Abstract
Type 2 diabetes mellitus (T2DM) is the world’s most common metabolic disease. The development of T2DM is mainly caused by a combination of two factors: the failure of insulin secretion by the pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin (insulin resistance); therefore, the disease is indicated by a chronic increase in blood glucose. T2DM patients can be treated with mono- or combined therapy using oral antidiabetic drugs and insulin-replaced agents; however, the medication often leads to various discomforts, such as abdominal pain, diarrhea or constipation, nausea and vomiting, and hypersensitivity reactions. A biguanide drug, metformin, has been used as a first-line drug to reduce blood sugar levels. Sulfonylureas work by blocking the ATP-sensitive potassium channel, directly inducing the release of insulin from pancreatic β-cells and thus decreasing blood glucose concentrations. However, the risk of the failure of sulfonylurea as a monotherapy agent is greater than that of metformin or rosiglitazone (a thiazolidinedione drug). Sulfonylureas are used as the first-line drug of choice for DM patients who cannot tolerate metformin therapy. Other antidiabetic drugs, thiazolidinediones, work by activating the peroxisome proliferator-activated receptor gamma (PPARγ), decreasing the IR level, and increasing the response of β-cells towards the glucose level. However, thiazolidines may increase the risk of cardiovascular disease, weight gain, water retention, and edema. This review article aims to discuss case reports on the use of metformin, sulfonylureas, and thiazolidinediones in DM patients. The literature search was conducted on the PubMed database using the keywords ‘metformin OR sulfonylureas OR thiazolidinediones AND case reports’, filtered to ‘free full text’, ‘case reports’, and ‘10 years publication date’. In some patients, metformin may affect sleep quality and, in rare cases, leads to the occurrence of lactate acidosis; thus, patients taking this drug should be monitored for their kidney status, plasma pH, and plasma metformin level. Sulfonylureas and TZDs may cause a higher risk of hypoglycemia and weight gain or edema due to fluid retention. TZDs may be associated with risks of cardiovascular events in patients with concomitant T2DM and chronic obstructive pulmonary disease. Therefore, patients taking these drugs should be closely monitored for adverse effects.
1. Introduction
The WHO defines diabetes mellitus (DM) as a chronic metabolic disease that is characterized by an increase in blood glucose and causes defects to the heart, blood vessels, kidneys, and nerves [1,2]. The International Diabetes Federation (IDF) recently reported that of approximately 415 million DM patients, 91% were categorized as T2DM [3]. Moreover, Indonesian Basic Health Research 2018 reported that the prevalence of T2DM in Indonesia had increased remarkably [4]. Patients with T2DM tend to have 15% higher mortality risks compared to those without DM [5,6].
More than 90% of DM cases are type 2 (T2DM), which is mainly caused by a combination of two factors: the failure of insulin secretion by the pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin (insulin resistance) [3,5]. Insulin resistance (IR) is a condition whereby insulin-targeted tissues, e.g., adipose, liver, and muscle, encounter metabolic disorders, thus leading to metabolic syndrome diseases, non-alcoholic fatty liver disease (NAFLD), atherosclerosis, and T2DM [7,8,9].
T2DM is a metabolic and endocrine disorder initiated by complex interactions between genetic factors, unhealthy lifestyle, and pancreatic β-cell dysfunction, along with other endocrine abnormalities [10,11,12]. T2DM is always associated with IR, a condition whereby pancreatic β-cells fail to compensate for insulin [13,14,15], leading to a continuous increase in blood glucose; thus, the demand for more insulin release is needed [3,16]. The distorted function of insulin induces the elevation of lipogenesis but fails to inhibit gluconeogenesis [17]. Gluconeogenesis is the specific characteristic of IR and T2DM: the elevation of hepatic glucose production whereby glycogen synthesis in the liver keeps maintaining the gluconeogenic activity without decreasing the high level of blood glucose [14,17]. The accumulation of fat in the liver eventually escalates the blood glucose levels through the reduction in insulin sensitivity and the occurrence of IR [18]. IR stimulates the increase in fatty acids in the blood, reduces the transportation of glucose to the muscle cells, and increases lipolysis, thus leading to the rise in hepatic glucose production [19,20].
