A Comparative Evaluation of Gait between Children with Autism and Typically Developing Matched Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrumentation
2.3. Procedures
2.4. Data Reduction
2.5. Statistical Analysis
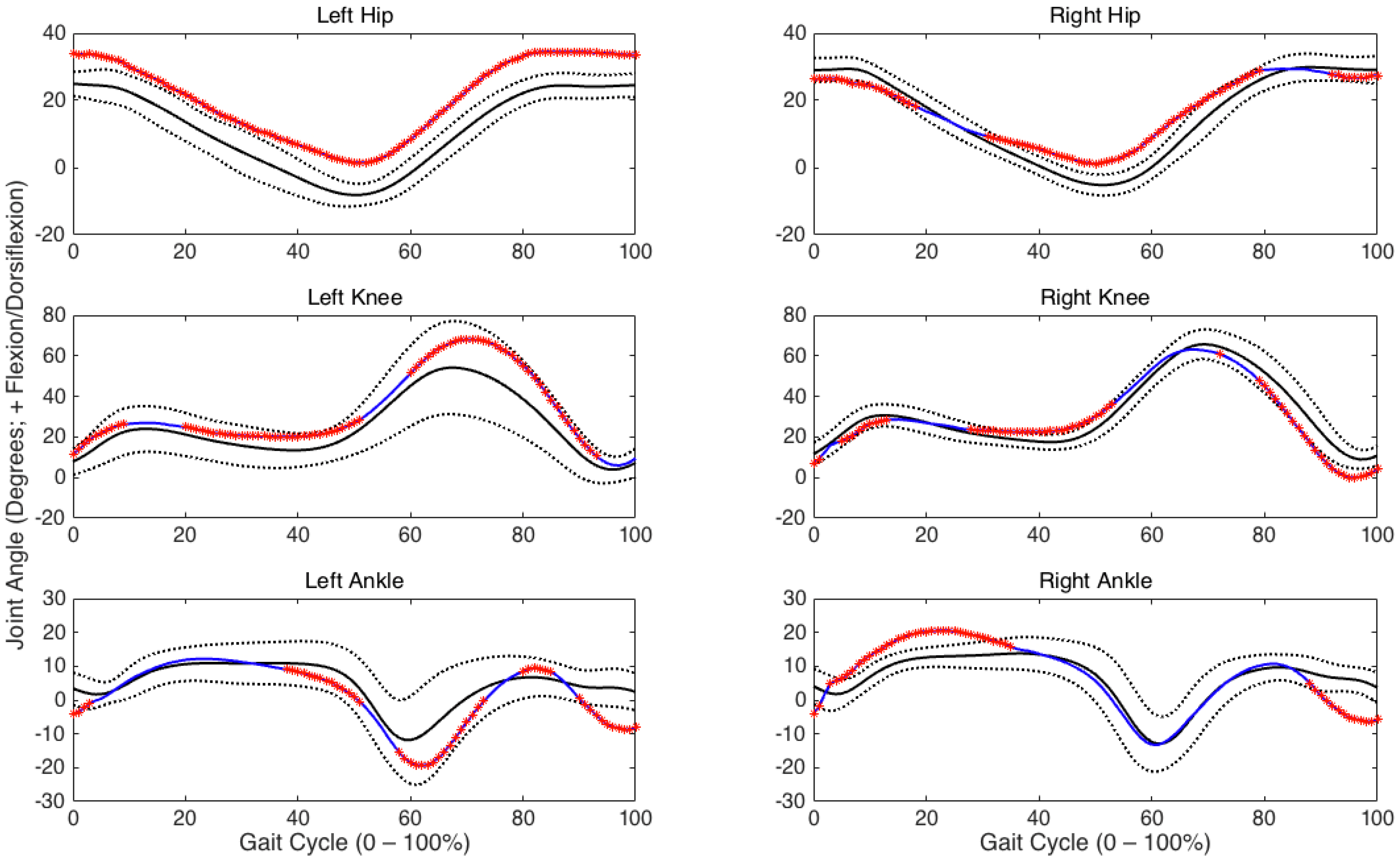
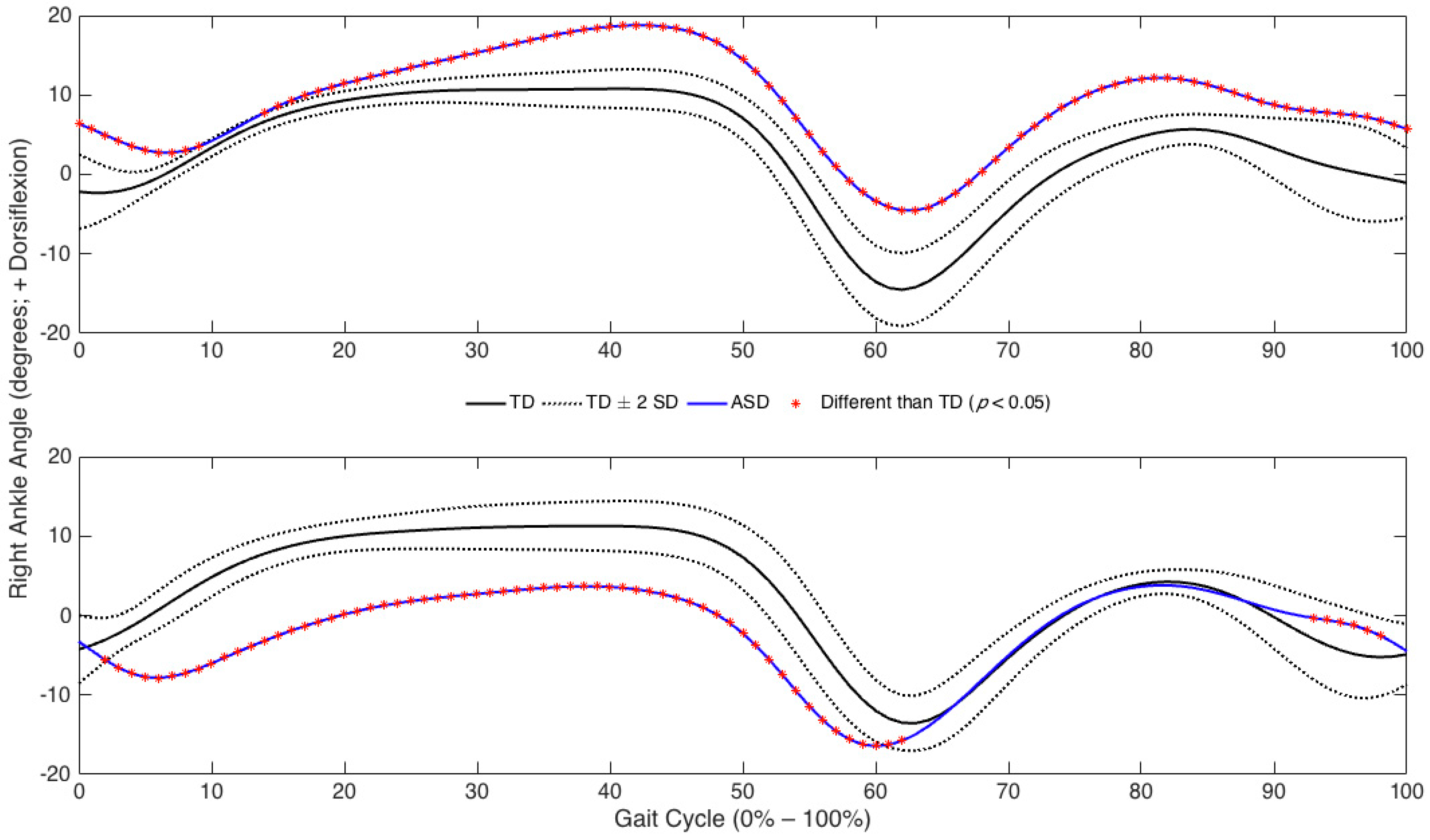
3. Results
3.1. Ground Reaction Forces
3.2. Joint Angles
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2010, 63, 1–21. [Google Scholar]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Blumberg, S.J. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 national health interview survey. Natl. Health Stat. Rep. 2015, 87, 1–20. [Google Scholar]
- Bradshaw, J.; Steiner, A.M.; Gengoux, G.; Koegel, L.K. Feasibility and effectiveness of very early intervention for infants at-risk for autism spectrum disorder: A systematic review. J. Autism Dev. Disord. 2015, 45, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, T.P.; Farley, M.; Speer, L.; Villalobos, M.; Baker, C.N.; Miller, J. Identifying autism in a brief observation. Pediatrics 2015, 135, e330–e338. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Kim, S.H.; Lord, C. The behavioral manifestations of autism spectrum disorders. In The Neuroscience of Autism Spectrum Disorders; Academic Press: Cambridge, MA, USA, 2013; pp. 25–37. [Google Scholar]
- Torres, E.B.; Donnellan, A.M. Editorial for research topic “Autism: The movement perspective”. Front. Integr. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, P.; Teitelbaum, O.; Nye, J.; Fryman, J.; Maurer, R.G. Movement analysis in infancy may be useful for early diagnosis of autism. Proc. Natl. Acad. Sci. USA 1998, 95, 13982–13987. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Data and Statistics. Available online: http://www.cdc.gov/ncbddd/autism/data/html (accessed on 16 June 2016).
