Quantification of Age‐Related Lung Tissue Mechanics under Mechanical Ventilation
Abstract
:1. Introduction
2. Materials and Methods
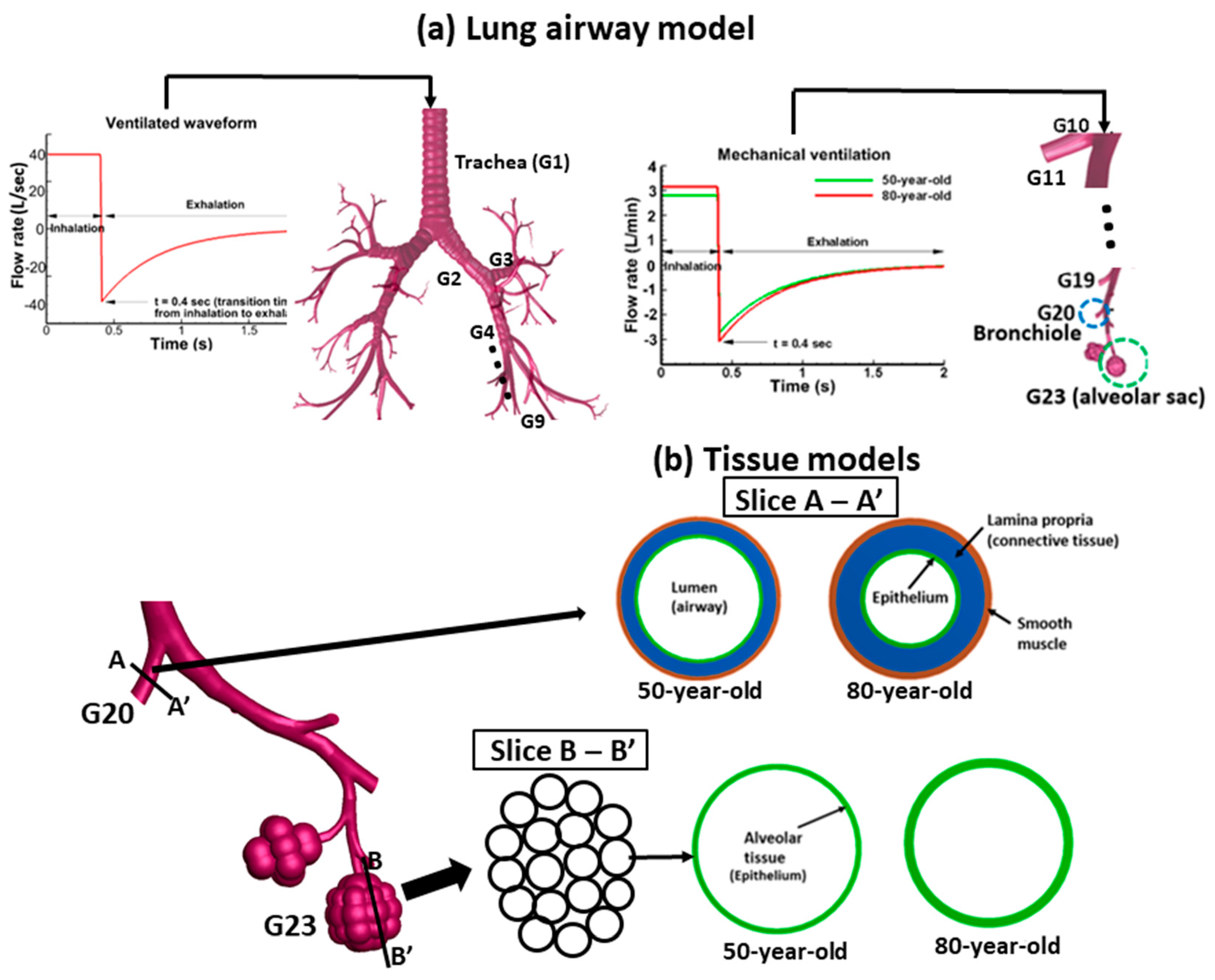
2.1. Tissue Models
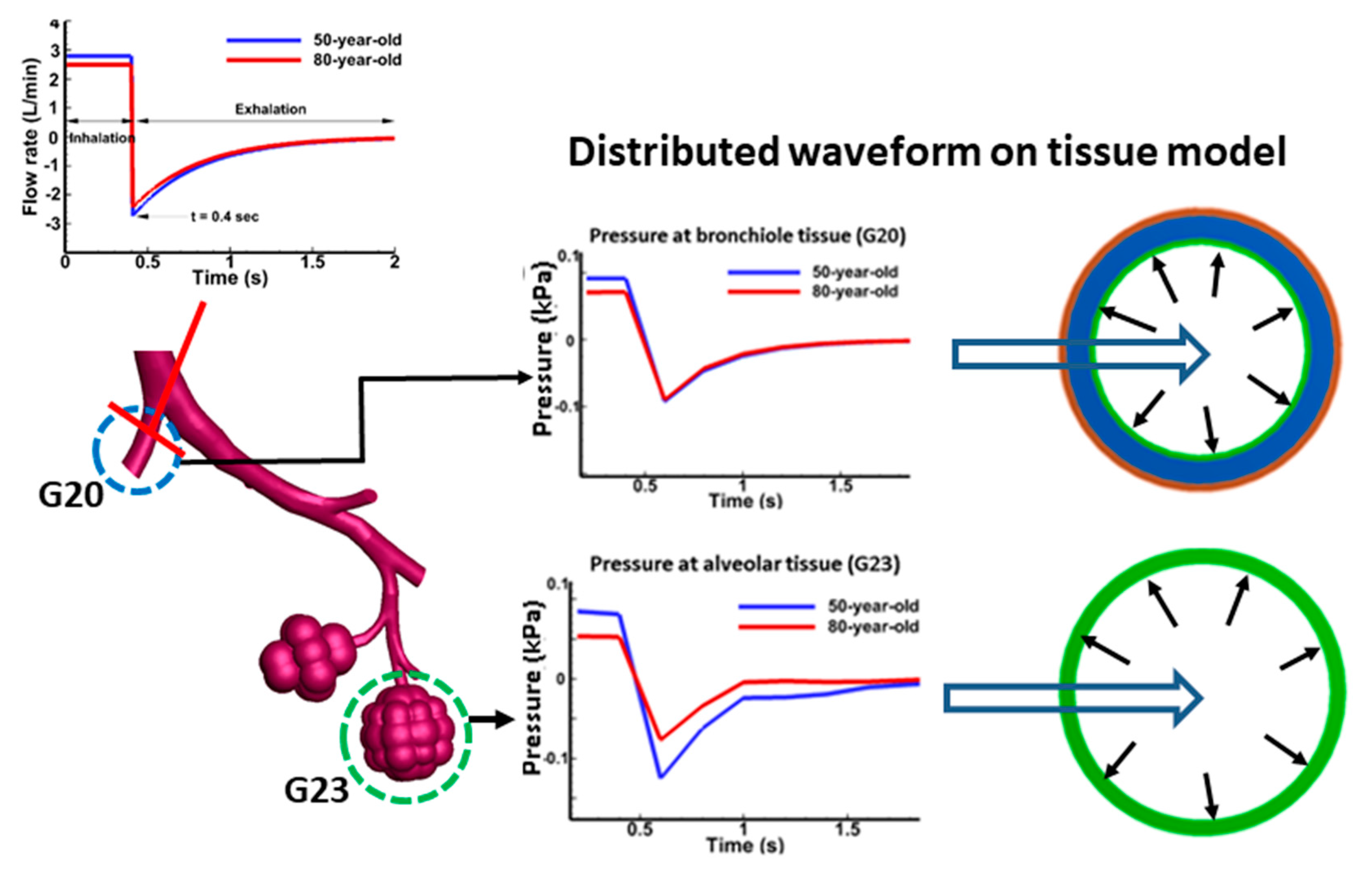
2.2. Tissue Dynamics
2.3. Material Model
2.4. Computational Simulations
3. Results
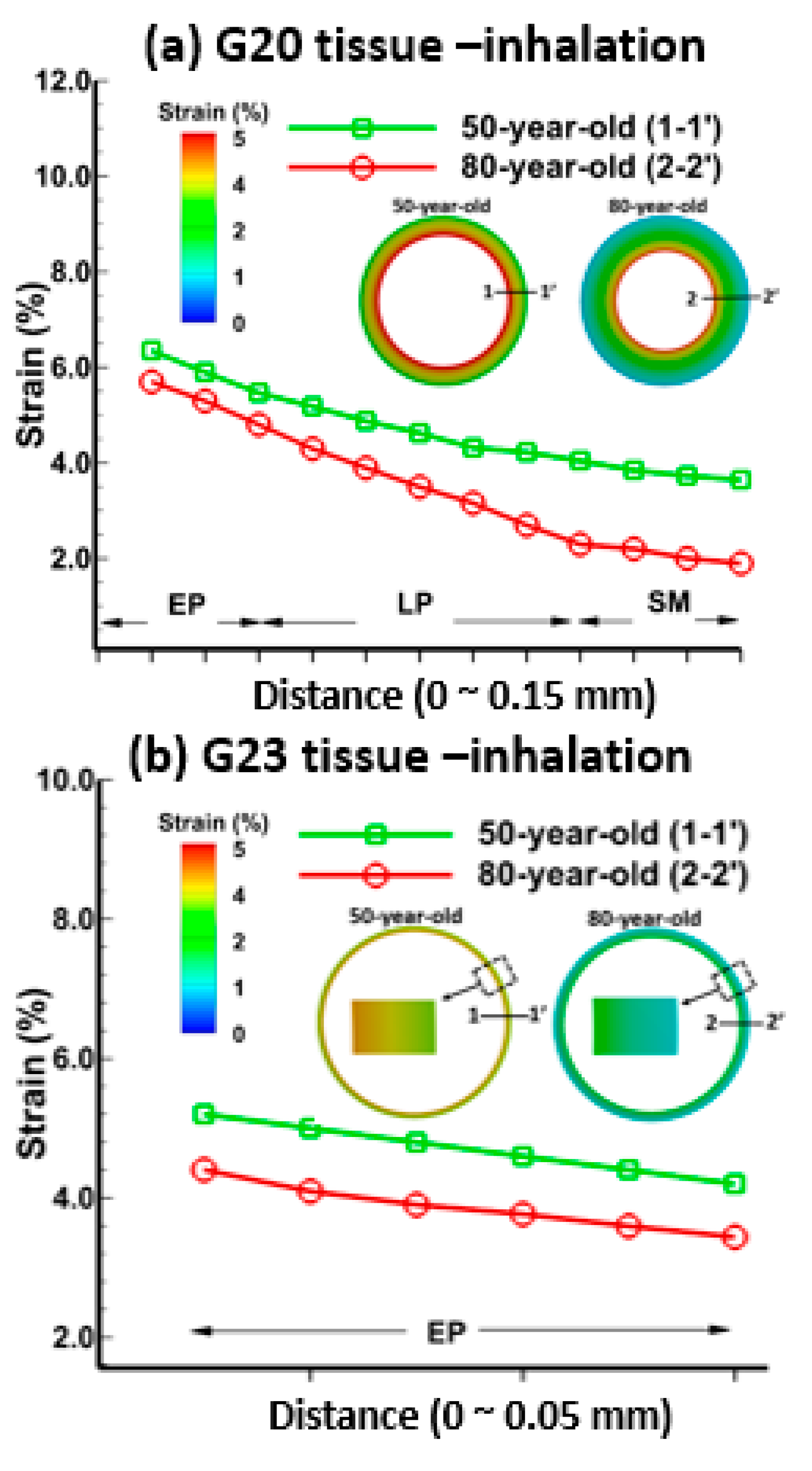
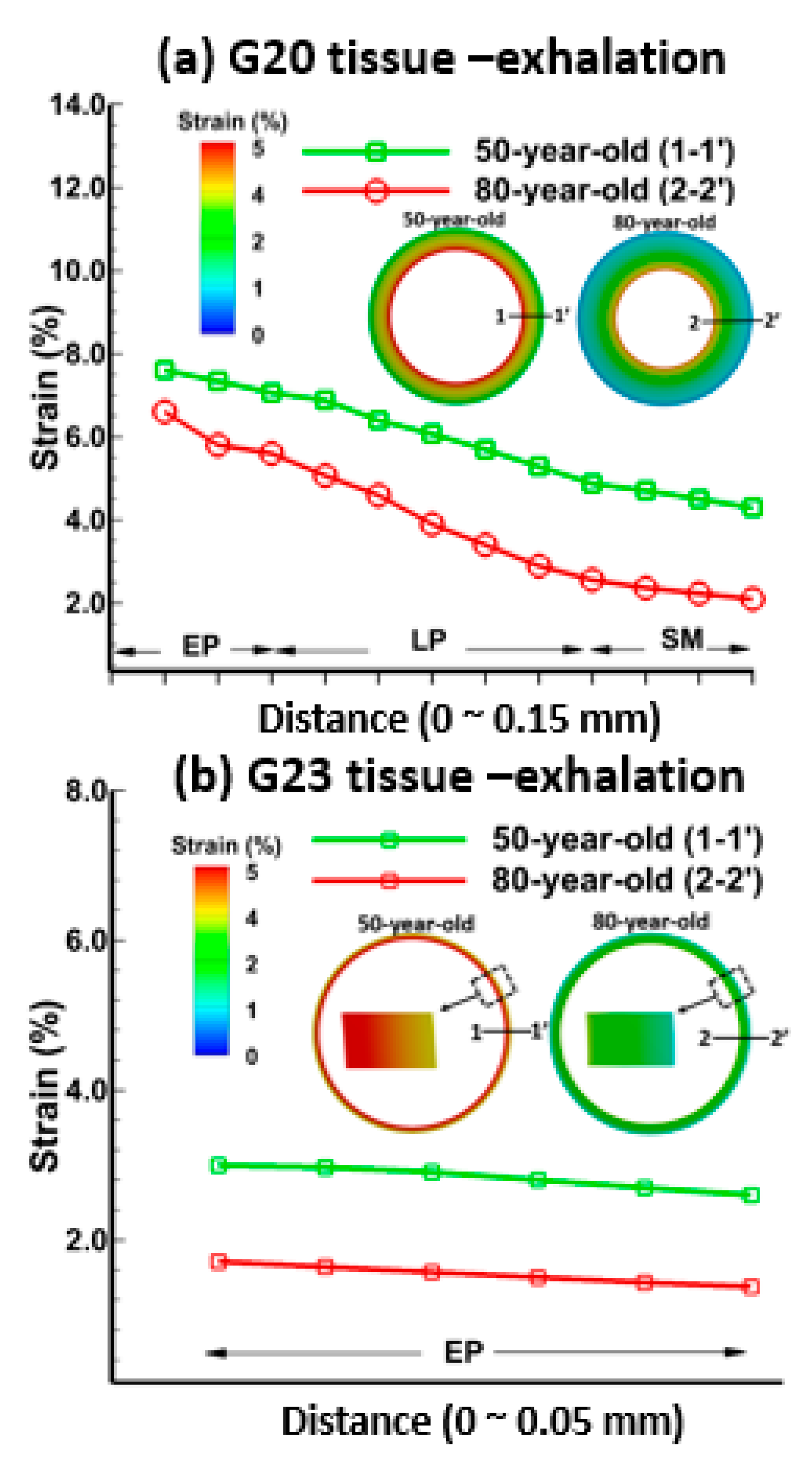
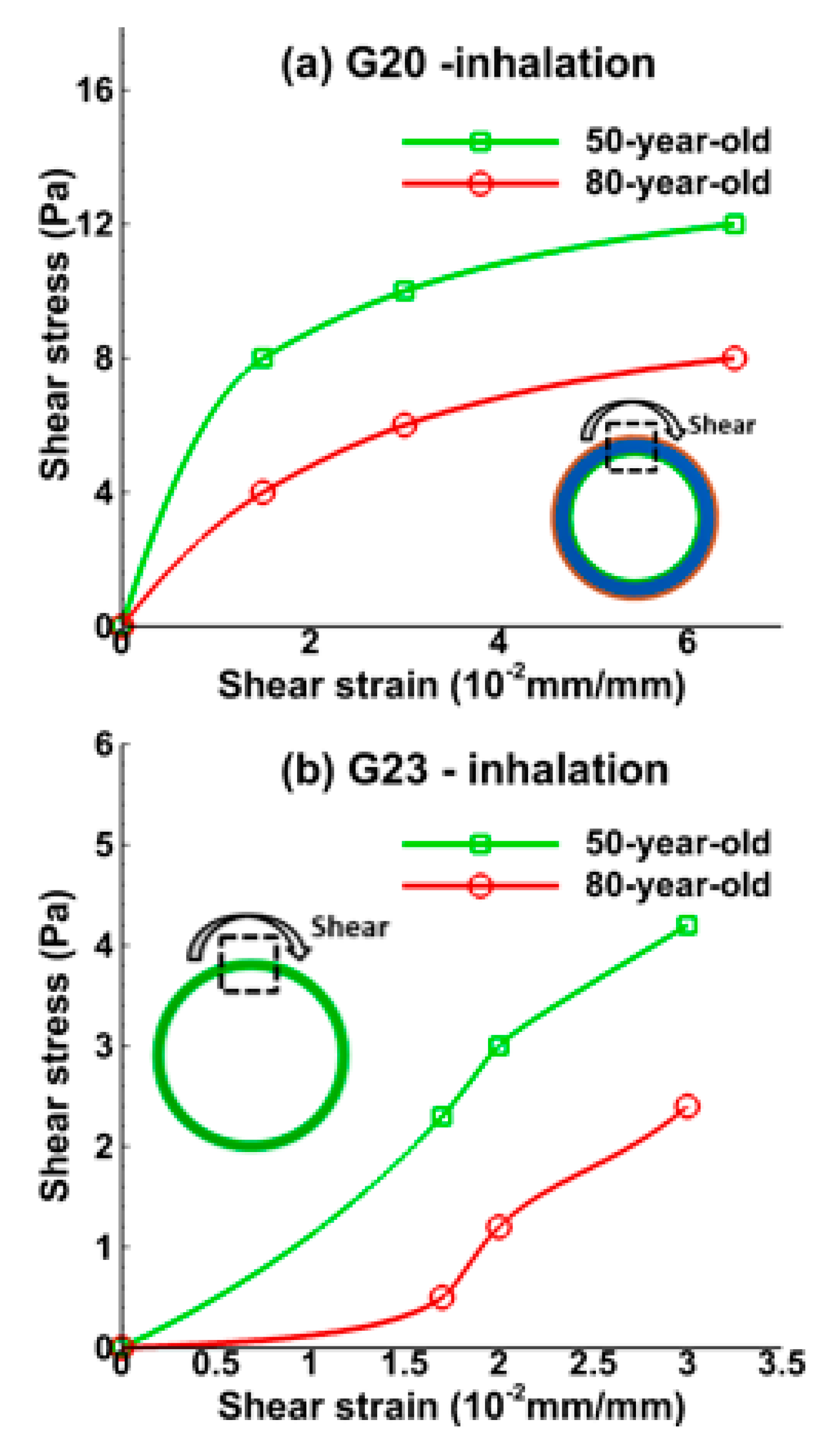
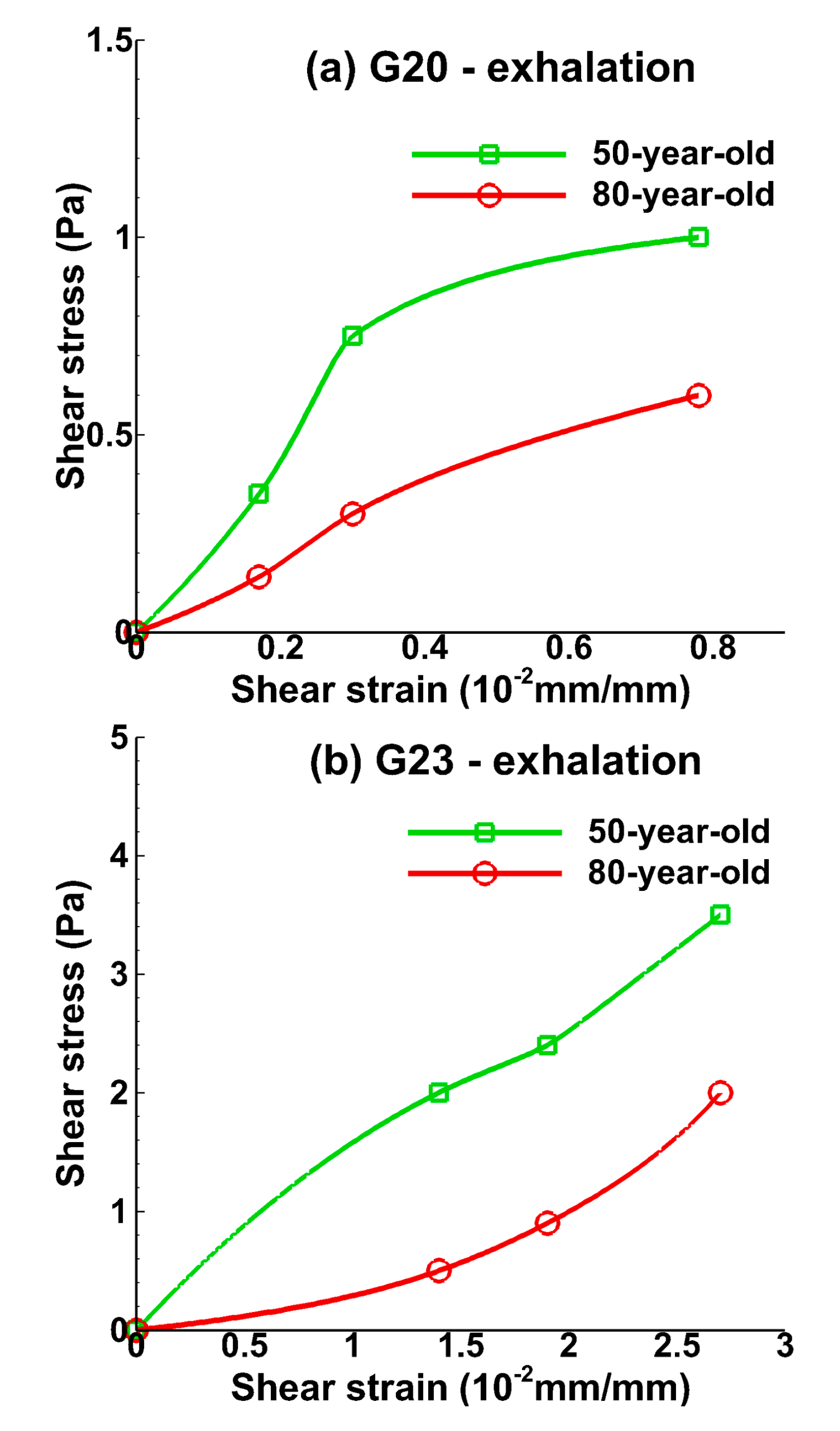
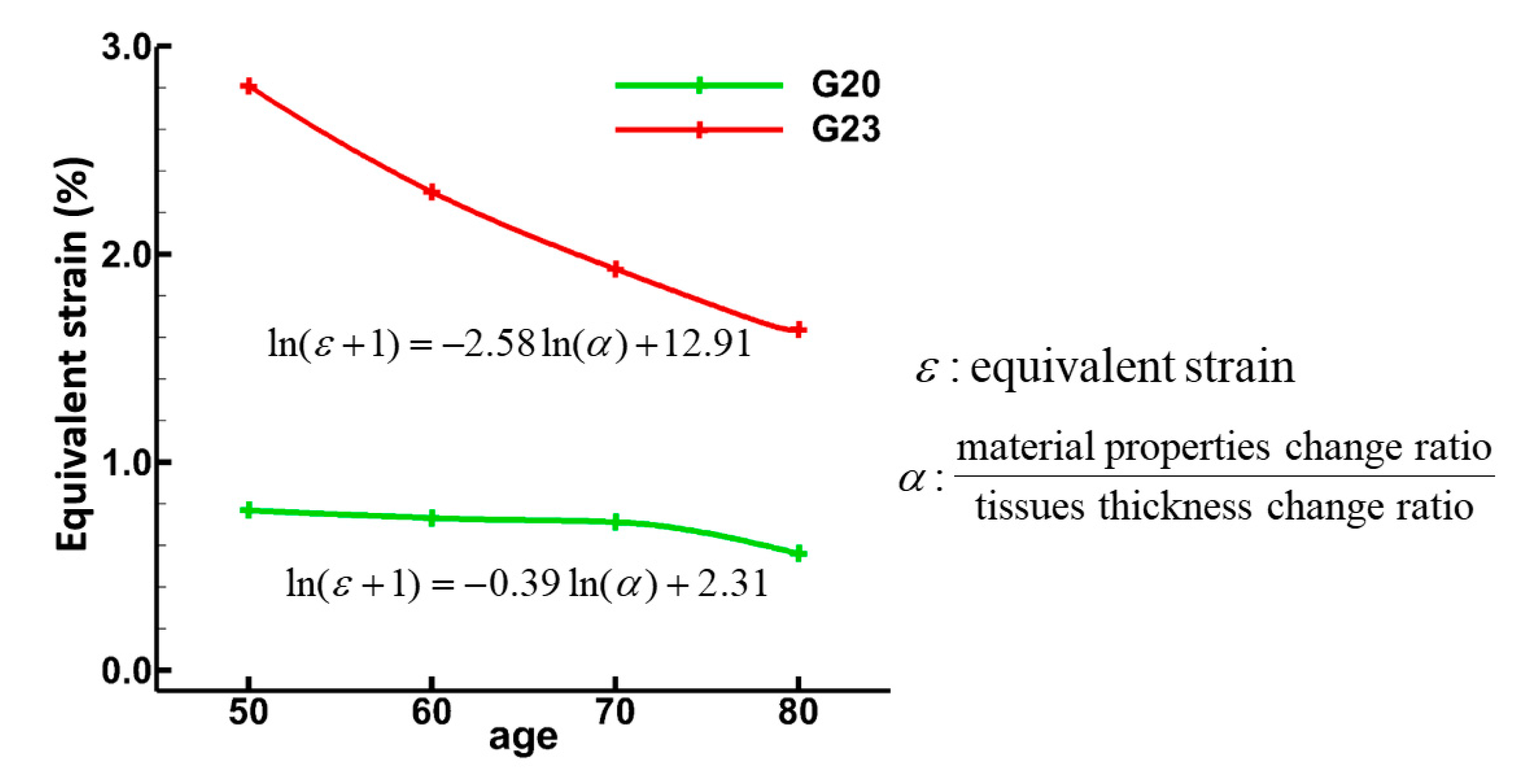
3.1. Tissue Strains
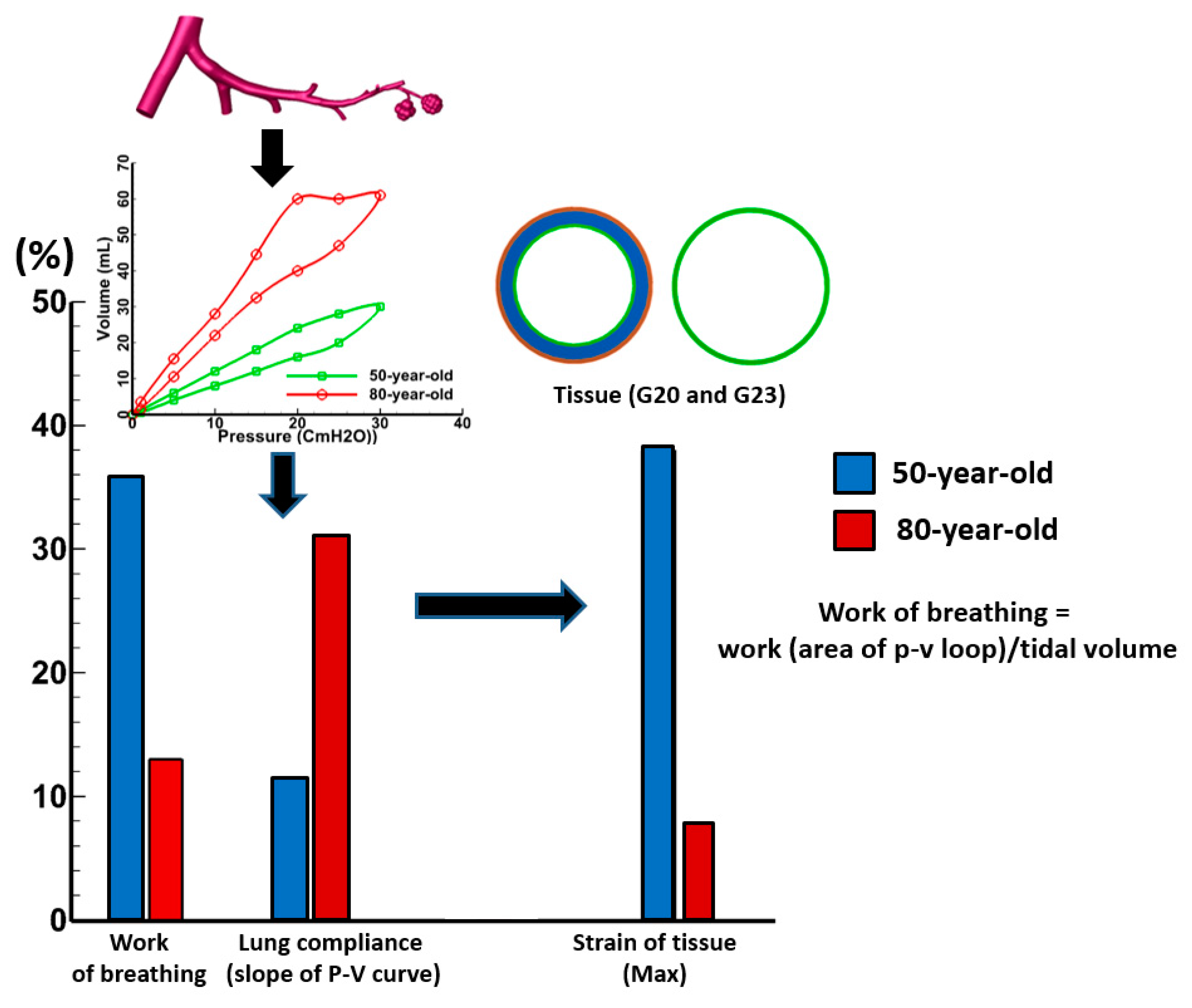
3.2. Sensitivity of Respiratory Mechanics Parameters
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wunsch, H.; Linde-Zwirble, W.T.; Angus, D.C.; Hartman, M.E.; Milbrandt, E.B.; Kahn, J.M. The epidemiology of mechanical ventilation use in the united states. Crit. Care Med. 2010, 38, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Protti, A.; Caironi, P.; Carlesso, E. Ventilator-induced lung injury: The anatomical and physiological framework. Crit. Care Med. 2010, 38, S539–S548. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J. Methods to prevent ventilator-associated lung injury: A summary. Intensive Crit. Care Nurs. 2004, 20, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Plötz, F.B.; Slutsky, A.S.; van Vught, A.J.; Heijnen, C.J. Ventilator-induced lung injury and multiple system organ failure: A