Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling
Abstract
:1. Introduction
2. Extracellular Vesicles
2.1. Definition and Classification
2.2. Exosomes
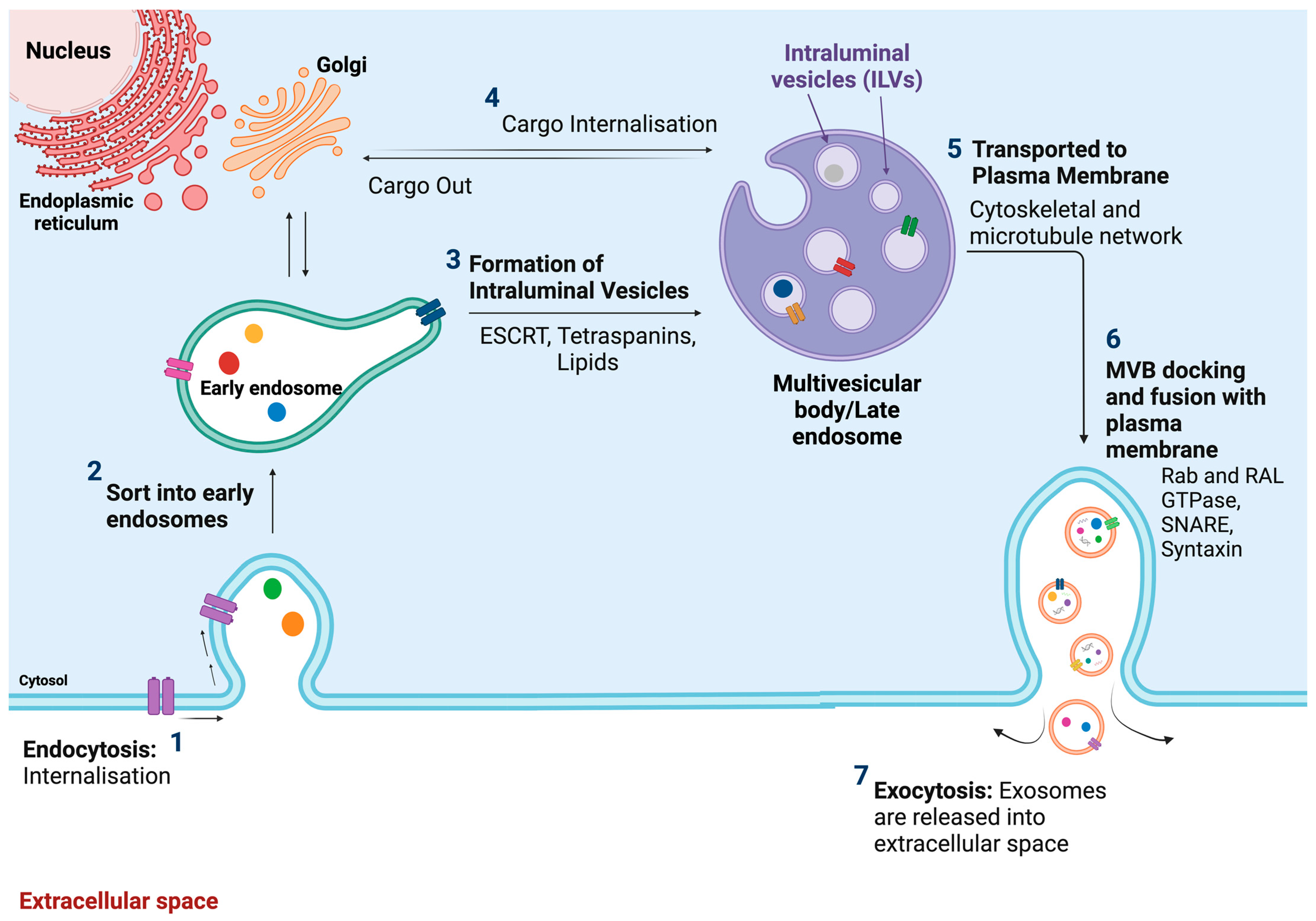
2.2.1. Definition, Size, and Origin
2.2.2. Composition/Contents
2.2.3. Biological Function
2.3. Microvesicles
2.3.1. Definition, Size, and Biogenesis/Formation
2.3.2. Composition and Contents
2.3.3. Biological Function
2.4. Apoptotic Bodies
2.4.1. Definition, Size, and Biogenesis
2.4.2. Composition and Contents
2.4.3. Biological Function
3. Angiogenesis
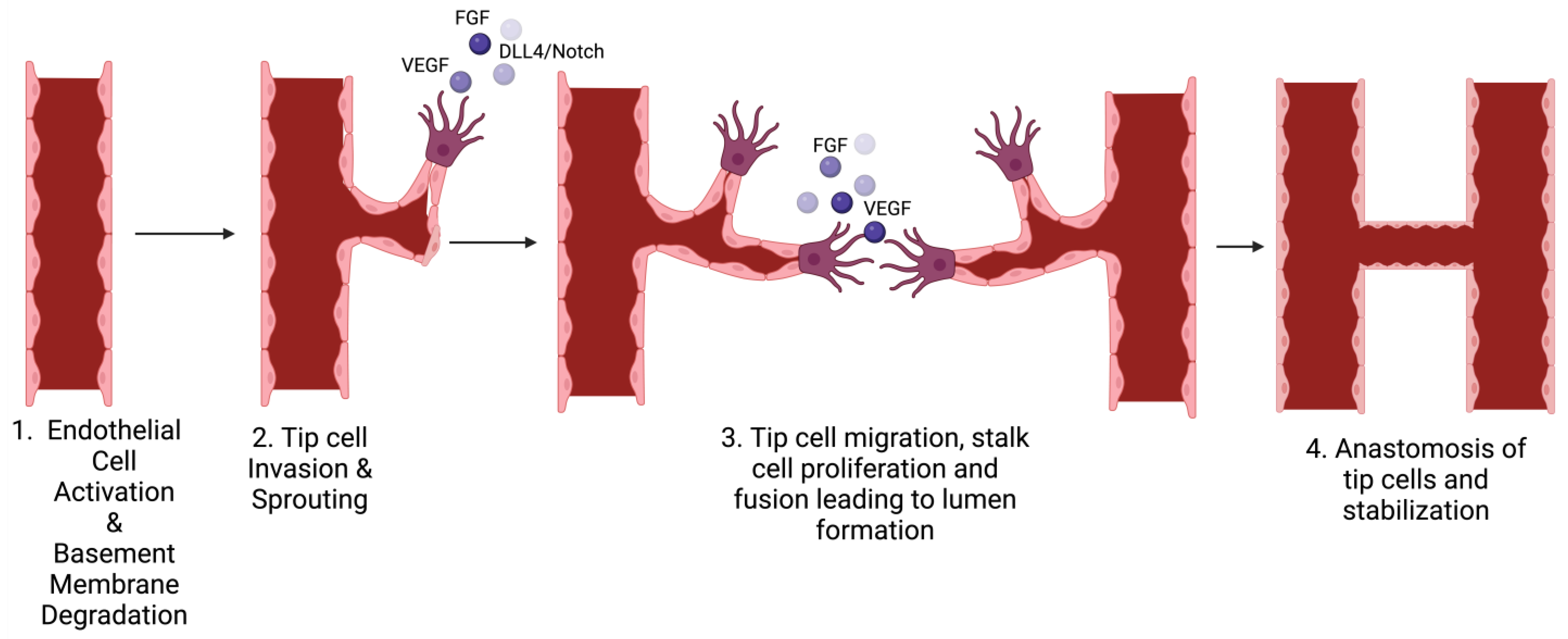
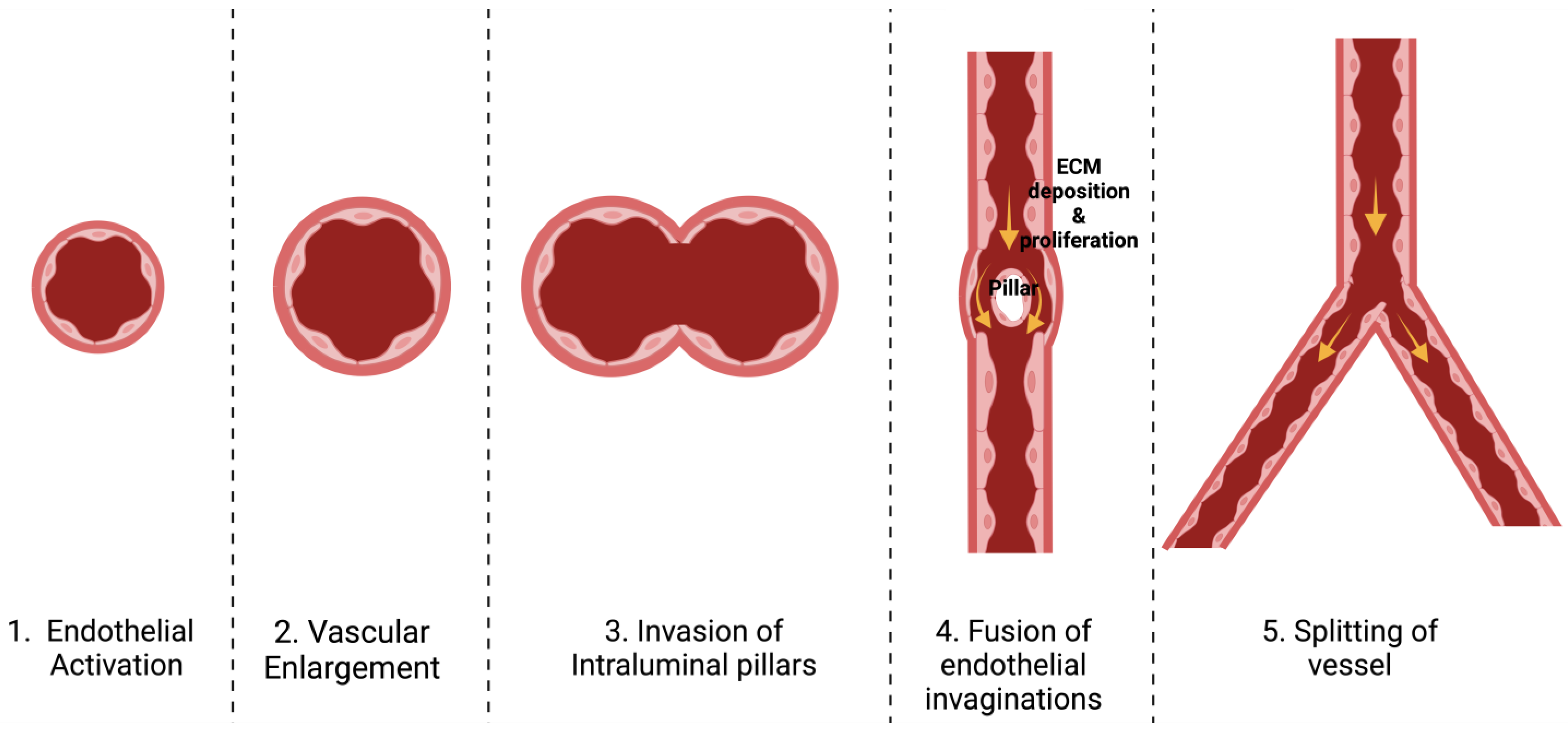
3.1. Definition and Types
3.2. Stages of Each Type
3.3. Mechanisms Involved in Angiogenesis
3.4. Key Mediators of Angiogenesis
4. Role of EVs in Angiogenesis
4.1. Main Sources of EVs
4.2. Evidence of EV Involvement in Angiogenesis
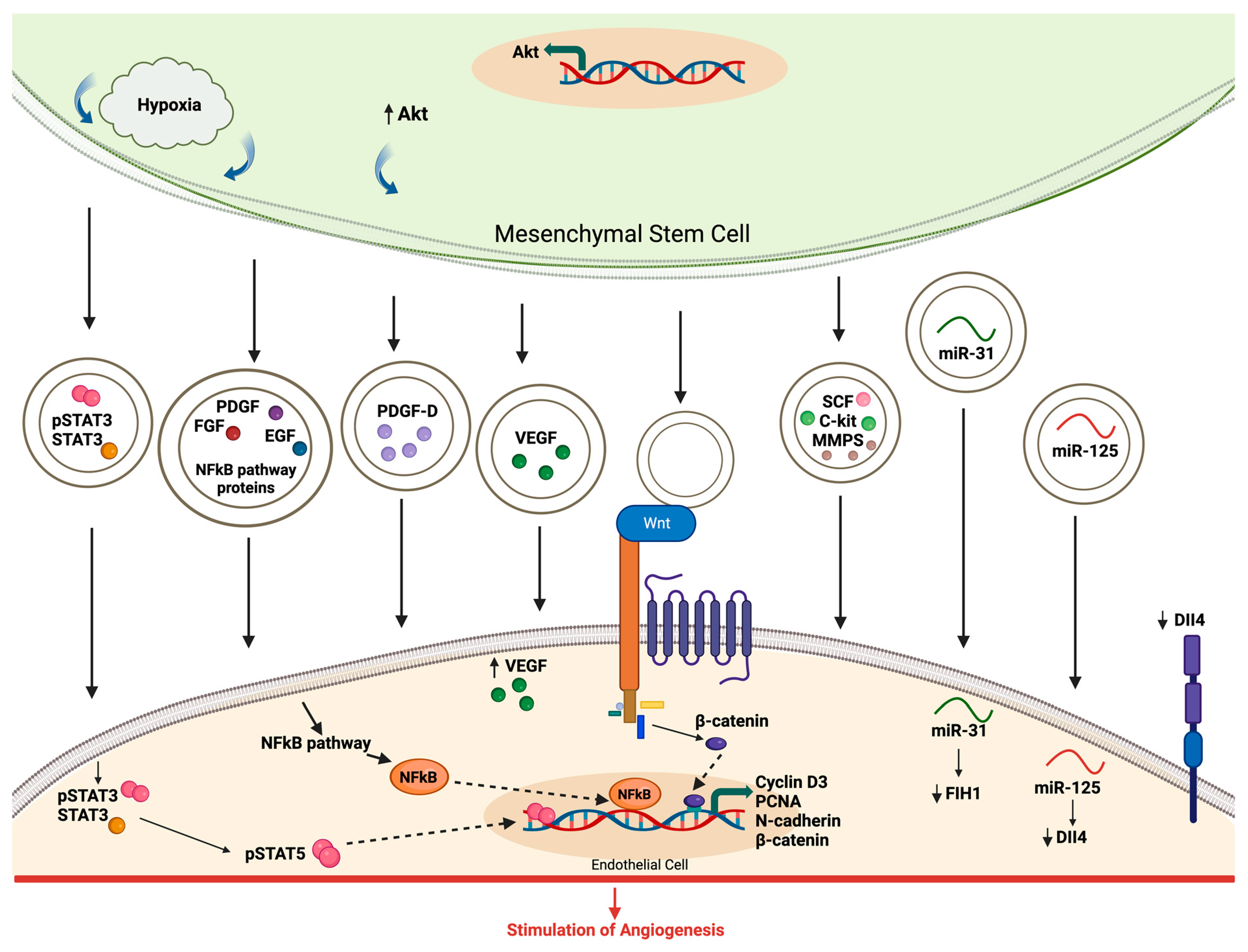
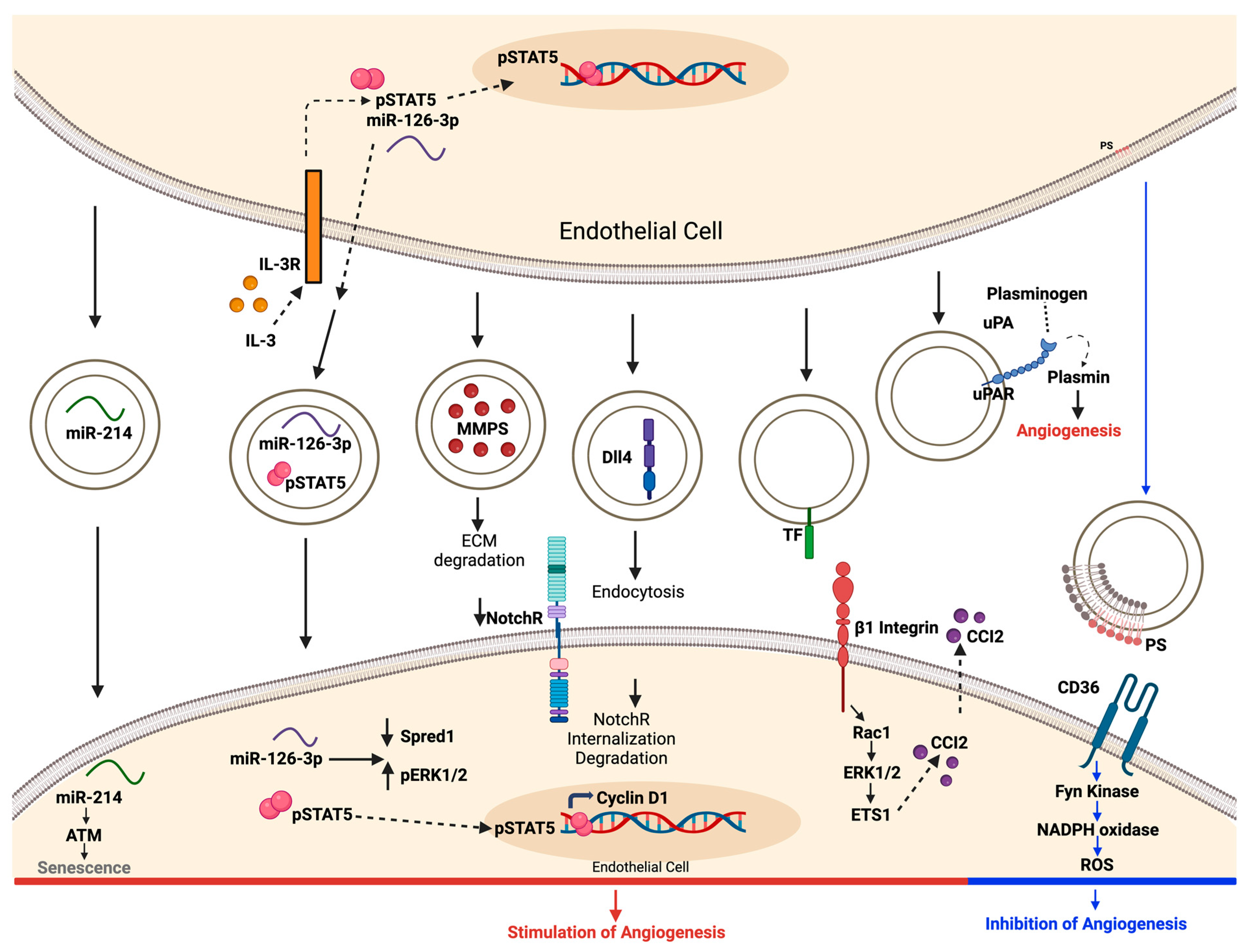
4.3. Modulation of Angiogenic Signaling Pathways by EVs
4.4. Advantages and Disadvantages of EV-Mediated Angiogenesis
5. Clinical Application
5.1. Diagnostic and Prognostic Implications of EVs in Angiogenesis-Related Diseases
5.2. Therapeutic Applications of EVs in Modulating Angiogenesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schleich, J.-M.; Abdulla, T.; Summers, R.; Houyel, L. An overview of cardiac morphogenesis. Arch. Cardiovasc. Dis. 2013, 106, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.; Adams, J.W.; Vaccarezza, M. The vertebrate heart: An evolutionary perspective. J. Anat. 2017, 231, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Simons, M.; Martin, J.F.; Zachary, I.C. Role of angiogenesis in cardiovascular disease: A critical appraisal. Circulation 2005, 112, 1813–1824. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef]
- Gai, C.; Carpanetto, A.; Deregibus, M.C.; Camussi, G. Extracellular vesicle-mediated modulation of angiogenesis. Histol. Histopathol. 2016, 31, 379–391. [Google Scholar] [CrossRef]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood–brain barrier. FASEB BioAdv. 2021, 3, 665–675. [Google Scholar] [CrossRef]
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Gao, Y.; Lin, C.; An, Q.; Feng, Y.; Liu, Y.; Liu, D.; Luo, H.; Wang, D. The Biology and Function of Extracellular Vesicles in Cancer Development. Front. Cell Dev. Biol. 2021, 9, 777441. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. Mesenchymal stem cell-derived extracellular vesicles in the failing heart: Past, present, and future. Am. J. Physiol. Circ. Physiol. 