- Weiss, M.J.; Moran, M.F.; Parker, M.E.; Foley, J.T. Gait analysis of teenagers and young adults diagnosed with autism and severe verbal communication disorders. Front. Integr. Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, L.; Molteni, M.; Ronconi, L. So close yet so far: Motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci. Biobehav. Rev. 2016, 63, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Baird, G.; Barnett, A.L.; Henderson, L.; Huber, J.; Henderson, S.E. The severity and nature of motor impairment in asperger’s syndrome: A comparison with specific developmental disorder of motor function. J. Child Psychol. Psychiatry 2002, 43, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Provost, B.; Heimerl, S.; Lopez, B.R. Levels of gross and fine motor development in young children with autism spectrum disorder. Phys. Occup. Ther. Pediatr. 2007, 27, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R.; Maurer, R.G. A neurological model for childhood autism. Arch. Neurol. 1978, 35, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Kohen-Raz, R.; Volkmar, F.R.; Cohen, D.J. Postural control in children with autism. J. Autism Dev. Disord. 1992, 22, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Dziuk, M.; Larson, J.; Apostu, A.; Mahone, E.; Denckla, M.; Mostofsky, S. Dyspraxia in autism: Association with motor, social, and communicative deficits. Dev. Med. Child Neurol. 2007, 49, 734–739. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, L.K.; Mostofsky, S.H. Specificity of dyspraxia in children with autism. Neuropsychology 2012, 26, 165. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, S.H.; Dubey, P.; Jerath, V.K.; Jansiewicz, E.M.; Goldberg, M.C.; Denckla, M.B. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. 2006, 12, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.A.; Harbourne, R.T.; Dusing, S.C.; McCoy, S.W. Grounding early intervention: Physical therapy cannot just be about motor skills anymore. Phys. Ther. 2013, 93, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, A.G.; Danielson, D.K.; Kandhadai, P.; Werker, J.F. Sensorimotor influences on speech perception in infancy. Proc. Natl. Acad. Sci. USA 2015, 112, 13531–13536. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.; Pinto, A.I. Identification of core functioning features for assessment and intervention in autism spectrum disorders. Disabil. Rehabil. 2013, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Nebel, M.B.; Joel, S.E.; Muschelli, J.; Barber, A.D.; Caffo, B.S.; Pekar, J.J.; Mostofsky, S.H. Disruption of functional organization within the primary motor cortex in children with autism. Hum. Brain Mapp. 2014, 35, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, N.J.; Tonge, B.J.; Iansek, R.; McGinley, J.; Brereton, A.V.; Enticott, P.G.; Bradshaw, J.L. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Dev. Med. Child Neurol. 2006, 48, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.K.; Crocetti, D.; Hulst, T.; Donchin, O.; Shadmehr, R.; Mostofsky, S.H. Behavioural and neural basis of anomalous motor learning in children with autism. Brain 2015, 138 Pt 3, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, M.; Longworth, M.; Chester, V.L. Gait patterns in children with autism. Clin. Biomech. (Bristol, Avon) 2011, 26, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Lebiedowska, M.K.; Thomas, S.L.; Stanhope, S.J.; Denckla, M.B.; Rumsey, J. Locomotion of autistic adults. Arch. Neurol. 1993, 50, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Kindregan, D.; Gallagher, L.; Gormley, J. Gait deviations in children with autism spectrum disorders: A review. Autism Res. Treat. 2015, 2015, 741480. [Google Scholar] [CrossRef] [PubMed]
- Brasic, J.R.; Gianutsos, J.G. Neuromotor assessment and autistic disorder. Autism 2000, 4, 287–298. [Google Scholar] [CrossRef]
- Bates, B.T. Single-subject methodology: An alternative approach. Med. Sci. Sports Exerc. 1996, 28, 631–638. [Google Scholar] [PubMed]
- Dufek, J.S.; Bates, B.T.; Stergiou, N.; James, C.R. Interactive effects between group and single-subject response patterns. Hum. Mov. Sci. 1995, 14, 301–323. [Google Scholar] [CrossRef]
- Rinehart, N.J.; Bellgrove, M.A.; Tonge, B.J.; Brereton, A.V.; Howells-Rankin, D.; Bradshaw, J.L. An examination of movement kinematics in young people with high-functioning Autism and Asperger’s disorder: Further evidence for a motor planning deficit. J. Autism Dev. Disord. 2006, 36, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.; Dufek, J.; James, C.; Harry, J.; Eggleston, J. The influence of experimental design on the detection of performance differences. Meas. Phys. Educ. Exerc. Sci. 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Manjiviona, J.; Prior, M. Comparison of Asperger syndrome and high-functioning autistic children on a test of motor impairment. J. Autism Dev. Disord. 1995, 25, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Dufek, J.S.; Harry, J.R.; Soucy, M.; Guadagnoli, M.; Lounsbery, M.A.F. Effects of active workstation use on walking mechanics and work efficiency. J. Nov. Physiother. 2016, 6, 2. [Google Scholar] [CrossRef]
- Bates, B.T.; James, C.R.; Dufek, J.S. Single subject analysis. In Innovative Analyses of Human Movement; Stergiou, N., Ed.; Human Kinetics: Champaign, IL, USA, 2004; pp. 3–28. [Google Scholar]
