Estimation of Possible Climate Change Impact on Methane Hydrate in the Arctic Ocean. IOP Conf. Ser. Earth Environ. Sci. 2018, 211. [Google Scholar] [CrossRef]





| Sample | Sampling Site | Mineralogy, % | Salinity, % | |
|---|---|---|---|---|
| Sand | Arctic shelf (Buor Khaya Bay) | Quartz | 55 | 0.4 |
| Albite | 18 | |||
| Microcline | 9 | |||
| Silt | Arctic shelf (Buor Khaya Bay) | Quartz | 42.5 | 0.1 |
| Albite | 14.9 | |||
| Illite | 9.1 | |||
| Chlorite | 6.9 | |||
| Microcline | 5.9 | |||
| Hydromica | 3.9 | |||
| Clay silt | South Tambei GCF | Quartz | 46.4 | 0.7 |
| Albite | 25.3 | |||
| Chlorite | 10.4 | |||
| Mirror stone | 7.4 | |||
| Orthoclase | 6.5 | |||
| Kaolinite | 4.2 | |||
| Sample | Particle Size Distribution, % | Lithology * | ||
|---|---|---|---|---|
| 1–0.05 mm | 0.05–0.001 mm | <0.001 mm | ||
| Sand | 96.7 | 2.0 | 1.3 | Fine sand |
| Silt | 63.4 | 32.6 | 4.0 | Silty sand |
| Clay silt | 21.1 | 55.8 | 23.1 | Clay silt (loam) |
| Sample | Water Content, % | Density, g/cm3 | Hydrate Saturation | ||||
|---|---|---|---|---|---|---|---|
| Equilibrium | Self-Preservation | ||||||
| Sh initial, % | Kh initial, u.f. | Sh final, % | Kh final, u.f. | Ksc, % | |||
| Sand | 14 | 1.80 | 47 | 0.59 | 4 | 0.05 | 8.5 |
| Silt | 15 | 1.82 | 25 | 0.29 | 6.9 | 0.05 | 26 |
| Loam | 18 | 1.20 | 11.5 | 0.29 | 7 | 0.17 | 61 |
| Sample | Sh d., % | CH4 Emission (m3) from 1 m3 of Thawing Hydrate-Bearing Rock |
|---|---|---|
| Sand | 2.3 | 1.5 |
| Silt | 4.8 | 3.2 |
| Clay silt | 5.3 | 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Davletshina, D.; Ekimova, V.; Bukhanov, B.; Shakhova, N.; Semiletov, I. Role of Warming in Destabilization of Intrapermafrost Gas Hydrates in the Arctic Shelf: Experimental Modeling. Geosciences 2019, 9, 407. https://doi.org/10.3390/geosciences9100407
Chuvilin E, Davletshina D, Ekimova V, Bukhanov B, Shakhova N, Semiletov I. Role of Warming in Destabilization of Intrapermafrost Gas Hydrates in the Arctic Shelf: Experimental Modeling. Geosciences. 2019; 9(10):407. https://doi.org/10.3390/geosciences9100407
Chicago/Turabian StyleChuvilin, Evgeny, Dinara Davletshina, Valentina Ekimova, Boris Bukhanov, Natalia Shakhova, and Igor Semiletov. 2019. "Role of Warming in Destabilization of Intrapermafrost Gas Hydrates in the Arctic Shelf: Experimental Modeling" Geosciences 9, no. 10: 407. https://doi.org/10.3390/geosciences9100407








