Desiccation Cracking Behavior of MICP-Treated Bentonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Bacteria and Cementation Solution
2.3. Sample Preparation
2.4. Testing Procedure
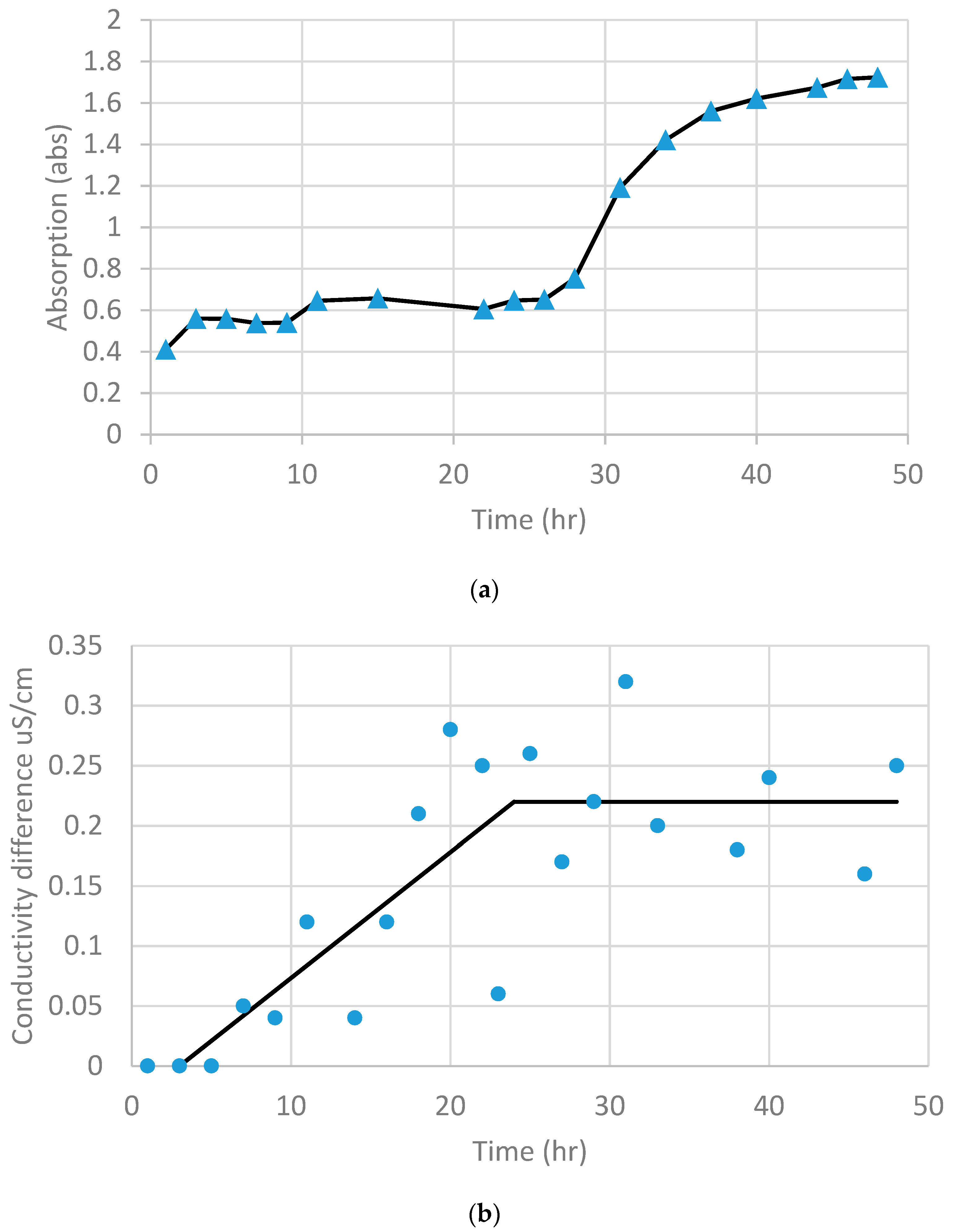
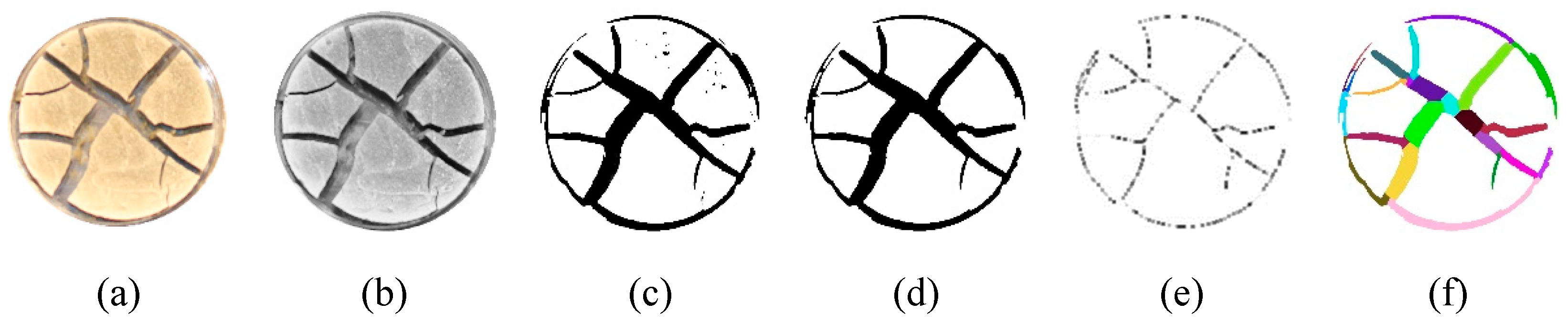
2.5. Image Processing and Quantitative Analysis
3. Results
3.1. Experimental Observation of Desiccation Cracks
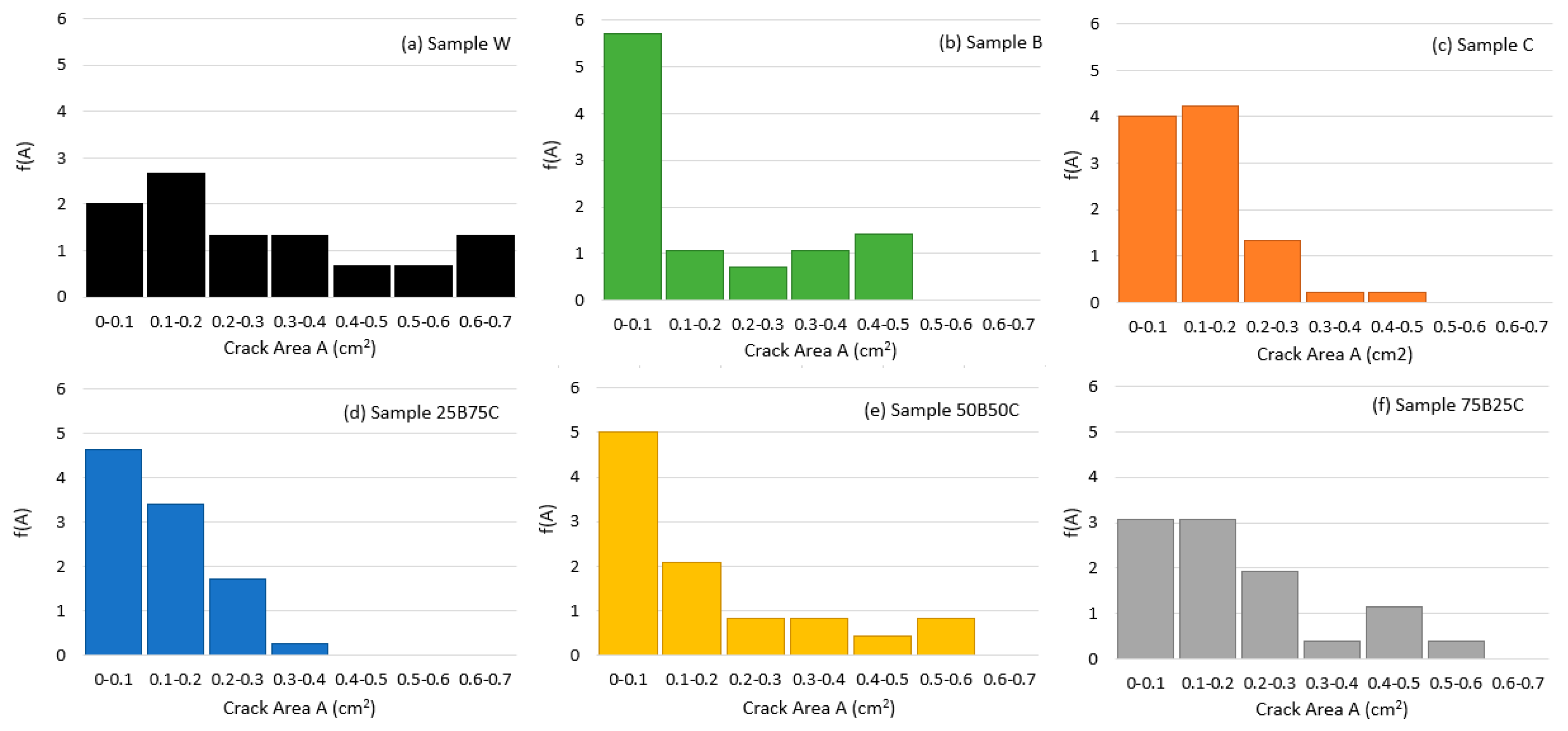
3.2. Quantitative Analysis of Crack Patterns
4. Discussion
4.1. Effect of Solution Type on Water Evaporation
4.2. Effect of Solution Type on Crack Pattern
4.3. Significance and Limitation of Current Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pusch, R. Highly compacted sodium bentonite for isolating rock-deposited radioactive waste products. Nucl. Technol. 1979, 45, 153–157. [Google Scholar] [CrossRef]
- Villar, M.V.; Lloret, A. Influence of dry density and water content on the swelling of a compacted bentonite. Appl. Clay Sci. 2008, 39, 38–49. [Google Scholar] [CrossRef]
- Chertkov, V.Y.; Ravina, I. Tortuosity of crack networks in swelling clay soils. Soil Sci. Soc. Am. 1996, 63, 1523–1530. [Google Scholar] [CrossRef]
- Hallett, P.D.; Newson, T.A. Describing soil crack formation using elastic-plastic fracture mechanics. Eur. J. Soil Sci. 2005, 56, 31–38. [Google Scholar] [CrossRef]
- Boynton, S.S.; Daniel, D.E. Hydraulic Conductivity Tests on Compacted Clay. J. Geotech. Eng. 1985, 111, 465–478. [Google Scholar] [CrossRef]
- Albrecht, B.A.; Benson, C.H. Effect of Desiccation on Compacted Natural Clays. J. Geotech. Geoenviron. Eng. 2001, 127, 67–75. [Google Scholar] [CrossRef]
- Miller, C.J.; Mi, H.; Yesiller, N. Experimental analysis of desiccation crack propagation in clay liners. JAWRA J. Am. Water Resour. Assoc. 1998, 34, 677–686. [Google Scholar] [CrossRef]
- Alonso, E.; Gens, A.; Lloret, A.; Delahaye, C. Effect of rain infiltration on the stability of slopes. In Proceedings of the First International Conference on Unsaturated Soils, Paris, France, 6–8 September 1995. [Google Scholar]
- Groenevelt, P.; Grant, C. Analysis of soil shrinkage data. Soil Tillage Res. 2004, 79, 71–77. [Google Scholar] [CrossRef]