- Dufek, J.S.; Bates, B.T.; Davis, H.P.; Malone, L.A. Dynamic performance assessment of selected sport shoes on impact forces. Med. Sci. Sports Exerc. 1991, 23, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Rancho Los Amigos National Rehabilitation Center. Observational Gait Analysis, 4th ed.; Los Amigos Research and Education Institute, Rancho Los Amigos National Rehabilitation Center: Downey, CA, USA, 2001. [Google Scholar]
- Brincker, M.; Torres, E.B. Noise from the periphery in autism. Front. Integr. Neurosci. 2003, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, W.R.; Hillstrom, H.J. The distributed plantar vertical force of neutrally aligned and pes planus feet. Gait Posture 2002, 15, 1–9. [Google Scholar] [CrossRef]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [PubMed]
- James, C.R. Considerations of movement variability in biomechanics research. In Innovative Analyses of Human Movement; Stergiou, N., Ed.; Human Kinetics: Champaign, IL, USA, 2004; pp. 29–62. [Google Scholar]
- Hamill, J.; Knutzen, K.M. Biomechanical Basis of Human Movement, 2nd ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Bernstein, N.A. The Co-Ordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- McDonald, P.; van Emmerik, R.; Newell, K. The effects of practice on limb kinematics in a throwing task. J. Mot. Behav. 1989, 21, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.; van Emmerik, R. The acquisition of coordination: Preliminary analysis of learning to write. Hum. Mov. Sci. 1989, 8, 17–32. [Google Scholar] [CrossRef]
- Sofuwa, O.; Nieuwboer, A.; Desloovere, K.; Willems, A.; Chavret, F.; Jonkers, I. Quantitative gait analysis in parkinson’s disease: Comparison with a healthy control group. Arch. Phys. Med. Rehabil. 2005, 86, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
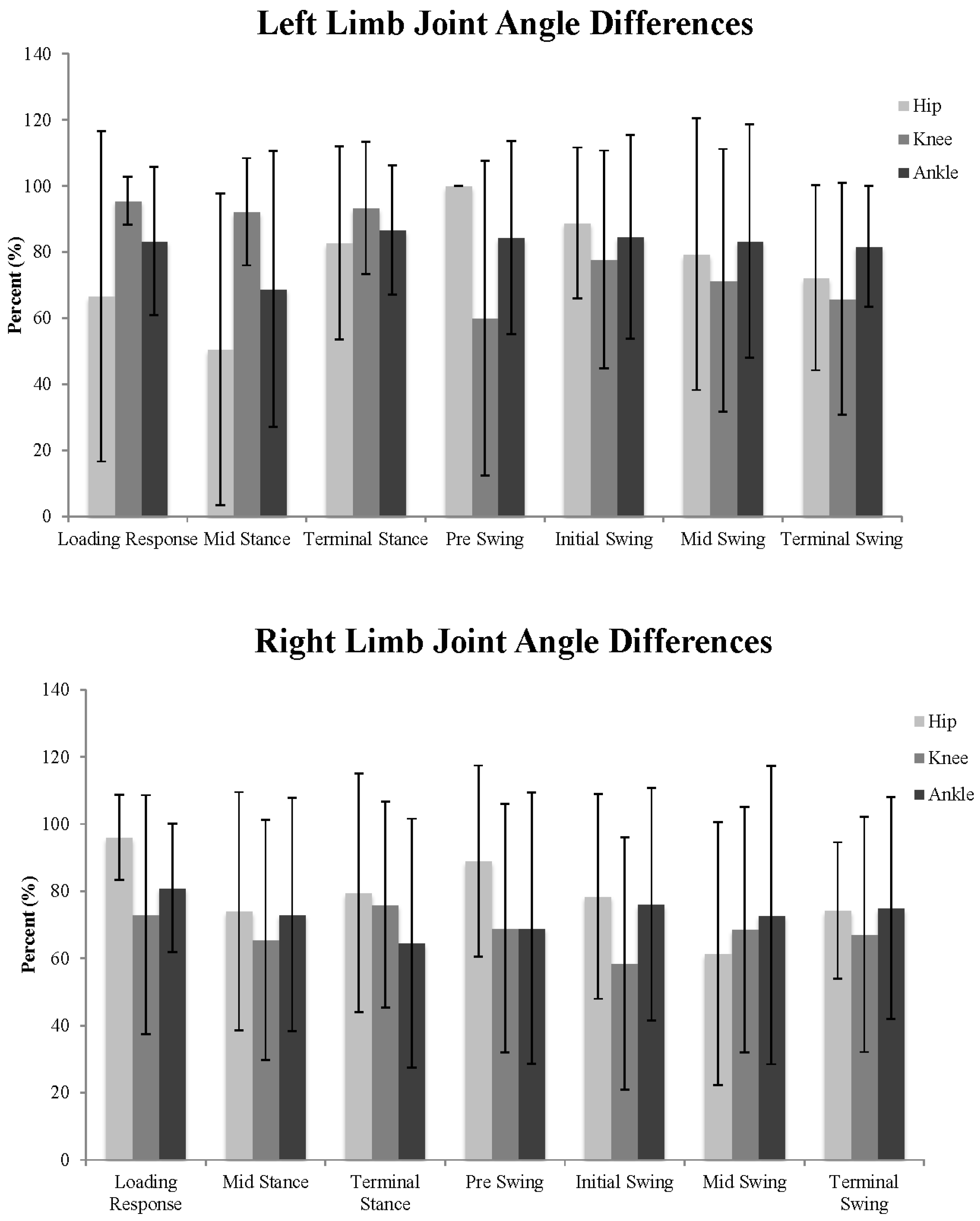
| Hip Position | Stance Sub-Phases | Swing Sub-Phases | Complete Gait Cycle | |||||||
| Matched Pair | Left | Right | Left | Right | Left | Right | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | |
| 1 | 75% | 35% | 29% | 37% | 52% | 16% | 63% | 14% | 58% | 42% |
| 2 | x | x | 89% | 11% | x | x | 90% | 39% | x | 90% |
| 3 | 100% | 4% | 100% | 4% | 98% | 48% | 98% | 48% | 100% | 100% |
| 4 | 100% | 4% | 85% | 27% | 98% | 48% | 73% | 26% | 100% | 76% |
| 5 | 36% | 43% | 83% | 19% | 63% | 11% | 77% | 30% | 48% | 77% |
| 6 | 54% | 41% | 100% | 46% | 36% | 8% | 67% | 23% | 45% | 83% |
| 7 | 33% | 18% | 76% | 32% | 74% | 24% | 66% | 20% | 52% | 71% |
| 8 | 78% | 4% | 100% | 4% | 93% | 41% | 98% | 48% | 82% | 100% |