2021, 320, H1999–H2010. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Tschuschke, M.; Kocherova, I.; Bryja, A.; Mozdziak, P.; Volponi, A.A.; Janowicz, K.; Sibiak, R.; Piotrowska-Kempisty, H.; Iżycki, D.; Bukowska, D.; et al. Inclusion Biogenesis, Methods of Isolation and Clinical Application of Human Cellular Exosomes. J. Clin. Med. 2020, 9, 436. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Bai, H.; Ren, L.; Zhang, L. The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection. Int. J. Mol. Sci. 2021, 22, 9060. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, A.; Ronquist, G. Role of Exosomes in Myocardial Remodeling. Circ. Res. 2014, 114, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Abdullah, S.T.; Rasul, M.F.; Salihi, A.; Ghafouri-Fard, S.; Hidayat, H.J.; Taheri, M. MicroRNAs: Important Players in Breast Cancer Angiogenesis and Therapeutic Targets. Front. Mol. Biosci. 2021, 8, 764025. [Google Scholar] [CrossRef]
- Fang, L.-L.; Wang, X.-H.; Sun, B.-F.; Zhang, X.-D.; Zhu, X.-H.; Yu, Z.-J.; Luo, H. Expression, regulation and mechanism of action of the miR-17-92 cluster in tumor cells (Review). Int. J. Mol. Med. 2017, 40, 1624–1630. [Google Scholar] [CrossRef]
- Le Sage, C.; Nagel, R.; Egan, D.A.; Schrier, M.; Mesman, E.; Mangiola, A.; Anile, C.; Maira, G.; Mercatelli, N.; Ciafrè, S.A.; et al. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007, 26, 3699–3708. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Yan, C.; Chen, J.; Wang, C.; Yuan, M.; Kang, Y.; Wu, Z.; Li, W.; Zhang, G.; Machens, H.-G.; Rinkevich, Y.; et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022, 29, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Yahya, S.M.M.; Yahya, S.M.M. The Effect of miR-98 and miR-214 on Apoptotic and Angiogenic Pathways in Hepatocellular Carcinoma HepG2 Cells. Indian J. Clin. Biochem. 2020, 35, 353–358. [Google Scholar] [CrossRef]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Günther, S.; et al. Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation 2017, 136, 65–79. [Google Scholar] [CrossRef]
- He, C.; Yang, W.; Yang, J.; Ding, J.; Li, S.; Wu, H.; Zhou, F.; Jiang, Y.; Teng, L.; Yang, J. Long Noncoding RNA MEG3 Negatively Regulates Proliferation and Angiogenesis in Vascular Endothelial Cells. DNA Cell Biol. 2017, 36, 475–481. [Google Scholar] [CrossRef]
- Li, W.-D.; Zhou, D.-M.; Sun, L.-L.; Xiao, L.; Liu, Z.; Zhou, M.; Wang, W.-B.; Li, X.-Q. LncRNA WTAPP1 Promotes Migration and Angiogenesis of Endothelial Progenitor Cells via MMP1 Through MicroRNA 3120 and Akt/PI3K/Autophagy Pathways. Stem Cells 2018, 36, 1863–1874. [Google Scholar] [CrossRef]