- Osinubi, K.J.; Nwaiwu, C.M.O. Desiccation-induced Shrinkage in Compacted Lateritic Soils. Geotech. Geol. Eng. 2008, 26, 603–611. [Google Scholar] [CrossRef]
- Steinberg, M.L. Geomembranes and the Control of Expansive Soils in Construction. In Proceedings of the Geosynthetics 1999, Specifying Geosynthetics and Developing Design Details, Boston, MA, USA, 28–30 April 1999. [Google Scholar]
- Leung, M.; Vipulanandan, C. Treating Contaminated, Cracked and Permeable Field Clay with Grouts. In Proceedings of the Specialty Conference on Geotechnical Practice in Waste Disposal, Geotechnical Special Publication. Ann Arbor, MI, USA, 13–15 June 1977; ASCE: Reston, VA, USA, 1995; pp. 829–843. [Google Scholar]
- Al-Taie, A.; Disfani, M.M.; Evans, R.; Arulrajah, A.; Horpibulsuk, S. Swell-shrink Cycles of Lime Stabilized Expansive Subgrade. Procedia Eng. 2016, 143, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Omidi, G.H.; Prasad, T.V.; Thomas, J.C.; Brown, K.W. The influence of amendments on the volumetric shrinkage and integrity of compacted clay soils used in landfill liners. Water Air Soil Pollut. 1996, 86, 263–274. [Google Scholar] [CrossRef]
- Harianto, T.; Hayashi, S.; Du, Y.-J.; Suetsugu, D. Effects of Fiber Additives on the Desiccation Crack Behavior of the Compacted Akaboku Soil as A Material for Landfill Cover Barrier. Water Air Soil Pollut. 2008, 194, 141–149. [Google Scholar] [CrossRef]
- Tang, C.-S.; Shi, B.; Cui, Y.-J.; Liu, C.; Gu, K. Desiccation cracking behavior of polypropylene fiber–reinforced clayey soil. Can. Geotech. J. 2012, 49, 1088–1101. [Google Scholar] [CrossRef]
- Reddy, N.G.; Tahasildar, J.; Rao, B.H. Evaluating the influence of additives on swelling characteristics. Int. J. Geosynth. Ground Eng. 2015, 7, 2–13. [Google Scholar]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Jiang, N.J.; Soga, K.; Kuo, M. Microbially induced carbonate precipitation for seepage-induced internal erosion control in sand–clay mixtures. J. Geotech. Geoenviron. Eng. 2016, 143, 04016100. [Google Scholar] [CrossRef]
- Bang, S.S.; Lippert, J.J.; Yerra, U.; Mulukutla, S.; Ramakrishnan, V. Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int. J. Smart Nano Mater. 2010, 1, 28–39. [Google Scholar] [CrossRef]
