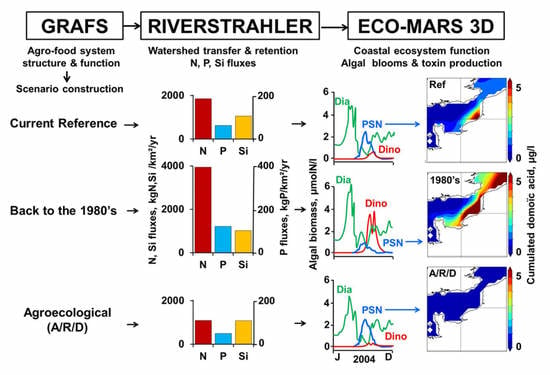
Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel
Abstract
:1. Introduction
2. Development of A Modelling Chain for Aquatic Continuums: From River Headwaters to Coastal Marine Ecosystems
2.1. The GRAFS Model
2.2. The RIVERSTRAHLER River Model
2.3. The ECO-MARS3D Coastal Zone Model
3. Nutrient Fluxes Delivered at the Coast
3.1. Inter-Annual Variations of Riverine Water Flow and Quality
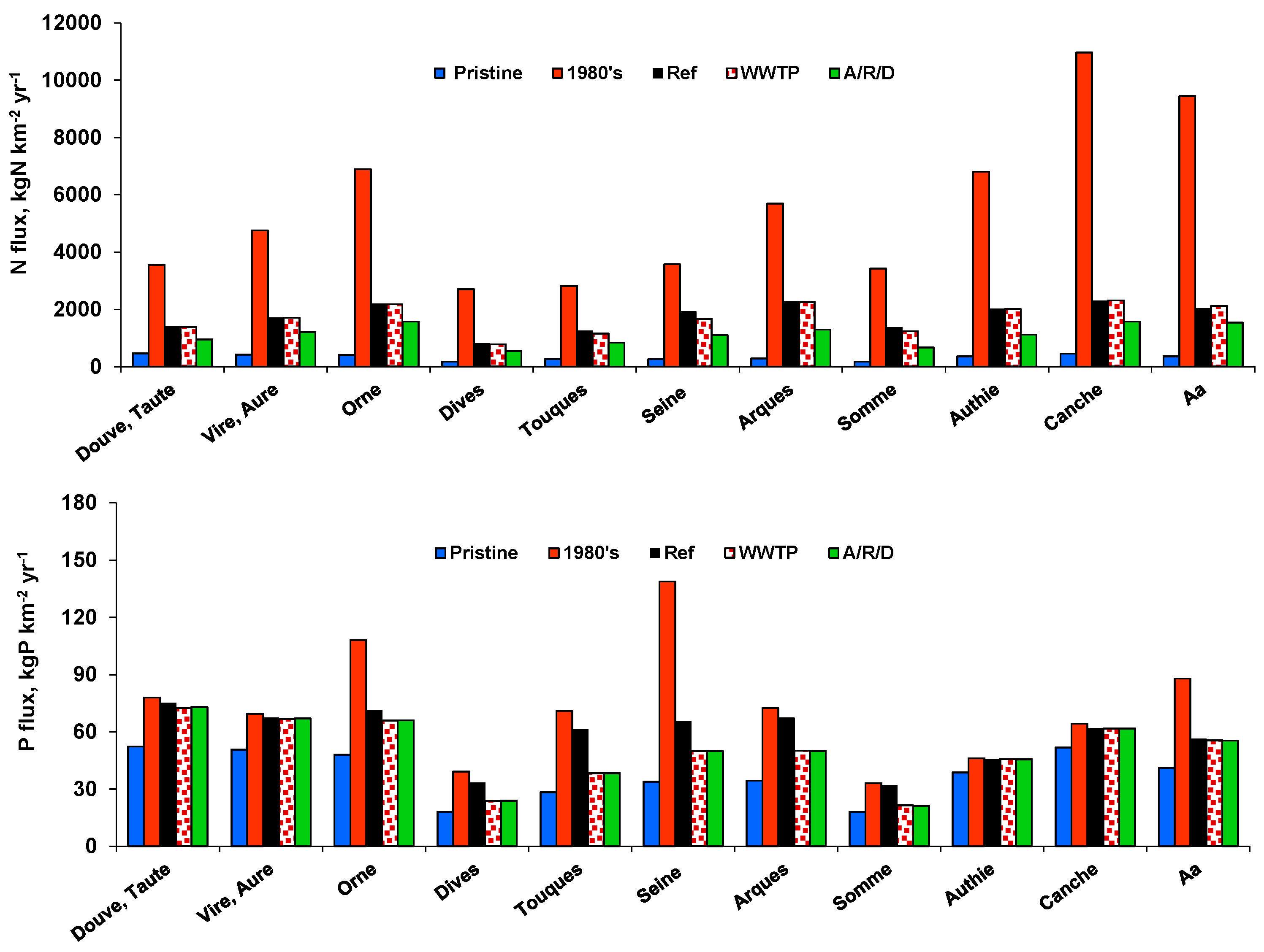
3.2. Fluxes Delivered to the Coastal Zones
4. Impact of the Riverine Nutrient Deliveries at the Coastal Zone
4.1. Coastal Water Quality and Model Evaluation
4.2. Pseudo-nitzschia Simulations
5. Exploring Different Scenarios in the Land-to-Sea Continuum
6. Discussion
6.1. Pseudo-nitzschia, Potentially Toxic Diatom Algae at Low Silica Concentration
6.2. A Model Chain for the Land-to-Sea Continuum
6.3. Construction and Exploration of Scenarios
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Ferreira, J.G.; Bricker, S.B.; Nunes, J.P.; Zhu, M.; Zhang, X. Trophic assessment in Chinese coastal systems-review of methods and application to the Changjiang (Yangtze) estuary and Jiaozhou Bay. Estuar. Coasts 2007, 30, 901–918. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.-C.; Ridoutt, B.G.; Wang, X.-C.; Ren, P.-A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Díaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused coastal hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Ulloa, M.J.; Álvarez-Torres, P.; Horak-Romo, K.P.; Ortega-Izaguirre, R. Harmful algal blooms and eutrophication along the mexican coastof the Gulf of Mexico large marine ecosystem. Environ. Dev. 2017, 22, 120–128. [Google Scholar] [CrossRef]
- Conley, D.J.; Humborg, C.; Rahm, L.; Savchuk, O.P.; Wulff, F. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 2002, 36, 5315–5320. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Vilicic, D.; Kuzmic, M.; Tomazi, I.; Ljubesic, Z.; Bosak, S.; Precali, R.; Djakovac, T.; Maric, D.; Godrijan, J. Northern Adriatic phytoplankton response to short Po River discharge pulses during summer stratified conditions. Mar. Ecol. 2013, 34, 451–466. [Google Scholar] [CrossRef]
- Radach, G.; Patsch, J. Variability of Continental Riverine Freshwater and Nutrient Inputs into the North Sea for the Years 1977–2000 and Its Consequences for the Assessment of Eutrophication. Estuar. Coasts 2007, 30, 66–81. [Google Scholar] [CrossRef]
- Peperzak, L.; Poelman, M. Mass mussel mortality in The Netherlands after a bloom of Phaeocystis globosa (prymnesiophyceae). J. Sea Res. 2008, 60, 220–222. [Google Scholar] [CrossRef]
- Lancelot, C.; Thieu, V.; Polard, A.; Garnier, J.; Billen, G.; Hecq, W.; Gypens, N. Ecological and economic effectiveness of nutrient reduction policies on coastal Phaeocystis colony blooms in the Southern North Sea: An integrated modeling approach. Sci. Total Environ. 2011, 409, 2179–2191. [Google Scholar] [CrossRef]
- Desmit, X.; Thieu, V.; Billen, G.; Campuzano, F.; Dulière, V.; Garnier, J.; Lassaletta, L.; Ménesguen, A.; Neves, R.; Pinto, L.; et al. Reducing marine eutrophication may require a paradigmatic change. Sci. Total Environ. 2018, 635, 1444–1466. [Google Scholar] [CrossRef] [PubMed]