- Chaput, N.; Théry, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts As an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.-A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom. Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The ins and outs of microvesicles. FASEB BioAdv. 2021, 3, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.-M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Sinha, A.; Ignatchenko, V.; Ignatchenko, A.; Mejia-Guerrero, S.; Kislinger, T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem. Biophys. Res. Commun. 2014, 445, 694–701. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12 (Suppl. S2), 1463–1467. [Google Scholar] [CrossRef]
- Li, M.; Liao, L.; Tian, W. Extracellular Vesicles Derived From Apoptotic Cells: An Essential Link Between Death and Regeneration. Front. Cell Dev. Biol. 2020, 8, 573511. [Google Scholar] [CrossRef]
- Wang, J.; Yue, B.-L.; Huang, Y.-Z.; Lan, X.-Y.; Liu, W.-J.; Chen, H. Exosomal RNAs: Novel Potential Biomarkers for Diseases—A Review. Int. J. Mol. Sci. 2022, 23, 2461. [Google Scholar] [CrossRef] [PubMed]
- Patel-Hett, S.; D’Amore, P.A. Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 2011, 55, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Houdek, J.P.; Gibney, B.C.; Ysasi, A.; Wagner, W.; Belle, J.; Schittny, J.C.; Enzmann, F.; Tsuda, A.; Mentzer, S.J.; et al. Sprouting and intussusceptive angiogenesis in postpneumonectomy lung growth: Mechanisms of alveolar neovascularization. Angiogenesis 2014, 17, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive Angiogenesis in Human Metastatic Malignant Melanoma. Am. J. Pathol. 2021, 191, 2023–2038. [Google Scholar] [CrossRef]
- Baum, O.; Suter, F.; Gerber, B.; Tschanz, S.A.; Buergy, R.; Blank, F.; Hlushchuk, R.; Djonov, V. VEGF-A promotes intussusceptive angiogenesis in the developing chicken chorioallantoic membrane. Microcirculation 2010, 17, 447–457. [Google Scholar] [CrossRef]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef]
- Gupta, M.K.; Qin, R.Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn. Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer 2000, 1 (Suppl. S1), S80–S84. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Chen, X. Vascular Endothelial Growth Factor as an Anti-Angiogenic Target for Cancer Therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Gerwins, P.; Sköldenberg, E.; Claesson-Welsh, L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit. Rev. Oncol./Hematol. 2000, 34, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Czerwinski, P.; Hortmann, M.; Sohn, H.-Y.; Förstermann, U.; Li, H. Protein kinase C α promotes angiogenic activity of human endothelial cells via induction of vascular endothelial growth factor. Cardiovasc. Res. 2008, 78, 349–355. [Google Scholar] [CrossRef]