The most effective management of T2DM needs an adjustment in both lifestyles, including a healthy diet and routine workouts, and oral antidiabetic drugs [21]. This review article aims to comprehensively discuss the case reports on metformin, sulfonylurea, and thiazolidinedione therapies in T2DM patients. Briefly, a literature search was conducted on the PubMed database (https://pubmed.ncbi.nlm.nih.gov/ accessed from 10 January to 30 May 2023) using the keywords ‘metformin OR sulfonylureas OR thiazolidinediones AND case reports’, filtered to ‘free full text’, ‘case reports’, and ’10 years publication date’. The initial search resulted in 496 articles, which were further screened by the title and the abstract for their relevance to the topic of interest. Review articles, systematic reviews, and clinical trials were excluded. The case report articles included in this review totaled four for sulfonylureas and two for thiazolidinediones; for metformin, only the cases that involved nightmares were included in this review. An additional search was conducted on Google using the same keywords.
2. Pharmacology of Sulfonylureas
Sulfonylureas are classified as both first- (e.g., tolbutamide and chlorpropamide) and second-generation antidiabetic drugs (e.g., glyburide, gliclazide, glipizide, and glimepiride). The second generation of anti-DM drugs has shown higher potency with a lower dose [22]. Both generations are able to significantly diminish glycosylated hemoglobin (HbA1c) levels. HbA1c represents the blood glucose status over the previous 90 days [23,24].
Sulfonylureas work by blocking the ATP-sensitive potassium channel, directly inducing the release of insulin from pancreatic β-cells, thus decreasing blood glucose concentrations [7,25]. However, the failure levels of sulfonylureas as monotherapy drugs are greater compared to those of metformin (biguanide) or rosiglitazone (thiazolidinedione). Sulfonylureas are the first-line drugs of choice for patients who cannot tolerate metformin [22].
More than 90% of sulfonylureas in the blood are bound to the plasma proteins, which eventually cause drug–drug interactions with salicylates, sulfonamides, and warfarin [22]. The main side effects of sulfonylureas are a higher risk of hypoglycemia and weight gain [26]. The increase in BW is associated with the anabolic effect of higher insulin levels and the decrease in glycosuria [14,15]. Several cases of the use of sulfonylureas in DM patients are summarized in Table 1.

Table 1.
Cases of the use of sulfonylureas in DM patients.
Sulfonylureas are the first-line drugs of choice for patients who cannot tolerate metformin. These drugs may cause mild to severe adverse events in T1DM or T2DM patients [27,28,29,30]. However, it should be noted that although oral hypoglycemic drugs are not recommended during pregnancy, sulfonylureas can be used in gestational diabetes mellitus with a normal outcome of the pregnancy because hyperglycemia often occurs during organogenesis and is linked with an elevated risk of congenital malformations, preeclampsia, premature delivery, intrauterine infections, and perinatal mortality [27].
3. Pharmacology of Thiazolidinediones
Pioglitazone and rosiglitazone, which are both thiazolidinediones (TZDs), work by activating the peroxisome proliferator-activated receptor gamma (PPARγ) [31], decreasing the IR level, and increasing the response of β-cells towards the glucose level [22,32,33]. However, thiazolidines may increase the risk of cardiovascular disease [26], weight gain, water retention, and edema [14]. Pioglitazone (15 mg/day) effectively reduced aminotransferase and bilirubin levels in a nonalcoholic steatohepatitis patient within three months of therapy [31]. Rosiglitazone has been withdrawn due to its effect on the bones and because it may cause an ischemic heart attack [33]. The most reported side effect of pioglitazone is the occurrence of pleural effusion in diabetic patients [34]. Several cases of the use of thiazolidinediones in DM patients are presented in Table 2.

Table 2.
Cases of the use of thiazolidinediones in DM patients.
It was estimated that 1.5–2.6% of cases experienced a worsening of DME after TZD (pioglitazone and/or rosiglitazone) therapy. Fluid retention was observed in 5–15% of patients treated with glitazones, and drug discontinuation resulted in the rapid resolution of both peripheral and macular edema [37]. TZDs may directly alter water reabsorption in the kidneys by affecting tubular transport, sodium retention, and vascular hyperpermeability or indirectly by affecting renal hemodynamics [38]. It was shown that the exposure of primary collecting duct cells to TZDs induced an increase in sodium transport, body weight gain, and plasma volume expansion [39].