- Justić, D.; Rabalais, N.N.; Turner, R.E. Stoichiometric nutrient balance and origin of coastal eutrophication. Mar. Pollut. Bull. 1995, 30, 41–46. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J. The Phison River Plume: Coastal eutrophication in response to changes in land use and water management in the watershed. Aquat. Microb. Ecol. 1997, 13, 3–17. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J. River basin nutrient delivery to the coastal sea: Assessing its potential to sustain new production of non siliceous algae. Mar. Chem. 2007, 106, 148–160. [Google Scholar] [CrossRef]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.M.; Parsons, M.L.; Rensel, J.E.J.; Townsend, D.W.; et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Romero, E.; Garnier, J.; Lassaletta, L.; Billen, G.; Le Gendre, R.; Riou, P.; Cugier, P. Large-scale patterns of river inputs in SW Europe: Seasonal and interannual variations and potential eutrophication effects at the coastal zone. Biogeochemistry 2013, 113, 481–505. [Google Scholar] [CrossRef]
- Guillaud, J.-F.; Ménesguen, A. Modélisation sur vingt ans (1976–1995) de la production phytoplanctonique en baie de Seine. Oceanol. Acta 1998, 21, 887–906. [Google Scholar] [CrossRef]
- Amzil, Z.; Fresnel, J.; Gal, D.L.; Billard, C. Domoic acid accumulation in French shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- Nézan, E.; Antoine, E.; Fiant, L.; Amzil, Z.; Billard, C. Identification of Pseudo-nitzschia australis and P. multiseries in the Bay of Seine. Was there a relation to presence of domoic acid in king scallops in autumn 2004? Harmful Algae News 2006, 31, 1–3. [Google Scholar]
- Husson, B.; Hernandez-Farinas, T.; Le Gendre, R.; Schapira, M.; Chapelle, A. Two decades of Pseudo-nitzschia spp. blooms and king scallop (Pecten maximus) contamination by domoic acid along the French Atlantic and English Channel coasts: Seasonal dynamics, spatial heterogeneity and interannual variability. Harmful Algae 2016, 51, 26–39. [Google Scholar] [CrossRef]
- Thorel, M.; Claquin, P.; Schapira, M.; Le Gendre, R.; Riou, P.; Goux, D.; Le Roy, B.; Raimbault, V.; Deton-Cabanillas, A.-F.; Bazin, P.; et al. Nutrient ratios influence variability in Pseudo-nitzschia species diversity and particulate domoic acid production in the Bay of Seine (France). Harmful Algae 2017, 68, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Charlier, R.H.; Morand, P.; Finkl, C.W.; Thys, A. Green Tides on the Brittany Coasts. Environ. Res. Eng. Manag. 2007, 41, 52–59. [Google Scholar]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordarok, J.; DeLong, R.; De Vogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Bargu, S.; Silver, M.W.; Ohman, M.D.; Benitez-Nelson, C.R.; Garrison, D.L. Mystery behind Hitchcock’s birds. Nat. Geosci. 2012, 5, 2–3. [Google Scholar] [CrossRef]
- Soliño, L.; Ferrer-Obiol, J.; Navarro-Herrero, L.; González-Solís, J.; Costa, P.R. Are pelagic seabirds exposed to amnesic shellfish poisoning toxins? Harmful Algae 2019, 84, 172–180. [Google Scholar] [CrossRef]
- Blanco, J.; Acosta, C.P.; Bermudez de la Puente, M.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Amzil, Z.; Royer, F.; Sibat, M.; Fiant, L.; Gelin, M.; Le Gal, D.; Francoise, S. First report on amnesic and diarrhetic toxins detection in French scallops during 2004–2005 monitoring surveys. In Proceedings of the Sixth International Conference on Molluscan Shellfish Safety, Blenheim, New Zealand, 18–23 March 2009; Available online: https://archimer.ifremer.fr/doc/00015/12577/ (accessed on 11 October 2019).
- Fehling, J.; Davidson, K.; Bolch, C.J.; Bates, S.S. Growth and domoic acid production of Pseudo-nitzschia seriata under phosphate and silicate limitation. J. Phycol. 2004, 40, 674–683. [Google Scholar] [CrossRef]
- Davidson, K.; Fehling, J. Modelling the influence of silicon and phosphorus limitation on the growth and toxicity of Pseudo-nitzschia seriata. Afr. J. Mar. Sci. 2006, 28, 357–360. [Google Scholar]