- Kanda, S.; Landgren, E.; Ljungström, M.; Claesson-Welsh, L. Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 1996, 7, 383–395. [Google Scholar]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef]
- Heo, J.; Kang, H. Platelet-derived growth factor-stimulated pulmonary artery smooth muscle cells regulate pulmonary artery endothelial cell dysfunction through extracellular vesicle miR-409-5p. Biol. Chem. 2023. [Google Scholar] [CrossRef]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor, during Embryonic Angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts in vivo Angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Klagsbrun, M.; D’Amore, P.A. Regulators of angiogenesis. Annu. Rev. Physiol. 1991, 53, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R. Cell-extracellular matrix interactions in morphogenesis: An in vitro approach. Experientia 1986, 42, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, C.M.; Blevins, K.S.; Hughes, C.C.; George, S.C.; Putnam, A.J. Mesenchymal Stem Cells Enhance Angiogenesis in Mechanically Viable Prevascularized Tissues via Early Matrix Metalloproteinase Upregulation. Tissue Eng. 2006, 12, 2875–2888. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.; Collen, A.; Koolwijk, P. Role of Fibrin Matrix in Angiogenesis. Ann. N. Y. Acad. Sci. 2001, 936, 426–437. [Google Scholar] [CrossRef]
- Francis, M.E.; Uriel, S.; Brey, E.M. Endothelial cell-matrix interactions in neovascularization. Tissue Eng. B Rev. 2008, 14, 19–32. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Zhong, H.; Yang, L.; Li, Q.; Su, C.; Gu, W.; Qian, Y. Extracellular Vesicles (EVs) from Lung Adenocarcinoma Cells Promote Human Umbilical Vein Endothelial Cell (HUVEC) Angiogenesis through Yes Kinase-associated Protein (YAP) Transport. Int. J. Biol. Sci. 2019, 15, 2110–2118. [Google Scholar] [CrossRef]
- Beez, C.M.; Schneider, M.; Haag, M.; Pappritz, K.; Van Linthout, S.; Sittinger, M.; Seifert, M. Cardiac Extracellular Vesicles (EVs) Released in the Presence or Absence of Inflammatory Cues Support Angiogenesis in Different Manners. Int. J. Mol. Sci. 2019, 20, 6363. [Google Scholar] [CrossRef]