Moreover, the prevalence of bladder cancer in T2DM patients treated with pioglitazone was recently studied. All the patients (n = 6440) were of Asian Indian ethnicity. It was concluded that pioglitazone is not correlated with a bladder cancer risk. However, this drug should not be given to patients with a history of hematuria [40].
The meta-analysis of randomized control trials revealed that TZDs had significantly decreased HbA1c, fasting blood glucose, and elevated HDL levels [41]. In a retrospective analysis, TZD administered as an add-on to metformin was reported safe for patients with mild renal impairment and normal renal function [42]. Another study reported that rosiglitazone therapy was related to a high risk of major cardiovascular events. In patients with concomitant T2DM and chronic obstructive pulmonary disease, TZDs may be associated with more risks of cardiovascular events, ventilation use, pneumonia, and lung cancer [43].
All things considered, thiazolidine pioglitazone has advantages in terms of the decreasing of triglycerides, the increasing of HDL, and the decreasing of the LDL concentration, which is thought to be due to the activation of PPAR-α [44].
4. Pharmacology of Metformin
Metformin (dimethyl biguanide) has been used to reduce blood sugar levels; however, other guanidines (phenformin and buformin) were withdrawn due to a lactate acidosis risk [22,45].
Metformin or Glucophage is the first-line therapy for T2DM management. This drug has been approved by the FDA (Food and Drug Administration) [8,45,46,47]. Metformin ameliorates glycemic control in patients with T2DM by amending insulin sensitivity in the liver. The main outcome is a decrease in glucose synthesis in the liver and an elevation in glucose excretion in the skeletal muscles [25,47,48]. The oral bioavailability of metformin is 40–60% with T1/2 plasma of 4–9 h. T2DM patients treated with metformin usually experience a reduction in the fasting plasma glucose of 2–4 mmol/L and in HbA1c of 1–2%, independently of their age, body weight, and the duration of therapy [49]. The main side effects of metformin therapy are nausea and other gastrointestinal inconveniences [22]. A correlation was reported between long-term metformin use and vitamin B12 deficiency [50,51,52]. Metformin causes depression, numbness, vision disorders, and mouth ulcers [52]. Although metformin is the oral drug of choice for patients with T2DM, several cases of the use of metformin that involved nightmares have been reported globally and are summarized in Table 3.

Table 3.
Cases of the use of metformin in T2DM Patients.
A cross-sectional study of more than 200 patients with childhood-onset T1DM reported that 26% of individuals had nightmares and 41% of them had poor sleep quality. The participants with uncontrolled glycemia revealed a higher frequency of nightmares compared to the patients with better glycemic control [56].
It was decided that the nocturnal decrease in cerebral blood glucose levels was the cause of the occurrence of nightmares and abnormal dreams [57]. The occurrence of abnormal dreams and the low quality of sleep among T2DM patients were found to be associated with poor glycemic control in such patients [58]. Studies in humans suggested that the endocannabinoid system was overactivated in patients with obesity and/or DM. The activation of the cannabinoid-1 receptors stimulates weight gain and is associated with metabolic changes [59]. Moreover, in a recent study, the HbA1c level was reported to correlate with the duration of sleep in T2DM patients. It was suggested that patients with T2DM should have an appropriate range of sleep duration [60].
In addition to sleep disorders, metformin therapy may also cause lactic acidosis. A case of a 70-year-old male Caucasian diagnosed with T2DM who had taken a deliberate metformin overdose revealed a profound lactic acidosis with a pH of 6.93 and a lactate level > 20 mmol/L. The patient was admitted to the ICU and was given continuous hemodiafiltration with an average blood flow rate of 150 mL/hour for 48 h and 8.4% sodium bicarbonate to neutralize the acid [61].
A recent study also reported 4241 cases of lactic acidosis in T2DM patients who were metformin users. Metformin-associated lactic acidosis (MaLA), although it rarely occurs, has been of great concern due to its life-threatening prevalence. Key information to have when evaluating a case of suspected MaLA includes the times and doses of metformin medication, the kidney condition, plasma pH, concentrations of metformin in the plasma, and details of concomitant drugs and comorbid risk factors for lactate acidosis [62]. A very rare case of metformin-induced hemolytic anemia was reported in a 17-year-old male patient [63].