- Billen, G.; Garnier, J.; Ficht, A.; Cun, C. Modelling the response of water quality in the Seine Estuary to human activity in its watershed over the last 50 years. Estuaries 2001, 24, 977–993. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J.; Lassaletta, L. Modelling the nitrogen cascade from watershed soils to the sea: From regional to global scales. Phil. Trans. R. Soc. B 2013, 368, 20130123. [Google Scholar] [CrossRef]
- Cugier, P.h.; Billen, G.; Guillaud, J.F.; Garnier, J.; Ménesguen, A. Modelling the eutrophication of the Seine Bight (France) under historical, present and future riverine nutrient loading. J. Hydrol. 2005, 304, 381–396. [Google Scholar] [CrossRef]
- Passy, P.; Gypens, N.; Billen, G.; Garnier, J.; Lancelot, C.; Thieu, V.; Rousseau, V.; Callens, J. A model reconstruction of riverine nutrient fluxes and eutrophication in the Belgian Coastal Zone since 1984. J. Mar. System. 2013, 128, 106–122. [Google Scholar] [CrossRef]
- Passy, P.; Le Gendre, R.; Garnier, J.; Cugier, P.; Callens, J.; Paris, F.; Billen, G.; Riou, P.; Romero, E. Eutrophication modelling chain for improved management strategies to prevent algal blooms in the Seine Bight. Mar. Ecol. Prog. Ser. 2016, 543, 107–125. [Google Scholar] [CrossRef] [Green Version]
- Garnier, J.; Billen, G.; Némery, J.; Sebilo, M. Transformations of nutrients (N, P, Si) in the turbidity maximum zone of the Seine estuary and export to the sea. Estuar. Coast. Shelf Sci. 2010, 90, 129–141. [Google Scholar] [CrossRef]
- Billen, G.; Le Noë, J.; Garnier, J. Two contrasted future scenarios for the French agro-food system. Sci. Total Environ. 2018, 637, 695–705. [Google Scholar] [CrossRef]
- European Commission. UWWTD EU Directive (Urban Wastewater Treatment Directive. 91/271/CEE du Conseil; du 21mai 1991; Relative au Traitement des Eaux Urbaines Résiduaires; JO L135 du 30.5.1991; European Commission: Brussels, Belgium, 1991; pp. 40–52. [Google Scholar]
- Romero, E.; Garnier, J.; Billen, G.; Ramarson, A.; Riou, P.; Le Gendre, R. The biogeochemical functioning of the Seine estuary and the nearby coastal zone: Export, retention and transformations. A modelling approach. Limnol. Oceanogr 2018, 64, 895–912. [Google Scholar] [CrossRef]
- Le Noë, J.; Billen, G.; Garnier, J. Nitrogen, phosphorus and carbon fluxes through the French Agro-Food System: An application of the GRAFS approach at the territorial scale. Sci. Total Environ. 2017, 586, 42–55. [Google Scholar] [CrossRef]
- Anglade, J.; Billen, G.; Garnier, J.; Makridis, T.; Puech, T.; Tittel, C. Agro-environmental performance of organic compared to conventional cash crop farming in the Seine watershed. Agric. Syst. 2015, 139, 82–92. [Google Scholar] [CrossRef]
- Anglade, J.; Billen, G.; Garnier, J. Reconquérir la qualité de l’eau en régions de grande culture: Agriculture biologique et reconnexion avec l’élevage. Fourrages 2017, 231, 257–268. [Google Scholar]
- Ruelland, D.; Billen, G.; Brunstein, D.; Garnier, J. SENEQUE 3: A GIS interface to the RIVERSTRAHLER model of the biogeochemical functioning of river systems. Sci. Total Environ. 2007, 375, 257–273. [Google Scholar] [CrossRef]
- Lyne, V.; Hollick, M. Stochastic time-variable rainfall–runoff modelling. In Proceedings of the Hydrology and Water Resources Symposium, Perth, Australia, 10–12 September 1979; National Committee on Hydrology and Water Resources of the Institution of Engineers: Barton, Australia; pp. 89–92. [Google Scholar]
- Arnold, J.G.; Allen, P.M. Automated methods for estimating baseflow and ground water recharge from streamflow records. J. Am. Water Resour. Assoc. 1999, 35, 411–424. [Google Scholar] [CrossRef]
- Billen, G.; Ramarson, A.; Thieu, V.; Théry, S.; Silvestre, M.; Pasquier, C.; Hénault, C.; Garnier, J. Nitrate retention at the river–watershed interface: A new conceptual modeling approach. Biogeochemistry 2018, 139, 31–51. [Google Scholar] [CrossRef]