- Potz, B.A.; Scrimgeour, L.A.; Pavlov, V.I.; Sodha, N.R.; Abid, M.R.; Sellke, F.W. Extracellular Vesicle Injection Improves Myocardial Function and Increases Angiogenesis in a Swine Model of Chronic Ischemia. J. Am. Heart Assoc. 2018, 7, e008344. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Roefs, M.T.; Bauzá-Martinez, J.; van de Wakker, S.I.; Qin, J.; Olijve, W.T.; Tuinte, R.; Rozeboom, M.; Blok, C.S.; Mol, E.A.; Wu, W.; et al. Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins. Commun. Biol. 2023, 6, 800. [Google Scholar] [CrossRef]
- Guarino, B.; Katari, V.; Adapala, R.; Bhavnani, N.; Dougherty, J.; Khan, M.; Paruchuri, S.; Thodeti, C. Tumor-Derived Extracellular Vesicles Induce Abnormal Angiogenesis via TRPV4 Downregulation and Subsequent Activation of YAP and VEGFR2. Front. Bioeng. Biotechnol. 2021, 9, 790489. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, X.; Jiang, L.; Li, Y.; Zheng, Q. Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling. Cell Commun. Signal. 2022, 20, 97. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Potz, B.A.; Malhotra, A.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Sellke, F.W. Comparative Analysis of Normoxia- and Hypoxia-Modified Extracellular Vesicle Therapy in Function, Perfusion, and Collateralization in Chronically Ischemic Myocardium. Int. J. Mol. Sci. 2023, 24, 2076. [Google Scholar] [CrossRef]
- Xu, C.M.; Karbasiafshar, C.; Teixeira, R.B.; Ahsan, N.; Corssac, G.B.; Sellke, F.W.; Abid, M.R. Proteomic Assessment of Hypoxia-Pre-Conditioned Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Demonstrates Promise in the Treatment of Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1674. [Google Scholar] [CrossRef]
- Sabe, S.A.; Scrimgeour, L.A.; Xu, C.M.; Sabra, M.; Karbasiafshar, C.; Aboulgheit, A.; Abid, M.R.; Sellke, F.W. Extracellular vesicle therapy attenuates antiangiogenic signaling in ischemic myocardium of swine with metabolic syndrome. J. Thorac. Cardiovasc. Surg. 2023, 166, e5–e14. [Google Scholar] [CrossRef]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.; Herkenne, S.; Ek, O.; Paquot, A.; Boeckx, A.; Paques, C.; Nivelles, O.; Thiry, M.; Struman, I. Extracellular Vesicles Mediate Communication between Endothelial and Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 23, 331. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Phng, L.-K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.-K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harb. Perspect. Med. 2012, 3, a006569. [Google Scholar] [CrossRef]