critical review of facts and hypotheses. Intensive Care Med. 2004, 30, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Pan, W.X.; Ma, G.; Cai, N.; Leong, K.W.; Liao, K. Viscoelastic behaviour of human mesenchymal stem cells. BMC Cell Biol. 2008, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.; Eidelman, D.H.; Hogg, J.C.; James, A.L.; Lambert, R.K.; Ludwig, M.S.; Martin, J.; McDonald, D.M.; Mitzner, W.A.; Okazawa, M. Proposed nomenclature for quantifying subdivisions of the bronchial wall. J. Appl. Physiol. 1994, 77, 1011–1014. [Google Scholar] [PubMed]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farré, R.; Navajas, D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 2003, 84, 2071–2079. [Google Scholar] [CrossRef] [Green Version]
- Suki, B.; Bartolák-Suki, E. Biomechanics of the aging lung parenchyma. In Mechanical Properties of Aging Soft Tissues; Springer: Berlin, Germany, 2015; pp. 95–133. [Google Scholar]
- Sokolov, I.; Iyer, S.; Woodworth, C.D. Recovery of elasticity of aged human epithelial cells in vitro. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Safshekan, F.; Tafazzoli-Shadpour, M.; Abdouss, M.; Shadmehr, M.B. Mechanical characterization and constitutive modeling of human trachea: Age and gender dependency. Materials 2016, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Heise, R.L.; Reynolds, A.M.; Pidaparti, R.M. Aging effects on airflow dynamics and lung function in human bronchioles. PLoS ONE 2017, 12, e0183654. [Google Scholar] [CrossRef] [PubMed]