- Garnier, J.; Billen, G.; Coste, M. Seasonnal succession of diatoms and Chlorophyceae in the drainage network of the river Seine: Observations and modelling. Limnol. Oceanogr. 1995, 40, 750–765. [Google Scholar]
- Garnier, J.; Billen, G.; Akopian, M.; Némery, J.; Pinault, S.; Desruelle, M.; Dufayt, O.; Flipo, N.; Even, S.; Poulin, M. Développement des Peuplements Végétaux Dans le Bassin de la Marne: Dynamique des Nutriments et Eutrophisation. Contrôle par les Organismes Filtreurs; Rapport synthèse Piren-Seine. 1998–2001; PIREN Seine: Paris, France, 2002; p. 43. Available online: http://piren16.metis.upmc.fr/?q=webfm_send/447 (accessed on 11 October 2019).
- Garnier, J.; Billen, G.; Hannon, E.; Fonbonne, S.; Videnina, Y.; Soulie, M. Modeling transfer and retention of nutrients in the drainage network of the Danube River. Estuar. Coast. Shelf Sci. 2002, 54, 285–308. [Google Scholar] [CrossRef]
- Lazure, P.; Dumas, F. An external-internal mode coupling for a 3D hydrodynamical model for applications at regional scale (MARS). Adv. Water Resour. 2008, 31, 233–250. [Google Scholar] [CrossRef]
- Ménesguen, A.; Hoch, T. Modelling the biogeochemical cycles of elements limiting primary production in the English Channel, I. Role of thermohaline stratification. Mar. Ecol. Prog. Ser. 1997, 146, 173–188. [Google Scholar] [CrossRef]
- Ménesguen, A.; Desmit, X.; Dulière, V.; Lacroix, G.; Thouvenin, B.; Thieu, V.; Dussauze, M. How to avoid eutrophication in coastal seas? A new approach to derive river-specific combined nitrate and phosphate maximum concentrations. Sci. Total Environ. 2018, 628, 400–414. [Google Scholar] [CrossRef]
- Ménesguen, A.; Dussauze, M.; Dumas, F.; Thouvenin, B.; Garnier, V.; Lecornu, F.; Répécaud, M. Ecological model of the Bay of Biscay and English Channel shelf for environmental status assessment part 1: Nutrients, phytoplankton and oxygen. Ocean Model. 2018, 133, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Belin, C.; Raffin, B. Les Espèces Phytoplanctoniques Toxiques et Nuisibles sur le Littoral Français de 1984 à 1995, Résultats du REPHY (Réseau de Surveillance du Phytoplancton et des Phycotoxines); Rapport Ifremer RST.DEL/MP-AO 98-16; Institut Français de Recherche Pour L’Exploitation de la Mer: Issy-les-Moulineaux, France, 1998; p. 283. [Google Scholar]
- Busch, J.A.; Andree, K.B.; Diogene, J.; Fernandez-Tejedor, M.; Toebe, K.; John, U.; Krock, B.; Tillmann, U.; Cembella, A.D. Toxigenic algae and associated phycotoxins in two coastal embayments in the Ebro Delta (NW Mediterranean). Harmful Algae 2016, 55, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Lelong, A.; Hegaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Parsons, M.L.; Scholin, C.A.; Miller, P.E.; Doucette, G.J.; Powell, C.L.; Fryxell, G.A.; Dortch, Q.; Soniat, T.M. Pseudo-nitzschia species (Bacillariophyceae) in Louisiana coastal waters: Molecular probe field trials, genetic variability, and domoic acid analyses. J. Phycol. 1999, 35, 1368–1378. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G.; Cochlan, W.P.; Hughe, M.P.; Trainer, V.L. Domoic acid: The synergy of iron; copper; and the toxicity of diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef]
- Trainer, V.L.; Pitcher, G.C.; Reguera, B.; Smayda, T.J. The distribution and impacts of harmful algal bloom species in eastern boundary upwelling systems. Prog. Oceanogr. 2010, 85, 33–52. [Google Scholar] [CrossRef]