- Peng, Z.; Pang, H.; Wu, H.; Peng, X.; Tan, Q.; Lin, S.; Wei, B. CCL2 promotes proliferation, migration and angiogenesis through the MAPK/ERK1/2/MMP9, PI3K/AKT, Wnt/βcatenin signaling pathways in HUVECs. Exp. Ther. Med. 2023, 25, 77. [Google Scholar] [CrossRef]
- Ramakrishnan, D.P.; Hajj-Ali, R.A.; Chen, Y.; Silverstein, R.L. Extracellular Vesicles Activate a CD36-Dependent Signaling Pathway to Inhibit Microvascular Endothelial Cell Migration and Tube Formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 534–544. [Google Scholar] [CrossRef]
- Janiszewski, M.; Carmo, A.O.D.; Pedro, M.A.; Silva, E.; Knobel, E.; Laurindo, F.R.M. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit. Care Med. 2004, 32, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mwaikambo, B.R.; Zhu, T.; Gagnon, C.; Lafleur, J.; Seshadri, S.; Lachapelle, P.; Lavoie, J.-C.; Chemtob, S.; Hardy, P.; et al. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R467–R476. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Racchetti, G.; Meldolesi, J. Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered with Extraordinary Success. Biomedicines 2021, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Ben, X.-Y.; Wang, Y.-R.; Zheng, H.-H.; Li, D.-X.; Ren, R.; Ni, P.-L.; Zhang, H.-Y.; Feng, R.-J.; Li, Y.-Q.; Li, Q.-F.; et al. Construction of Exosomes that Overexpress CD47 and Evaluation of Their Immune Escape. Front. Bioeng. Biotechnol. 2022, 10, 936951. [Google Scholar] [CrossRef]
- Kennedy, T.L.; Russell, A.J.; Riley, P. Experimental limitations of extracellular vesicle-based therapies for the treatment of myocardial infarction. Trends Cardiovasc. Med. 2021, 31, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, V.; Yang, X.; Ma, Y.; Wu, M.H.; Yuan, S.Y. Extracellular vesicles: New players in regulating vascular barrier function. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1181–H1196. [Google Scholar] [CrossRef]
- Pardo, F.; Villalobos-Labra, R.; Sobrevia, B.; Toledo, F.; Sobrevia, L. Extracellular vesicles in obesity and diabetes mellitus. Mol. Asp. Med. 2018, 60, 81–91. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Scrimgeour, L.A.; Potz, B.A.; Gheit, A.A.; Shi, G.; Stanley, M.; Zhang, Z.; Sodha, N.R.; Ahsan, N.; Abid, M.R.; Sellke, F.W. Extracellular Vesicles Promote Arteriogenesis in Chronically Ischemic Myocardium in the Setting of Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012617. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Ma, R.; Cai, W.; Huang, W.; Paul, C.; Liang, J.; Wang, Y.; Zhao, T.; Kim, H.W.; Xu, M.; et al. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells Int. 2015, 2015, 659890. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, W.; Ma, Y.; Feng, D.; Chen, H.; Cai, B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int. J. Biol. Sci. 2015, 11, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zheng, S.; Zhou, C.; Yuan, W.; Wang, J.; Wang, T. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators. J. Cell. Mol. Med. 2012, 16, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, X.; Liu, Q.; Yang, T.; Qu, H.; Zhou, H. Extracellular vesicles mediate biological information delivery: A double-edged sword in cardiac remodeling after myocardial infarction. Front. Pharmacol. 2023, 14, 1067992. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic–Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, e2302622. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; Van der Pol, E.; Hajji, N.; Ćwiek, A.; Pluta, K.; Konwerski, M.; Filipiak, K.J. P2Y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol. J. 2019, 26, 782–789. [Google Scholar] [CrossRef]
- Bian, X.; Ma, K.; Zhang, C.; Fu, X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: An emerging approach for treatment of ischemic diseases. Stem Cell Res. Ther. 2019, 10, 158. [Google Scholar] [CrossRef]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Merino-González, C.; Zuñiga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomón, C.; Aguayo, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lei, Y.; Zhong, Y.; Chung, C.; Wang, M.; Hu, M.; Deng, L. New Insights Into the Regulatory Roles of Extracellular Vesicles in Tumor Angiogenesis and Their Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 791882. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, J.; Liu, C.-G.; Goh, R.M.W.J.; Yu, F.; Ma, Z.; Wang, L. Noncoding RNAs of Extracellular Vesicles in Tumor Angiogenesis: From Biological Functions to Clinical Significance. Cells 2022, 11, 947. [Google Scholar] [CrossRef]

| Origin | Size | Contents | Characteristics | Biological Function | |
|---|---|---|---|---|---|
| Exosomes | Endosomal pathway, released from Multivesicular Bodies (MVBs) | 30–150 nm | miRNAs, mRNAs, proteins, lipids and nucleic acids | Small, stable and enriched in tetraspanins (CD63, CD9, CD81) | Cell- cell communication, immune modulation, transfer of genetic material & contribute to disease progression |
| Microvesicles | Plasma Membrane Budding | 100 nm–1 μm | Proteins, Lipids, miRNAs, mRNAs | Heterogenous is size and content | Intercellular communication, inflammation, transmission of signals and immune response |