- Kalén, A.; Appelkvist, E.-L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Schneider, E.L.; Mitsui, Y. The relationship between in vitro cellular aging and in vivo human age. Proc. Natl. Acad. Sci. USA 1976, 73, 3584–3588. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; DiPietro, L. Fibrogenic cytokines and connective tissue production. FASEB J. 1994, 8, 854–861. [Google Scholar] [PubMed]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, S. Ventilator induced lung injury and infection in the critically ill. Thorax 2001, 56, ii50–ii57. [Google Scholar] [PubMed]
- Caironi, P.; Langer, T.; Carlesso, E.; Protti, A.; Gattinoni, L. Time to generate ventilator-induced lung injury among mammals with healthy lungs: A unifying hypothesis. Intensiv. Care Med. 2011, 37, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Halbertsma, F.; Vaneker, M.; Scheffer, G.; Van der Hoeven, J. Cytokines and biotrauma in ventilator-induced lung injury: A critical review of the literature. Neth. J. Med. 2005, 63, 382–392. [Google Scholar] [PubMed]
- Putensen, C.; Theuerkauf, N.; Zinserling, J.R.; Wrigge, H.; Pelosi, P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann. Intern. Med. 2009, 151, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Paxson, J.A.; Gruntman, A.; Parkin, C.D.; Mazan, M.R.; Davis, A.; Ingenito, E.P.; Hoffman, A.M. Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS ONE 2011, 6, e23232. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.; Oschatz, K.; Hueter, L.; Schmidt, B.; Schwarzkopf, K.; Schreiber, T. Susceptibility to ventilator induced lung injury is increased in senescent rats. Crit. Care 2013, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, D.E.; Kleinerman, J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J. Appl. Physiol. 1974, 36, 412–418. [Google Scholar] [PubMed]
- Turner, J.M.; Mead, J.; Wohl, M.E. Elasticity of human lungs in relation to age. J. Appl. Physiol. 1968, 25, 664–671. [Google Scholar] [PubMed]
- Navajas, D.; Maksym, G.N.; Bates, J. Dynamic viscoelastic nonlinearity of lung parenchymal tissue. J. Appl. Physiol. 1995, 79, 348–356. [Google Scholar] [PubMed]
- Maksym, G.N.; Bates, J.H. A distributed nonlinear model of lung tissue elasticity. J. Appl. Physiol. 1997, 82, 32–41. [Google Scholar] [PubMed]
- Bates, J.H.; Ma, B. A progressive rupture model of soft tissue stress relaxation. Ann. Biomed. Eng. 2013, 41, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Simakov, S.; Kholodov, A. Computational study of oxygen concentration in human blood under low frequency disturbances. Math. Models Comput. Simul. 2009, 1, 283–295. [Google Scholar] [CrossRef]
