NKT Cell Responses to B Cell Lymphoma
Abstract
:1. Introduction
2. Experimental Section
2.1. Peripheral Blood Mononuclear Cells (PBMC)
2.2. Mice
2.3. Cell Lines
2.4. NKT Cell Assays
2.5. FACS Analysis
2.6. Histological Analysis
3. Results and Discussion
3.1. B Cell Lymphoma Lines Do Not Present an Endogenous Activating Antigen to NKT Cells
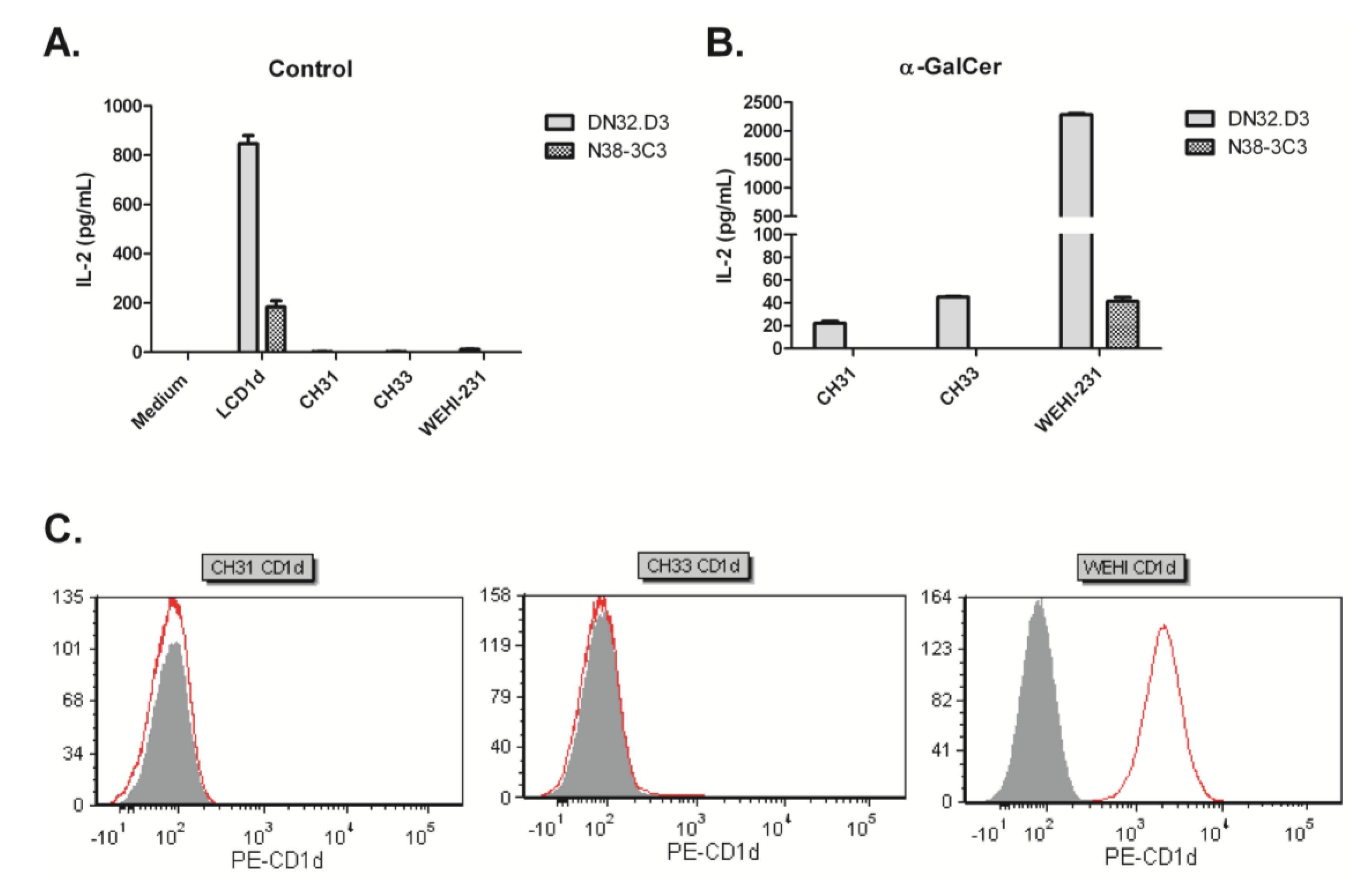
3.2. Human NKT Cells Respond to B Cell Lymphoma in the Presence of α-GalCer

3.3. NKT Cell Responses Are Reduced during Lymphomagenesis

3.4. Treatment of Tumor-Bearing Mice with α-GalCer Results in Reduced MCL Pathology


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michallet, A.S.; Lebras, L.; Coiffier, B. Maintenance therapy in diffuse large B-cell lymphoma. Curr. Opin. Oncol. 2012, 24, 461–465. [Google Scholar] [CrossRef]
- Williams, M.E.; Densmore, J.J. Biology and therapy of mantle cell lymphoma. Curr. Opin. Oncol. 2005, 17, 425–431. [Google Scholar] [CrossRef]
- Marcus, R.; Sweetenham, J.W.; Williams, M.E. Lymphoma: Pathology, Diagnosis, and Treatment; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. xiv, 277. [Google Scholar]
- Perez-Galan, P.; Dreyling, M.; Wiestner, A. Mantle cell lymphoma: Biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Finn, O.J. Cancer immunology. N. Engl. J. Med. 2008, 358, 2704–2715. [Google Scholar] [CrossRef]
- Bhardwaj, N. Harnessing the immune system to treat cancer. J. Clin. Investig. 2007, 117, 1130–1136. [Google Scholar]
- Cerundolo, V.; Kronenberg, M. The role of invariant NKT cells at the interface of innate and adaptive immunity. Semin. Immunol. 2010, 22, 59–60. [Google Scholar] [CrossRef]
- Singh, A.K.; Gaur, P.; Das, S.N. Natural killer T cell anergy, co-stimulatory molecules and immunotherapeutic interventions. Hum. Immunol. 2014, 75, 250–260. [Google Scholar] [CrossRef]
- Monteiro, M.; Graca, L. iNKT cells: Innate lymphocytes with a diverse response. Crit. Rev. Immunol. 2014, 34, 81–90. [Google Scholar] [CrossRef]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Berzins, S.P.; Smyth, M.J.; Baxter, A.G. Presumed guilty: Natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011, 11, 131–142. [Google Scholar] [CrossRef]
- Dellabona, P.; Padovan, E.; Casorati, G.; Brockhaus, M.; Lanzavecchia, A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J. Exp. Med. 1994, 180, 1171–1176. [Google Scholar] [CrossRef]
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol. Immunother. 2014, 63, 199–213. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. The role of NKT cells in tumor immunity. Adv. Cancer Res. 2008, 101, 277–348. [Google Scholar] [CrossRef]
- Swann, J.B.; Uldrich, A.P.; van Dommelen, S.; Sharkey, J.; Murray, W.K.; Godfrey, D.I.; Smyth, M.J. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood 2009, 113, 6382–6385. [Google Scholar] [CrossRef]
- Illes, Z.; Kondo, T.; Newcombe, J.; Oka, N.; Tabira, T.; Yamamura, T. Differential expression of NK T cell V alpha 24J alpha Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 2000, 164, 4375–4381. [Google Scholar]