| Apoptotic bodies | Apoptotic cell fragmentation | 500 nm–2 μm | Cellular debris, nuclear fragments, organelles, cytoplasmic components | Large and irregular in shape | Clearance of dying cells, prevent inflammation and immune regulation |
| microRNA/lncRNA | Impact on Angiogenesis | Reference |
|---|---|---|
| Let-7 | Regulate sprout formation Increases EC-mediated angiogenesis | [26] |
| miR-10 | Enhance the ability of EC- mediated angiogenesis by suppressing anti-angiogenic genes. | [27] |
| miR-17-92 | Facilitates cell proliferation and angiogenesis via PI3K/AKT pathway | [28] |
| miR-221/222 | Suppress EC migration, tube formation and proliferation. Decreases EC-mediated angiogenesis | [29,30] |
| miR-31 | Stimulate growth, migration and EC mediated angiogenesis. | [31] |
| miR-125 | Stimulate angiogenesis by inhibiting DLL-4 | [32] |
| miR-126 | Enhance EC- mediated angiogenesis. Sustain vascular development, regeneration, and stability. | [33,34] |
| miR-214 | Regulates angiogenesis | [35] |
| MALAT1 | Stimulate angiogenesis | [36] |
| MANTIS | Induces angiogenesis | [37] |
| MEG3 | Decrease proliferation and angiogenesis | [38] |
| WTAPP1 | Mediate cell migration and angiogenesis | [39] |
| Trial Title | Phase | Status | Principle Therapy | Primary Endpoints | Outcome |
|---|---|---|---|---|---|
| Antiplatelet therapy effect on extracellular vesicles in acute myocardial infarction | Phase 4 | Completed | Ticagrelor and Clopidogrel | Basic science | P2Y12 antagonist ticagrelor reduces release of proinflammatory and procoagulant PEVs [119] |
| Effect of plasma-derived exosomes on cutaneous wound healing | Early phase 1 | Unknown status | Plasma derived exosomes | Treatment | Not reported |
| Treatment of nonischemic cardiomyopathies by intravenous extracellular vesicles of cardiovascular progenitor cells (SECRET-HF) | Phase 1 | Recruiting | Extracellular vesicle-enriched secretome of cardiovascular progenitor cells | Treatment | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateeq, M.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Med. Sci. 2024, 12, 4. https://doi.org/10.3390/medsci12010004
Ateeq M, Broadwin M, Sellke FW, Abid MR. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Medical Sciences. 2024; 12(1):4. https://doi.org/10.3390/medsci12010004
Chicago/Turabian StyleAteeq, Maryam, Mark Broadwin, Frank W. Sellke, and M. Ruhul Abid. 2024. "Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling" Medical Sciences 12, no. 1: 4. https://doi.org/10.3390/medsci12010004