There are very few cases of metformin use reported in Indonesia. However, a recent case of metformin in Lampung, Sumatra, is described as follows: a female patient (65 years old) was diagnosed with T2DM 2 years ago and had complaints of fatigue accompanied by weakness during activities. The fatigue was rarely felt, but it later became more frequent. The complaints were not accompanied by blurred vision or numbness. This patient had an HbA1C of 8.7%. It should be noted that for elderly T2DM patients, lifestyle adjustment is critical as a first step [64]. Metformin has been reported to cause a deficiency of vitamin B12 [45,65,66].
The safety of long-term use of metformin in 393 T2DM patients with contraindications has been studied. Of them, 67.68% were suffering from coronary heart disease, and 23.92% were from congestive heart failure. It was concluded that the patients who tolerate the drug well may continue taking it, even when mild kidney impairment evolves [67].
5. Conclusions
Metformin is the first-line therapy for patients with T2DM. This drug ameliorates glycemic control by amending insulin sensitivity in the liver. However, data about the metformin side effects are limited and based mainly on case reports. It should be noted that in some patients, metformin may affect sleep quality by inducing abnormal nightmares and, in rare cases, the occurrence of lactate acidosis. Patients taking this drug should be monitored for their kidney status, plasma pH, and plasma metformin level. Another antidiabetic drug, sulfonylurea, is used as the first-line drug of choice for DM patients who cannot tolerate metformin therapy. Other antidiabetic drugs, TZDs, work by activating the PPAR-γ, decreasing the IR level, and increasing the response of β-cells toward the glucose level. However, it should be noted that in some patients, sulfonylureas and TZDs may cause a higher risk of hypoglycemia and weight gain or edema due to fluid retention. TZDs may be associated with risks of cardiovascular events in patients with concomitant T2DM and chronic obstructive pulmonary disease. Therefore, patients taking these drugs should be closely monitored for adverse effects. Based on the case reports, the advantages and disadvantages of these drugs are summarized in Figure 1.
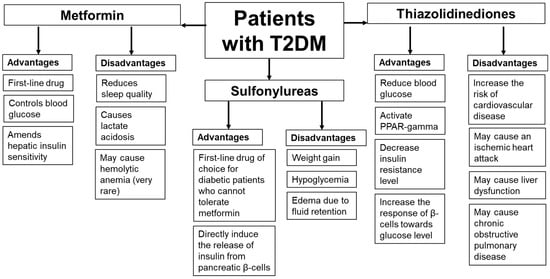
Figure 1.
The advantages and disadvantages of metformin, sulfonylurea, and thiazolidinedione therapies based on the case reports.
Author Contributions
Conceptualization, J.L. and S.A.S.; methodology, J.L. and E.S.; validation, S.A.S.; formal analysis, E.S.; data curation, J.L. and Y.S.; writing—original draft preparation, E.S.; writing—review and editing, J.L. and E.S.; supervision, S.A.S., J.L. and Y.S.; All authors have read and agreed to the published version of the manuscript.
Funding
The APC is funded by Padjadjaran University via the Directorate of Research and Community Engagement.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.; Sun, J.; Yang, Y.; Lou, X.; Wang, Y.; Wang, Y.; Ma, L. Cortical thinning in type 2 diabetes mellitus and recovering effects of insulin therapy. J. Clin. Neurosci. 2015, 22, 275–279. [Google Scholar] [CrossRef] [PubMed]
- WHO. Word Health Organization. 2022. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 5 March 2023).
- IDF. International Diabetes Federation. 2022. Available online: https://idf.org/aboutdiabetes/type-2-diabetes.html (accessed on 5 March 2023).
- The Republic of Indonesia Ministry of Health. Basic Health Research 2018. In Lembaga Penerbit Badan Penelitian dan Pengembangan Kesehatan; The Republic of Indonesia Ministry of Health: South Jakarta, Indonesia, 2019. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Diabetes Medications for Adults with Type 2 Diabetes: An Update. Comparative Effectiveness Review Number 173 (April 2016). AHRQ Publication No. 16-EHC013-EF. Available online: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/diabetes-update-2015_research.pdf (accessed on 5 March 2023).