- Golov, A.; Simakov, S.; Soe, Y.N.; Pryamonosov, R.; Mynbaev, O.; Kholodov, A. Multiscale CT-based computational modeling of alveolar gas exchange during artificial lung ventilation, cluster (biot) and periodic (cheyne-stokes) breathings and bronchial asthma attack. Computation 2017, 5, 11. [Google Scholar] [CrossRef]
- Pidaparti, R. Aged Human Airway Tissue Modeling and Analysis Using Coupled Mechanics. In Proceedings of the 2016 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM 16), Jeju Island, Korea, 28 August–1 September 2016. [Google Scholar]
- Politi, A.Z.; Donovan, G.M.; Tawhai, M.H.; Sanderson, M.J.; Lauzon, A.-M.; Bates, J.H.; Sneyd, J. A multiscale, spatially distributed model of asthmatic airway hyper-responsiveness. J. Theor. Biol. 2010, 266, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Lai-Fook, S.J.; Hyatt, R.E. Effects of age on elastic moduli of human lungs. J. Appl. Physiol. 2000, 89, 163–168. [Google Scholar] [PubMed]
- Montaudon, M.; Desbarats, P.; Berger, P.; De Dietrich, G.; Marthan, R.; Laurent, F. Assessment of bronchial wall thickness and lumen diameter in human adults using multi-detector computed tomography: Comparison with theoretical models. J. Anat. 2007, 211, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, J.N.; Yang, Y.; Werner, R.; Lu, W.; Low, D.; Guo, X.; Wang, J. Sensitivity of tumor motion simulation accuracy to lung biomechanical modeling approaches and parameters. Phys. Med. Biol. 2015, 60, 8833. [Google Scholar] [CrossRef] [PubMed]