- Fujita, Y.; Taylor, J.L.; Gresham, T.L.T.; Delwiche, M.E.; Colwell, F.S.; McLing, T.L.; Petzke, L.M.; Smith, R.W. Stimulation of microbial urea hydrolysis in groundwater to enhance calcite precipitation. Environ. Sci. Technol. 2008, 42, 3025–3032. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M. Assessment of different treatment methods by microbial-induced calcite precipitation for clayey soil improvement. In Proceedings of the 68th Canadian Geotechnical Conference, Quebec, QC, Canada, 20 September 2015. [Google Scholar]
- Cardoso, R.; Pires, I.; Duarte, S.O.; Monteiro, G.A. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Li, M.; Fang, C.; Kawasaki, S.; Achal, V. Fly ash incorporated with biocement to improve strength of expansive soil. Sci. Rep. 2018, 8, 2565. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Loria, M.; Rhoades, K.; Yu, X.B. Effects of Microbial Induced Calcite Precipitation on Bentonite Cracking Remediation. In Proceedings of the IFCEE 2018: Recent Developments in Geotechnical Engineering Practice, Orlando, FL, USA, 5–10 March 2018. [Google Scholar]
- ASTM International. ASTM D2487-17 Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System), ASTM Standard D2487; American Society for Testing and Materials: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- Mortensen, B.; Haber, M.; DeJong, J.; Caslake, L.; Nelson, D. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Montoya, B.; DeJong, J.; Boulanger, R. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique 2013, 63, 302–312. [Google Scholar] [CrossRef]
- Kodikara, J.K.; Barbour, S.L.; Fredlund, D.G. Desiccation cracking of soil layers. Unsaturated Soils Asia. 2000, 90, 139. [Google Scholar]
- Lakshmikantha, M.R.; Prat, P.C.; Ledesma, A. Boundary Effects in the Desiccation of Soil Layers with Controlled Environmental Conditions. Geotech. Test. J. 2018, 41, 675–697. [Google Scholar] [CrossRef]
- Decarlo, K.F.; Shokri, N. Effects of substrate on cracking patterns and dynamics in desiccating clay layers. Water Resour. Res. 2014, 50, 3039–3051. [Google Scholar] [CrossRef]
- Tang, C.-S.; Shi, B.; Liu, C.; Zhao, L.; Wang, B. Influencing factors of geometrical structure of surface shrinkage cracks in clayey soils. Eng. Geol. 2008, 101, 204–217. [Google Scholar] [CrossRef]