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef]
- Khand, P.; Feng, B. The association of obesity with type 2 diabetes: A review. Int. J. Sci. Invent. Today 2020, 9, 61–74. [Google Scholar]
- Rottenkolber, M.; Gar, C.; Then, C.; Wanger, L.; Sacco, V.; Banning, F.; Potzel, A.L.; Kern-Matschilles, S.; Nevinny-Stickel-Hinzpeter, C.; Grallert, H.; et al. A pathophysiology of type 2 diabetes unrelated to metabolic syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 1460–1471. [Google Scholar] [CrossRef]
- Zaghlool, S.B.; Halama, A.; Stephan, N.; Gudmundsdottir, V.; Gudnason, V.; Jennings, L.L.; Thangam, M.; Ahlqvist, E.; Malik, R.A.; Albagha, O.M.E.; et al. Metabolic and proteomic signatures of type 2 diabetes subtypes in an Arab population. Nat. Commun. 2022, 13, 7121. [Google Scholar] [CrossRef]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Suastika, K.; Eliana, F.; Kshanti, I.A.M.; Mardianto, M.; Mudjarnako, S.W.; Natalia, N.; Nugrohom, H.S.H.; Sibarani, R.P.; Soewondo, P.; Soelistijo, S.A.; et al. Expert opinion on diabetes management challenges and role of basal insulin/GLP-1 RA fixed-ratio combination in people with type 2 diabetes from Indonesia. Diabetes Metab. Syndr. Obes. 2022, 15, 2977–2990. [Google Scholar] [CrossRef]
- Kumar, K.G.S.; Sasidharan, P.K.; Anoop, N. A study of clinical, metabolic, and anthropometric profile and possible etiological factors among newly detected type 2 DM in North Kerala. J. Evol. Med. Dent. Sci. 2019, 8, 907–914. [Google Scholar] [CrossRef]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging targets in type 2 diabetes and diabetic complications. Adv. Sci. 2021, 8, e2100275. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, e21, Erratum in Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Khemka, V.K.; Banerjee, A. Metabolic risk factors in obesity and diabetes mellitus: Implications in the pathogenesis and therapy. Integr. Obes. Diabetes 2017, 3, 1–4. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, B. Drug development strategy for type 2 diabetes: Targeting positive energy balances. Curr. Drug Targets 2019, 20, 879–890. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef]
- Goswami, G.; Shinkazh, N.; Davis, N. Optimal pharmacologic treatment strategies in obesity and type 2 diabetes. J. Clin. Med. 2014, 3, 595–613. [Google Scholar] [CrossRef]
- Eyth, E.; Naik, R. Hemoglobin A1C. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549816/ (accessed on 10 April 2023).
- Eller-Vainicher, C.; Cairoli, E.; Grassi, G.; Grassi, F.; Catalano, A.; Merlotti, D.; Falchetti, A.; Gaudio, A.; Chiodini, I.; Gennari, L. Pathophysiology and management of type 2 diabetes mellitus bone fragility. J. Diabetes Res. 2020, 2020, 7608964. [Google Scholar] [CrossRef]
- Qaseem, A.; Barry, M.J.; Humphrey, L.L.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians; Fitterman, N.; Horwitch, C.; Kansagara, D.; McLean, R.M.; Wilt, T.J. Oral pharmacologic treatment of type 2 diabetes mellitus: A clinical practice guideline update from the American College of Physicians. Ann. Intern. Med. 2017, 166, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Klupa, T.; Kozek, E.; Nowak, N.; Cyganek, K.; Gach, A.; Milewicz, T.; Czajkowski, K.; Tolloczko, J.; Mlynarski, W.; Malecki, M.T. The first case report of sulfonylurea use in a woman with permanent neonatal diabetes mellitus due to KCNJ11 mutation during a high-risk pregnancy. J. Clin. Endocrinol. Metab. 2010, 95, 3599–3604. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Aljenaee, K.; Garrahy, A.; Byrne, M. Successful transition from insulin to sulfonylurea, on second attempt, in a 24-year-old female with neonatal diabetes secondary to KCNJ11 gene mutation. BMJ Case Rep. 2021, 14, e239973. [Google Scholar] [CrossRef]