- Pidaparti, R.M.; Koombua, K. Tissue strains induced in airways due to mechanical ventilation. Mol. Cell. Biomech. 2011, 81, 149–168. [Google Scholar]
- Koombua, K.; Pidaparti, R.M. Inhalation induced stresses and flow characteristics in human airways through fluid-structure interaction analysis. Model. Simul. Eng. 2008, 2008, 4. [Google Scholar] [CrossRef]
- Gefen, A.; Elad, D.; Shiner, R. Analysis of stress distribution in the alveolar septa of normal and simulated emphysematic lungs. J. Biomech. 1999, 32, 891–897. [Google Scholar] [CrossRef]
- Lewis, M.A.; Owen, M.R. The mechanics of lung tissue under high-frequency ventilation. SIAM J. Appl. Math. 2001, 61, 1731–1761. [Google Scholar] [CrossRef]
- Martin, C.; Chihara, S.; Chang, D. A comparative study of the mechanical properties in aging alveolar wall. Am. Rev. Respir. Dis. 1977, 115, 981–988. [Google Scholar] [PubMed]
- Kucukatay, V.; Bor-Kucukatay, M.; Gundogdu, G.; Erken, G.; Ozcan, T.; Miloglu, F.; Kadioglu, Y. Vitamin E treatment enhances erythrocyte deformability in aged rats. Folia Biol. 2012, 58, 157. [Google Scholar]
- Tschumperlin, D.J.; Margulies, S.S. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 275, L1173–L1183. [Google Scholar]