- Klein, C.; Claquin, P.; Bouchart, V.; Le Roy, B.; Veron, B. Dynamics of Pseudo- nitzschia spp. and domoic acid production in a macrotidal ecosystem of the Eastern English Channel (Normandy, France). Harmful Algae 2010, 9, 218–226. [Google Scholar] [CrossRef]
- Pénard, C. Détection Satellitaire et Modélisation Opérationnelle de la Production Végétale Non-Fixée (phytoplancton et ulves) Dans la Bande Côtière Bretonne. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2009; p. 227. [Google Scholar]
- Thorel, M.; Fauchot, J.; Morelle, J.; Raimbault, V.; Le Roy, B.; Miossec, C.; Kientz-Bouchart, V.; Claquin, P. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2014, 39, 232–241. [Google Scholar] [CrossRef]
- Blanchard, G.F.; Guarini, J.M.; Richard, P.; Gros, P.; Mornet, F. Quantifying the short-term temperature effect on light-saturated photosynthesis of intertidal microphytobenthos. Mar. Ecol. Prog. Ser. 1996, 134, 309–313. [Google Scholar] [CrossRef]
- Ménesguen, A.; Dussauze, M.; Thouvenin, B. Ecological model of the Bay of Biscay and English Channel shelf for ecological status assessment. Part 2: Three types of HAB (Karenia, Phaeocystis, Pseudo-nitzschia) and their link with nitrogen anthropogenic enrichment. Ocean Model. 2019. in revision. [Google Scholar]
- Aissa-Grouz, A.; Garnier, J.; Billen, G.; Mercier, B.; Martinez, A. The response of river nitrification to changes in wastewater treatment (The case of the lower Seine River downstream from Paris). Ann. Limnol. Int. J. Lim. 2015, 51, 351–364. [Google Scholar] [CrossRef] [Green Version]
- Aissa-Grouz, N.; Garnier, J.; Billen, G. Long trend reduction of phosphorus wastewater loading in the Seine: Determination of phosphorus speciation and sorption for modeling algal growth. Environ. Sci. Pollut. Res. 2018, 25, 23515. [Google Scholar] [CrossRef]
- Garnier, J.; Beusen, A.; Thieu, V.; Billen, G.; Bouwman, L. N:P:Si nutrient export ratios and ecological consequences in coastal seas evaluated by the ICEP approach. Special issue “Past and Future Trends in Nutrient Export from Global Watersheds and Impacts on Water Quality and Eutrophication”. Global Biogeochem. Cy. 2010, 24, GB0A05. [Google Scholar] [CrossRef]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of sea-water. In The Sea; Hill, M.N., Ed.; John Wiley: New York, NY, USA, 1963; pp. 12–37. [Google Scholar]
- Conley, D.J.; Kilham, S.S.; Theriot, E.C. Differences in silica content between marine and freshwater diatoms. Limnol. Oceanogr. 1989, 34, 205–213. [Google Scholar] [CrossRef]
- Hagström, J.A.; Graneli, E.; Moreira, M.O.P.; Odebrecht, C. Domoic acid production and elemental composition of two Pseudo-nitzschia multiseries strains, from the NW and SW Atlantic Ocean, growing in Phosphorus-or Nitrogen-limited chemostat cultures. J. Plankton Res. 2011, 33, 297–308. [Google Scholar] [CrossRef]
- Garnier, J.; Passy, P.; Thieu, V.; Callens, J.; Silvestre, M.; Billen, G. Fate of Nutrients in the Aquatic Continuum of the Seine River and its Estuary: Modelling the Impacts of Human Activity Changes. In The Watershed Biogeochemical Dynamics at Large River-Coastal Interfaces: Linkages with Global Climate Change; Bianchi, T.S., Allison, M.A., Cai, W.-J., Eds.; Cambridge University Press: Cambridge, UK, 2014; p. 671. ISBN 978-1-107-20257-7. [Google Scholar]
- Humborg, C.; Pastuszak, M.; Aigars, J.; Siegmund, H.; Morth, C.M.; Ittekkot, V. Decreased silica land–sea fluxes through damming in the Baltic Sea catchment—Significance of particle trapping and hydrological alterations. Biogeochemistry 2006, 77, 265–281. [Google Scholar] [CrossRef]
- Harrison, J.A.; Frings, P.J.; Beusen, A.H.W.; Conley, D.J.; McCrackin, M.L. Global importance, patterns, and controls of dissolved silica retention in lakes and reservoirs. Glob. Biogeochem. Cy. 2012, 26, GB2037. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraef, G.M., Eds.; Springer: Berlin, Germany, 1998; pp. 267–292. [Google Scholar]