- Mifsud, S.; Schembri, E.L.; Fava, S. A case of severe relapsing sulphonylurea-induced hypoglycaemia. BMJ Case Rep. 2019, 12, e231368. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, K.; Austin, E.; Wu, P.E. Unintentional sulfonylurea toxicity due to a drug-drug interaction: A case report. BMC Res. Notes 2018, 11, 331. [Google Scholar] [CrossRef]
- Yasmin, S.; Jayaprakash, V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: From past to present. Eur. J. Med. Chem. 2017, 126, 879–893. [Google Scholar] [CrossRef]
- Ohno, T.; Nishigaki, Y.; Yamada, T.; Wakahara, Y.; Sakai, H.; Yoshimura, K.; Shimizu, M.; Usui, T.; Saito, M.; Yasuda, I.; et al. Effects of pioglitazone on nonalcoholic steatohepatitis in a patient with anorexia nervosa: A case report. Exp. Ther. Med. 2014, 7, 811–815. [Google Scholar] [CrossRef]
- Chatterjee, S.; Davies, M.J.; Tarigopula, G. Pharmacological control of blood sugar. Anaesth. Intensive Care Med. 2017, 18, 532–534. [Google Scholar] [CrossRef]
- Xue, J.; Liu, W.; Shi, F.; Zheng, J.; Ma, J. Pleural effusion due to use of pioglitazone: A case report. Metab. Syndr. Relat. Disord. 2020, 18, 168–171. [Google Scholar] [CrossRef]
- Oshitari, T.; Asaumi, N.; Watanabe, M.; Kumagai, K.; Mitamura, Y. Severe macular edema induced by pioglitazone in a patient with diabetic retinopathy: A case study. Vasc. Health Risk Manag. 2008, 4, 1137–1140. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Z.; Chen, P.; Song, X.; Li, W.; Yu, X.; Xie, J. Case Report: A new peroxisome proliferator-activated receptor gamma mutation causes familial partial lipodystrophy type 3 in a Chinese patient. Front. Endocrinol. 2022, 13, 830708. [Google Scholar] [CrossRef]
- Ryan, E.H., Jr.; Han, D.P.; Ramsay, R.C.; Cantrill, H.L.; Bennett, S.R.; Dev, S.; Williams, D.F. Diabetic macular edema associated with glitazone use. Retina 2006, 26, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Clifton, L.G.; McNulty, J.A.; Chen, L.; Brown, K.K.; Baer, P.G. Effects of a PPARgamma agonist, GI262570, on renal filtration fraction and nitric oxide level in conscious rats. J. Cardiovasc. Pharmacol. 2003, 42, 436–441. [Google Scholar] [CrossRef]
- Yang, T.; Soodvilai, S. Renal and vascular mechanisms of thiazolidinedione-induced fluid retention. PPAR Res. 2008, 2008, 943614. [Google Scholar] [CrossRef]
- Malhotra, B.; Hiteshi, P.; Khalkho, P.; Malik, R.; Bhadada, S.K.; Bhansali, A.; Shafiq, N.; Malhotra, S.; Kumar, N.; Rajput, R.; et al. Bladder cancer with pioglitazone: A case–control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102637. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Kwong, J.S.W.; Li, L.; Li, Y.; Sun, X. Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1717. [Google Scholar] [CrossRef]
- Banerji, M.A.; Purkayastha, D.; Francis, B.H. Safety and tolerability of vildagliptin vs. thiazolidinedione as add-on to metformin in type 2 diabetic patients with and without mild renal impairment: A retrospective analysis of the GALIANT study. Diabetes Res. Clin. Pract. 2010, 90, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-S.; Wei, J.C.-C.; Yang, Y.-C.; Hsu, C.-C.; Hwu, C.-M. Thiazolidinedione use in individuals with type 2 diabetes and chronic obstructive pulmonary disease. Front. Med. 2021, 8, 729518. [Google Scholar] [CrossRef]
- Hurren, K.M.; Dunham, M.W. Are thiazolidinediones a preferred drug treatment for type 2 diabetes? Exp. Opin. Pharmacother. 2021, 22, 131–133. [Google Scholar] [CrossRef]
- Sanjay, C.; Patil, K.; Nagabhushana, D.; Panda, R.; Mahima, V. Long-term use of metformin and its effect on serum vitamin B12 with its oral manifestations: A review. J. Clin. Diagn. Res. 2022, 16, ZE01–ZE06. [Google Scholar] [CrossRef]
- Hsu, W.H.; Hsiao, P.J.; Lin, P.C.; Chen, S.C.; Lee, M.Y.; Shin, S.J. Effect of metformin on kidney function in patients with type 2 diabetes mellitus and moderate chronic kidney disease. Oncotarget 2017, 9, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Starakis, I.K. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res. Clin. Pract. 2012, 97, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, S.; Quan, H.; Li, J. Vitamin B12 status in metformin treated patients: Systematic review. PLoS ONE 2014, 9, e100379. [Google Scholar] [CrossRef] [PubMed]