- Tschumperlin, D.J.; Oswari, J.; Margulies, S.S. Deformation-induced injury of alveolar epithelial cells: Effect of frequency, duration, and amplitude. Am. J. Respir. Crit. Care Med. 2000, 162, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.; Kuhn, H.; Grasenack, T.; Gessner, C.; Wirtz, H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 2004, 30, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H.; Davis, G.S.; Majumdar, A.; Butnor, K.J.; Suki, B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am. J. Respir. Crit. Care Med. 2007, 176, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Schweitzer, K.S.; Caterina, M.J.; Shimoda, L.; King, L.S. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc. Natl. Acad. Sci. USA 2008, 105, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Pare, P. Composition changes in human tracheal cartilage in growth and aging, including changes in proteoglycan structure. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1991, 261, L92–L101. [Google Scholar]
- Mead, J. Volume displacement body plethysmograph for respiratory measurements in human subjects. J. Appl. Physiol. 1960, 15, 736–740. [Google Scholar]
- Mittman, C.; Edelman, N.H.; Norris, A.H.; Shock, N.W. Relationship between chest wall and pulmonary compliance and age. J. Appl. Physiol. 1965, 20, 1211–1216. [Google Scholar]
- Gattinoni, L.; Carlesso, E.; Caironi, P. Stress and strain within the lung. Curr. Opin. Crit. Care 2012, 18, 42–47. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Bronchiole Tissue (G20) | |