- Cochlan, W.P.; Herndon, J.; Kudela, R.M. Inorganic and organic nitrogen uptake by the toxigenic diatom. Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2008, 8, 111–118. [Google Scholar] [CrossRef]
- Radan, R.L.; Cochlan, W.P. Differential toxin response of Pseudo-nitzschia multiseries as a function of nitrogen speciation in batch and continuous cultures, and during a natural assemblage experiment. Harmful Algae 2018, 73, 12–29. [Google Scholar] [CrossRef]
- Trainer, V.L.; Wells, M.L.; Cochlan, W.P.; Trick, C.G.; Bill, B.D.; Baugh, K.A.; Beall, B.F.; Herndon, J.; Lundholm, N. An ecological study of a massive toxigenic bloom of Pseudo-nitzschia cuspidate off the Washington State coast. Limnol. Oceanogr. 2009, 54, 1461–1474. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Wikfors, G.H. Control of domoic acid toxin expression in Pseudo-nitzschia multiseries by copper and silica: Relevance to mussel aquaculture in New England (USA). Mar. Environ. Res. 2013, 83, 23–28. [Google Scholar] [CrossRef]
- Lema, K.A.; Latimier, M.; Nézan, E.; Fauchot, J.; Le Gac, M. Inter and intra-specific growth and domoic acid production in relation to nutrient ratios and concentrations in Pseudo-nitzschia: Phosphate an important factor. Harmful Algae 2017, 64, 11–19. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.; Garcés, E.; Flo, E.; Fernandez-Tejedor, M.; Diogène, J.; Camp, J. Bloom dynamics of the genus Pseudo-nitzschia (Bacillariophyceae) in two coastal bays (NW Mediterranean Sea). Sci. Mar. 2008, 72, 577–590. [Google Scholar]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology; phylogeny; toxicity; monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- European Commission. EU-WFD (European Water Framework Directive). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000; OLJ 327/1, 22.12; European Commission: Brussels, Belgium, 2000; pp. 1–72. [Google Scholar]
- Belin, C.; Chapelle, A.; Delmas, D.; Nezan, E.; Siano, R. DYNAPSE—DYNamiques des Efflorescences et de la Toxicité des Espèces Phytoplanctoniques Nuisibles du Genre Pseudo-Nitzschia en Région Loire Bretagne; ODE/DYNECO/PELAGOS, 2013-01; Institut Français de Recherche Pour L’Exploitation de la MerI: Issy-les-Moulineaux, France, 2013; Available online: https://archimer.ifremer.fr/doc/00189/30035/ (accessed on 11 October 2019).
- Salomon, J.-C.; Breton, M. An atlas of long-term currents in the channel. Oceanol. Acta 1993, 16, 439–448. Available online: https://archimer.ifremer.fr/doc/00099/21068/ (accessed on 11 October 2019).
- European Commission. EU-Nitrate Directive. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (91/676/ CEE, European Council); European Commission: Brussels, Belgium, 1991. [Google Scholar]
- Kerimoglu, O.; Große, F.; Kreus, M.; Lenhart, H.-L.; van Beusekom, J.E.E. A model-based projection of historical state of a coastal ecosystem: Relevance of phytoplankton stoichiometry. Sci. Total Environ. 2018, 639, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnier, J.; Marescaux, A.; Guillon, S.; Vilmin, L.; Rocher, V.; Billen, G.; Thieu, V.; Silvestre, M.; Passy, P.; Groleau, A.; et al. Ecological functioning of the Seine River: From long-term modelling approaches to high-frequency data analysis. Handb. Env. Chem. 2019. [Google Scholar] [CrossRef]
- Le Noë, J.; Billen, G.; Esculier, F.; Garnier, J. Long term socio-ecological trajectories of agro-food systems revealed by N and P flows: The case of French regions from 1852 to 2014. Agric. Ecosyst. Environ. 2018, 265, 132–143. [Google Scholar] [CrossRef]