- Kinjo, Y.; Wu, D.; Kim, G.; Xing, G.W.; Poles, M.A.; Ho, D.D.; Tsuji, M.; Kawahara, K.; Wong, C.H.; Kronenberg, M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005, 434, 520–525. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Hadinia, A.; Yousefi, M.; Jadidi-Niaragh, F. The role of natural killer T cells in B cell malignancies. Tumour Biol. 2013, 34, 1349–1360. [Google Scholar] [CrossRef]
- Burdin, N.; Brossay, L.; Koezuka, Y.; Smiley, S.T.; Grusby, M.J.; Gui, M.; Taniguchi, M.; Hayakawa, K.; Kronenberg, M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 1998, 161, 3271–3281. [Google Scholar]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Sato, H.; Kondo, E.; Harada, M.; Koseki, H.; Nakayama, T.; et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5690–5693. [Google Scholar] [CrossRef]
- Seino, K.; Fujii, S.; Harada, M.; Motohashi, S.; Nakayama, T.; Fujisawa, T.; Taniguchi, M. Valpha14 NKT cell-mediated anti-tumor responses and their clinical application. Springer Semin. Immunopathol. 2005, 27, 65–74. [Google Scholar] [CrossRef]
- Carnaud, C.; Lee, D.; Donnars, O.; Park, S.H.; Beavis, A.; Koezuka, Y.; Bendelac, A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999, 163, 4647–4650. [Google Scholar]
- Hermans, I.F.; Silk, J.D.; Gileadi, U.; Salio, M.; Mathew, B.; Ritter, G.; Schmidt, R.; Harris, A.L.; Old, L.; Cerundolo, V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003, 171, 5140–5147. [Google Scholar]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef]
- Mattarollo, S.R.; West, A.C.; Steegh, K.; Duret, H.; Paget, C.; Martin, B.; Matthews, G.M.; Shortt, J.; Chesi, M.; Bergsagel, P.L.; et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood 2012, 120, 3019–3029. [Google Scholar] [CrossRef]
- Bjordahl, R.L.; Gapin, L.; Marrack, P.; Refaeli, Y. iNKT cells suppress the CD8+ T cell response to a murine Burkitt’s-like B cell lymphoma. PLoS One 2012, 7, e42635. [Google Scholar]
- Chung, Y.; Qin, H.; Kang, C.Y.; Kim, S.; Kwak, L.W.; Dong, C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood 2007, 110, 2013–2019. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Khan, M.A.; Vieira, M.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood 2008, 111, 5637–5645. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Sriram, V.; Du, W.; Gervay-Hague, J.; van Kaer, L.; Brutkiewicz, R.R. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J. Cancer 2006, 118, 3045–3053. [Google Scholar] [CrossRef]
- Hong, S.; Lee, H.; Jung, K.; Lee, S.M.; Lee, S.J.; Jun, H.J.; Kim, Y.; Song, H.; Bogen, B.; Choi, I. Tumor cells loaded with alpha-galactosylceramide promote therapeutic NKT-dependent anti-tumor immunity in multiple myeloma. Immunol. Lett. 2013, 156, 132–139. [Google Scholar] [CrossRef]
- Webb, T.J.; Li, X.; Giuntoli, R.L., 2nd; Lopez, P.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar]