- Morris, P.H.; Graham, J.; Williams, D.J. Cracking in drying soils. Can. Geotech. J. 1992, 29, 263–277. [Google Scholar] [CrossRef]
- Zhu, C.-M.; Ye, W.-M.; Chen, Y.-G.; Chen, B.; Cui, Y.-J. Influence of salt solutions on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Eng. Geol. 2013, 166, 74–80. [Google Scholar] [CrossRef]
- Decarlo, K.F.; Shokri, N. Salinity effects on cracking morphology and dynamics in 3-D desiccating clays. Water Resour. Res. 2014, 50, 3052–3072. [Google Scholar] [CrossRef]
- Petry, T.M.; Little, D.N. Review of Stabilization of Clays and Expansive Soils in Pavements and Lightly Loaded Structures—History, Practice, and Future. J. Mater. Civ. Eng. 2002, 14, 447–460. [Google Scholar] [CrossRef]
- Weinberger, R. Initiation and growth of cracks during desiccation of stratified muddy sediments. J. Struct. Geol. 1999, 21, 379–386. [Google Scholar] [CrossRef]
- Nahlawi, H.; Kodikara, J.K.; Kodikara, J. Laboratory experiments on desiccation cracking of thin soil layers. Geotech. Geol. Eng. 2006, 24, 1641–1664. [Google Scholar] [CrossRef]
- Tang, C.-S.; Cui, Y.-J.; Shi, B.; Tang, A.-M.; Liu, C. Desiccation and cracking behaviour of clay layer from slurry state under wetting–drying cycles. Geoderma 2011, 166, 111–118. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Rapid Determination of Carbonate Content of Soils, ASTM D4373; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors Affecting Improvement of Engineering Properties of MICP-Treated Soil Catalyzed by Bacteria and Urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Bell, F. Lime stabilization of clay minerals and soils. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Garakani, A.A.; Haeri, S.M.; Cherati, D.Y.; Givi, F.A.; Tadi, M.K.; Hashemi, A.H.; Chiti, N.; Qahremani, F. Effect of road salts on the hydro-mechanical behavior of unsaturated collapsible soils. Transp. Geotech. 2018, 17, 77–90. [Google Scholar] [CrossRef]
- Egloffstein, T.A. Natural bentonites—Influence of the ion exchange and partial desiccation on permeability and self-healing capacity of bentonites used in GCLs. Geotext. Geomembr. 2001, 19, 427–444. [Google Scholar] [CrossRef]