- Passy, P.; Garnier, J.; Billen, G.; Fesneau, C.; Tournebize, J. Restoration of ponds in rural landscapes: Modelling the effect on nitrate contamination of surface water (the Seine watershed, France). Sci. Total Environ. 2012, 430, 280–290. [Google Scholar] [CrossRef]
- Direction Générale de la Santé. Abandons de Captages Utilisés Pour la Production d’eau Destinée à la Consommation Humaine; Bilan Février 2012; Secrétariat d’Etat Chargé de la Santé: Paris, France, 2012; p. 22. [Google Scholar]
- Garnier, J.; Le Noë, J.; Marescaux, A.; Sanz-Cobena, A.; Lassaletta, L.; Silvestre, M.; Thieu, V.; Billen, G. Long term changes in greenhouse gas emissions of French agriculture (1852–2014): From traditional agriculture to conventional intensive systems. Sci. Total Environ. 2019, 660, 1486–1501. [Google Scholar] [CrossRef]
- James, C.; Fisher, J.; Russel, V.; Collings, S.; Moss, B. Nitrate availability and hydrophyte species richness in shallow lakes. Freshw. Biol. 2005, 50, 1049–1063. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch–harmful algal bloom (HB–HAB) link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. (Eds.) ENA, European Nitrogen Assessment; Cambridge University Press: London, UK, 2011; p. 612. [Google Scholar]









| Data Sources | Normandy | Hauts-de-France |
|---|---|---|
| Hydrology | HYDRO database (http://www.hydro.eaufrance.fr) | |
| Point sources | ||
| WWTPs | Seine Normandy Water Agency | Artois Picardy Water Agency |
| Industries | Seine Normandy Water Agency | nd |
| Diffuse sources | ||
| Land use | Corine Land Cover | |
| N concentrations | Calculated from GRAFS | |
| Riparian retention | Temperature fonct (Topt = 40 °C; dti = 10 °C) | |
| Regions | River Names | Surface Area | Population | Cropland | Grassland |
|---|---|---|---|---|---|
| km2 | Inhabitants | % | % | ||
| Normandy | Douve & Taute | 1440 | 129,424 | 15.4 | 76.9 |
| Vire & Aure | 1575 | 440,402 | 22.7 | 69.9 | |
| Orne | 2903 | 440,757 | 35.7 | 48.7 | |
| Dives | 1776 | 152,532 | 38.4 | 51.1 | |
| Touques | 1273 | 165,723 | 22.9 | 62.9 | |
| Seine (Eure, Risle) | 73,224 | 2,0829,875 | 50.5 | 20.2 | |
| Arques | 1020 | 101,795 | 44.5 | 34.0 | |
| Hauts-de-France | Somme | 6029 | 632,989 | 77.3 | 7.2 |
| Authie | 1089 | 48,459 | 68.2 | 18.1 | |
| Canche | 1327 | 82,615 | 64.1 | 22.6 | |
| Aa | 1144 | 196,093 | 66.4 | 17.9 |
| Parameter | Meaning | Unit | Value |
|---|---|---|---|
| µmaxpsnz | Maximum growth rate | d−1 | 1.6 |
| Toptpsnz | Optimal temperature | degree C | 15 |
| Tlethalpsnz | Lethal temperature | degree C | 21 |
| βpsnz | Steepness parameter of Blanchard’s curve | unitless | 0.6 |
| KNO3psnz | Half-saturation constant for NO3 uptake | micromolN·L−1 | 1.5 |
| KNH4psnz | Half-saturation constant for NH4 uptake | micromolN·L−1 | 0.8 |
| KPO4psnz | Half-saturation constant for PO4 uptake | micromolP·L−1 | 0.03 |
| KSipsnz | Half-saturation constant for Si(OH)4 uptake | micromolSi·L−1 | 1 |
| vmaxSipsnz | Maximum Si(OH)4 uptake rate | micromolSi.micromolN−1·d−1 | 0.15 |
| qminSi | Minimum Si quota | micromolSi.micromol·N−1 | 0.85 |
| fSi_th | Si limitation threshold for DA toxin production | unitless | 0.3 |
| rda | DA toxin production rate | microgDA.micromolN−1·d−1 | 10 |
| dda | DA toxin destruction rate | d−1 | 0.02 |
| Ikpsnz | Ik parameter of Smith’s curve of light effect | W·m−2 | 40 |
| mpsnz | Mortality rate | d−1 | 0.015 |
| τpsnz_mesozoo | Edibility for the mesozooplankton | unitless | 0.3 |
| τpsnz_microzoo | Edibility for the microzooplankton | unitless | 0.3 |
| Poses | Abbeville | ||||||
|---|---|---|---|---|---|---|---|