- Ford, R.J.; Shen, L.; Lin-Lee, Y.C.; Pham, L.V.; Multani, A.; Zhou, H.J.; Tamayo, A.T.; Zhang, C.; Hawthorn, L.; Cowell, J.K.; et al. Development of a murine model for blastoid variant mantle-cell lymphoma. Blood 2007, 109, 4899–4906. [Google Scholar] [CrossRef]
- Tupin, E.; Kronenberg, M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006, 417, 185–201. [Google Scholar] [CrossRef]
- Brutkiewicz, R.R.; Bennink, J.R.; Yewdell, J.W.; Bendelac, A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995, 182, 1913–1919. [Google Scholar] [CrossRef]
- Roberts, T.J.; Sriram, V.; Spence, P.M.; Gui, M.; Hayakawa, K.; Bacik, I.; Bennink, J.R.; Yewdell, J.W.; Brutkiewicz, R.R. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J. Immunol. 2002, 168, 5409–5414. [Google Scholar]
- Park, S.H.; Roark, J.H.; Bendelac, A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 1998, 160, 3128–3134. [Google Scholar]
- Renukaradhya, G.J.; Webb, T.J.; Khan, M.A.; Lin, Y.L.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. Virus-induced inhibition of CD1d1-mediated antigen presentation: Reciprocal regulation by p38 and ERK. J. Immunol. 2005, 175, 4301–4308. [Google Scholar]
- Metelitsa, L.S.; Naidenko, O.V.; Kant, A.; Wu, H.-W.; Loza, M.J.; Perussia, B.; Kronenberg, M.; Seeger, R.C. Human NKT cells mediate anti-tumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J. Immunol. 2001, 167, 3114–3122. [Google Scholar]
- Nicol, A.; Nieda, M.; Koezuka, Y.; Porcelli, S.; Suzuki, K.; Tadokoro, K.; Durrant, S.; Juji, T. Human invariant Vα24+ natural killer T cells activated by α-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology 2000, 99, 229–234. [Google Scholar] [CrossRef]
- Brigl, M.; Brenner, M.B. CD1: Antigen presentation and T cell function. Annu. Rev. Immunol. 2004, 22, 817–890. [Google Scholar] [CrossRef]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Bosma, A.; Abdel-Gadir, A.; Isenberg, D.A.; Jury, E.C.; Mauri, C. Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity 2012, 36, 477–490. [Google Scholar] [CrossRef]
- Donnou, S.; Galand, C.; Touitou, V.; Sautes-Fridman, C.; Fabry, Z.; Fisson, S. Murine models of B-cell lymphomas: Promising tools for designing cancer therapies. Adv. Hematol. 2012, 2012, 701704. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.; Sun, W.; Subrahmanyam, P.B.; Page, C.; Younger, K.M.; Tiper, I.V.; Frieman, M.; Kimball, A.S.; Webb, T.J. NKT Cell Responses to B Cell Lymphoma. Med. Sci. 2014, 2, 82-97. https://doi.org/10.3390/medsci2020082
Li J, Sun W, Subrahmanyam PB, Page C, Younger KM, Tiper IV, Frieman M, Kimball AS, Webb TJ. NKT Cell Responses to B Cell Lymphoma. Medical Sciences. 2014; 2(2):82-97. https://doi.org/10.3390/medsci2020082
Chicago/Turabian StyleLi, Junxin, Wenji Sun, Priyanka B. Subrahmanyam, Carly Page, Kenisha M. Younger, Irina V. Tiper, Matthew Frieman, Amy S. Kimball, and Tonya J. Webb. 2014. "NKT Cell Responses to B Cell Lymphoma" Medical Sciences 2, no. 2: 82-97. https://doi.org/10.3390/medsci2020082