- Niafar, M.; Hai, F.; Porhomayon, J.; Nader, N.D. The role of metformin on vitamin B12 deficiency: A meta-analysis review. Intern. Emerg. Med. 2015, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; So, S.K.C.; Khallouq, B.B. The effect of metformin on vitamin B12 level in pediatric patients. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 223–228. [Google Scholar] [CrossRef]
- Moussa, A.M.; Sallam, A.M.; Alamri, H.A.; Alghamdi, J.A.; Almohussein, N.S.; Alshahrani, S.M.; Ali, N.S.M. Study on the side effects and complications of metformin on diabetic patients. Int. J. Pharm. Phytopharm. Res. 2021, 11, 28–33. [Google Scholar] [CrossRef]
- Voloshyna, D.; Sandhu, Q.I.; Khan, S.; Bseiso, A.; Mengar, J.; Nayudu, N.; Kumar, R.; Khemani, D.; Usama, M. An unusual association between metformin and nightmares: A case report. Cureus 2022, 14, e28974. [Google Scholar] [CrossRef]
- Yanto, T.A.; Huang, I.; Kosasih, F.N.; Lugito, N.P.H. Nightmare and abnormal dreams: Rare side effects of metformin? Case Rep. Endocrinol. 2018, 2018, 7809305. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Gautam, A.; Pursnani, N.; Parihar, A.; Singh, B. An unusual side effect of metformin—Nightmare and abnormal dreams. J. Diabetol. 2021, 12, 530–532. [Google Scholar] [CrossRef]
- Denic-Roberts, H.; Costacou, T.; Orchard, T.J. Subjective sleep disturbances and glycemic control in adults with long-standing type 1 diabetes: The Pittsburgh’s Epidemiology of Diabetes Complications study. Diabetes Res. Clin. Pract. 2016, 119, 1–12. [Google Scholar] [CrossRef]
- Wiwanitkit, S.; Wiwanitkit, V. Metformin and sleep disorders. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S63–S64. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Lee, H.; Ryu, O.H.; Choi, M.G.; Kim, S.W. Sleep disturbances and glucoregulation in patients with type 2 diabetes. J. Korean Med. Sci. 2014, 29, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. The endocannabinoid system: A promising target for the management of type 2 diabetes. Curr. Protein Pept. Sci. 2009, 10, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Kaneita, Y.; Yokoyama, E.; Harano, S.; Tamaki, T.; Ibuka, E.; Kaneko, A.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Association between sleep duration and hemoglobin A1c level. Sleep Med. 2008, 9, 745–752. [Google Scholar] [CrossRef]
- Timbrell, S.; Wilbourn, G.; Harper, J.; Liddle, A. Lactic acidosis secondary to metformin overdose: A case report. J. Med. Case Rep. 2012, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.M.G.; Schlachter, J.; Foch, C.; Boutmy, E. Quality and characteristics of 4241 case reports of lactic acidosis in metformin users reported to a large pharmacovigilance database. Ther. Clin. Risk Manag. 2022, 2022, 1037–1047. [Google Scholar] [CrossRef]
- Saftarina, F. Case report: Type 2 diabetes mellitus for the elderly with less family support. Rev. Prim. Care Pract. Educ. 2021, 4, 49–53. [Google Scholar] [CrossRef]
- Kirkiz, S.; Yarali, N.; Arman Bilir, O.; Tunc, B. Metformin-induced hemolytic anemia. Med. Princ. Pract. 2014, 23, 183–185. [Google Scholar] [CrossRef]
- Alhaji, J.H. Vitamin B12 deficiency in patients with diabetes on metformin: Arab countries. Nutrients 2022, 14, 2046. [Google Scholar] [CrossRef]
- Baig, F.A.; Khan, S.; Rizwan, A. Frequency of vitamin B12 deficiency in type 2 diabetic patients taking metformin. Cureus 2022, 14, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Rachmani, R.; Slavachevski, I.; Levi, Z.; Zadok, B.; Kedar, Y.; Ravid, M. Metformin in patients with type 2 diabetes mellitus: Reconsideration of traditional contraindications. Eur. J. Intern. Med. 2002, 13, 428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).