| Water Quality Variables | Units | Obs. (n) | NRMSE | Biais | Obs. (n) | NRMSE | Biais |
| % | % | % | % | ||||
| Discharge | m3·s−1 | 468 | 5 | −7 | 468 | 6 | −1 |
| Nitrate | mgN·L−1 | 368 | 9 | 9 | 108 | 59 | 36 |
| Phosphates | mgP·L−1 | 368 | 41 | 7 | 70 | 14 | 28 |
| Total phosphorus | mgP·L−1 | 368 | 18 | 3 | 0 | − | − |
| Silica | mgSi L−1 | 344 | 25 | 7 | 0 | − | − |
| Chlorophyll a | µg·L−1 | 354 | 18 | −33 | 0 | − | − |
| Suspended Solids | mg·L−1 | 347 | 16 | 23 | 116 | 15 | 6 |
| Regions | River Names | Average Discharge | kton N yr−1 | kton P yr−1 | kton Si yr−1 |
|---|---|---|---|---|---|
| m3 s−1 | |||||
| Normandy | Douve & Taute | 17.5 | 2.9 | 0.1 | 1.5 |
| Vire & Aure | 16.9 | 3.6 | 0.1 | 2.0 | |
| Orne | 31.9 | 5.8 | 0.1 | 4.5 | |
| Dives | 8.0 | 2.4 | 0.1 | 2.1 | |
| Touques | 9.7 | 2.9 | 0.1 | 2.1 | |
| Seine (Eure, Risle) | 532.1 | 141.7 | 4.8 | 69.6 | |
| Arques | 9.1 | 1.3 | 0.1 | 1.7 | |
| Hauts-de-France | Somme | 37.2 | 4.9 | 0.2 | 5.7 |
| Authie | 11.2 | 2.4 | 0.1 | 2.5 | |
| Canche | 17.4 | 2.3 | 0.1 | 3.2 | |
| Aa | 16.8 | 2.2 | 0.1 | 3.7 |
| Antifer | Gefosse | Dunkerque | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water quality variables | Units | Obs. (n) | NRMSE | Biais | Obs. (n) | NRMSE | Biais | Obs. (n) | NRMSE | Biais |
| % | % | % | % | % | % | |||||
| Temperature | °C | 1119 | 27 | −16 | 464 | 7 | 1 | 161 | 8 | 0 |
| Salinity | − | 698 | 13 | 4 | 466 | 10 | 0 | 164 | 21 | 1 |
| Nitrate | µM | 237 | 15 | −38 | 203 | 10 | 44 | 49 | 21 | −10 |
| Phosphates | µM | 238 | 17 | −72 | 203 | 17 | −33 | 136 | 16 | −12 |
| Silica | µM | 241 | 13 | −33 | 206 | 18 | 15 | 137 | 23 | 56 |
| Chlorophyll a | µg L−1 | 325 | 12 | −36 | 239 | 14 | 48 | 141 | 20 | −49 |
| N-ICEP, kgC km−2 d−1 | P-ICEP, kgC km−2 d−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pristine | 1980’s | Ref | WWTP | A/R/D | Pristine | 1980’s | Ref | WWTP | A/R/D | ||
| Normandy | Douve & Taute | −7.5 | 40.9 | 6.7 | 6.7 | −0.1 | −8.9 | −6.3 | −6.6 | −6.9 | −6.8 |
| Vire & Aure | −8.4 | 60.1 | 11.8 | 11.7 | 4.0 | −9.3 | −7.0 | −7.4 | −7.5 | −7.5 | |
| Orne | −7.7 | 94.5 | 20.2 | 19.9 | 10.5 | −8.7 | −1.8 | −6.3 | −6.9 | −6.9 | |
| Dives | −3.1 | 36.8 | 6.8 | 6.3 | 2.7 | −3.8 | −1.4 | −2.1 | −3.2 | −3.2 | |
| Touques | −6.0 | 34.2 | 9.2 | 7.6 | 2.8 | −7.0 | −2.3 | −3.5 | −6.1 | −6.1 | |
| Seine | −1.5 | 51.0 | 24.5 | 20.3 | 11.5 | −1.8 | 10.3 | 1.7 | −0.2 | −0.2 | |
| Arques | −5.6 | 79.6 | 25.4 | 25.2 | 10.2 | −6.2 | −1.9 | −2.6 | −4.5 | −4.5 | |
| Hauts-de-France | Somme | −4.4 | 46.7 | 14.2 | 12.2 | 3.4 | −5.2 | −3.5 | −3.7 | −4.8 | −4.8 |
| Authie | −4.0 | 97.5 | 21.9 | 21.9 | 7.8 | −5.3 | −4.5 | −4.6 | −4.6 | −4.6 | |
| Canche | −0.6 | 165.1 | 28.2 | 28.4 | 17.0 | −1.9 | −0.5 | −0.9 | −0.9 | −0.9 | |
| Aa | −0.5 | 142.6 | 25.5 | 26.6 | 17.6 | −1.6 | 3.6 | −0.2 | −0.3 | −0.3 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnier, J.; Riou, P.; Le Gendre, R.; Ramarson, A.; Billen, G.; Cugier, P.; Schapira, M.; Théry, S.; Thieu, V.; Ménesguen, A. Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel. Geosciences 2019, 9, 441. https://doi.org/10.3390/geosciences9100441
Garnier J, Riou P, Le Gendre R, Ramarson A, Billen G, Cugier P, Schapira M, Théry S, Thieu V, Ménesguen A. Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel. Geosciences. 2019; 9(10):441. https://doi.org/10.3390/geosciences9100441
Chicago/Turabian StyleGarnier, Josette, Philippe Riou, Romain Le Gendre, Antsiva Ramarson, Gilles Billen, Philippe Cugier, Mathilde Schapira, Sylvain Théry, Vincent Thieu, and Alain Ménesguen. 2019. "Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel" Geosciences 9, no. 10: 441. https://doi.org/10.3390/geosciences9100441