| 9 | 100% | 4% | 100% | 47% | 98% | 48% | 41% | 27% | 100% | 69% |
| 10 | 100% | 4% | 85% | 27% | 98% | 48% | 73% | 26% | 100% | 76% |
| Knee Position | Stance Sub-Phases | Swing Sub-Phases | Complete Gait Cycle | |||||||
| Matched Pair | Left | Right | Left | Right | Left | Right | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | |
| 1 | 98% | 4% | 39% | 26% | 98% | 47% | 82% | 25% | 99% | 60% |
| 2 | x | x | 90% | 23% | x | x | 75% | 31% | x | 83% |
| 3 | 100% | 4% | 100% | 39% | 98% | 48% | 74% | 27% | 100% | 90% |
| 4 | 63% | 41% | 68% | 32% | 87% | 20% | 92% | 26% | 75% | 79% |
| 5 | 75% | 41% | 69% | 0% | 28% | 21% | 0% | 35% | 61% | 47% |
| 6 | 100% | 34% | 56% | 21% | 67% | 29% | 83% | 26% | 87% | 66% |
| 7 | 100% | 42% | 100% | 37% | 55% | 25% | 53% | 28% | 74% | 82% |
| 8 | 89% | 32% | 65% | 30% | 60% | 25% | 42% | 15% | 78% | 49% |
| 9 | 75% | 52% | 63% | 15% | 69% | 12% | 93% | 33% | 79% | 76% |
| 10 | 69% | 39% | 60% | 35% | 83% | 19% | 53% | 11% | 77% | 59% |
| Ankle Position | Stance Sub-Phases | Swing Sub-Phases | Complete Gait Cycle | |||||||
| Matched Pair | Left | Right | Left | Right | Left | Right | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | |
| 1 | 65% | 34% | 95% | 4% | 40% | 28% | 98% | 44% | 96% | 54% |
| 2 | 51% | 4% | x | x | 98% | 46% | x | x | 0% | 64% |
| 3 | 98% | 48% | 95% | 43% | 18% | 33% | 22% | 31% | 67% | 66% |
| 4 | 50% | 24% | 81% | 4% | 98% | 34% | 98% | 41% | 88% | 68% |
| 5 | 75% | 24% | 84% | 28% | 80% | 27% | 77% | 26% | 80% | 76% |
| 6 | 85% | 29% | 100% | 4% | 98% | 33% | 98% | 47% | 100% | 94% |
| 7 | 78% | 44% | 73% | 39% | 98% | 23% | 98% | 26% | 86% | 91% |
| 8 | 95% | 11% | 96% | 18% | 93% | 41% | 88% | 38% | 93% | 94% |
| 9 | 68% | 4% | 63% | 4% | 98% | 40% | 98% | 40% | 74% | 77% |
| 10 | 55% | 43% | 41% | 24% | 29% | 12% | 74% | 21% | 54% | 47% |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dufek, J.S.; Eggleston, J.D.; Harry, J.R.; Hickman, R.A. A Comparative Evaluation of Gait between Children with Autism and Typically Developing Matched Controls. Med. Sci. 2017, 5, 1. https://doi.org/10.3390/medsci5010001
Dufek JS, Eggleston JD, Harry JR, Hickman RA. A Comparative Evaluation of Gait between Children with Autism and Typically Developing Matched Controls. Medical Sciences. 2017; 5(1):1. https://doi.org/10.3390/medsci5010001
Chicago/Turabian StyleDufek, Janet S., Jeffrey D. Eggleston, John R. Harry, and Robbin A. Hickman. 2017. "A Comparative Evaluation of Gait between Children with Autism and Typically Developing Matched Controls" Medical Sciences 5, no. 1: 1. https://doi.org/10.3390/medsci5010001