| 50-year-old | 80-year-old | |
| Lumen diameter (mm) | 0.45 | 0.32 |
| Epithelium thickness (mm) | 0.011 | 0.015 |
| Lamina propria (mm) | 0.056 | 0.073 |
| Smooth muscle (mm) | 0.016 | 0.021 |
| Alveolar sac tissue (G23) | ||
| 50-year-old | 80-year-old | |
| Alveolus diameter (mm) | 0.25 | 0.54 |
| Alveolar tissue thickness (mm) | 0.025 | 0.049 |
| Initial Shear Modulus (Pa) | ||||
|---|---|---|---|---|
| Epithelium | Lamina Propria | Smooth Muscle | ||
| 50-year-old [33,36,37] | Bronchiole (G20) | 112,000 | 145,600 | 160,000 |
| Alveolar sac (G23) | 35,714 | 46,428 | 51,000 | |
| 80-year-old [33,38,39] | Bronchiole (G20) | 123,760 | 160,000 | 177,000 |
| Alveolar sac (G23) | 39,464 | 51,300 | 56,400 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Heise, R.L.; Reynolds, A.M.; Pidaparti, R.M. Quantification of Age‐Related Lung Tissue Mechanics under Mechanical Ventilation. Med. Sci. 2017, 5, 21. https://doi.org/10.3390/medsci5040021
Kim J, Heise RL, Reynolds AM, Pidaparti RM. Quantification of Age‐Related Lung Tissue Mechanics under Mechanical Ventilation. Medical Sciences. 2017; 5(4):21. https://doi.org/10.3390/medsci5040021
Chicago/Turabian StyleKim, JongWon, Rebecca L. Heise, Angela M. Reynolds, and Ramana M. Pidaparti. 2017. "Quantification of Age‐Related Lung Tissue Mechanics under Mechanical Ventilation" Medical Sciences 5, no. 4: 21. https://doi.org/10.3390/medsci5040021