- Banagan, B.; Wertheim, B.; Roth, M.; Caslake, L. Microbial strengthening of loose sand. Lett. Appl. Microbiol. 2010, 51, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.H.; Daniel, D.E.; Eykholt, G.R. Calcium and Sodium Bentonite for Hydraulic Containment Applications. J. Geotech. Geoenviron. Eng. 1997, 123, 438–445. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Zhang, L. Geometric parameters and REV of a crack network in soil. Comput. Geotech. 2010, 37, 466–475. [Google Scholar] [CrossRef]





| Soil Properties | Value |
|---|---|
| Specific gravity | 2.6 |
| Consistency limit | |
| Liquid limit (%) | 276 |
| Plastic limit (%) | 37 |
| Plasticity index (%) | 239 |
| pH | 8.5 |
| USCS classification | CH |
| Clay (< 2 ) | 46% |
| Crack Parameters | Sample W | Sample B | Sample C | Sample 25B75C | Sample 50B50C | Sample 75B25C |
|---|---|---|---|---|---|---|
| Surface crack ratio (%) | 29.2 | 24 | 29 | 25.7 | 22.6 | 23.5 |
| Average crack width (cm) | 0.28 | 0.18 | 0.20 | 0.17 | 0.18 | 0.18 |
| Total crack length (cm) | 23.4 | 28.8 | 36.2 | 34.2 | 29.0 | 31.7 |
| Crack segment number (−) | 16 | 28 | 44 | 41 | 24 | 25 |
| Average crack length (cm) | 1.46 | 1.02 | 0.82 | 0.83 | 1.21 | 1.27 |
| Sample Type | W | B | C | 25B75C | 50B50C | 75B25C |
|---|---|---|---|---|---|---|
| Surface crack initiation time (hr) | 2.5 | 5 | 4.5 | 16.5 | 16 | 17 |
| Critical water content | 93.5% | 81% | 85% | 72% | 77.3% | 66.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vail, M.; Zhu, C.; Tang, C.-S.; Anderson, L.; Moroski, M.; Montalbo-Lomboy, M.T. Desiccation Cracking Behavior of MICP-Treated Bentonite. Geosciences 2019, 9, 385. https://doi.org/10.3390/geosciences9090385
Vail M, Zhu C, Tang C-S, Anderson L, Moroski M, Montalbo-Lomboy MT. Desiccation Cracking Behavior of MICP-Treated Bentonite. Geosciences. 2019; 9(9):385. https://doi.org/10.3390/geosciences9090385
Chicago/Turabian StyleVail, Mark, Cheng Zhu, Chao-Sheng Tang, Luke Anderson, Michael Moroski, and Melissa Tabada Montalbo-Lomboy. 2019. "Desiccation Cracking Behavior of MICP-Treated Bentonite" Geosciences 9, no. 9: 385. https://doi.org/10.3390/geosciences9090385





