Histologic Grade Is Predictive of Incidence of Epidermal Growth Factor Receptor Mutations in Metastatic Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Methods
3. Results
3.1. Incidence of EGFR Mutation by Clinical Characteristics
3.2. Histologic Grade Is a Predictive Factor for the Incidence of EGFR Mutation
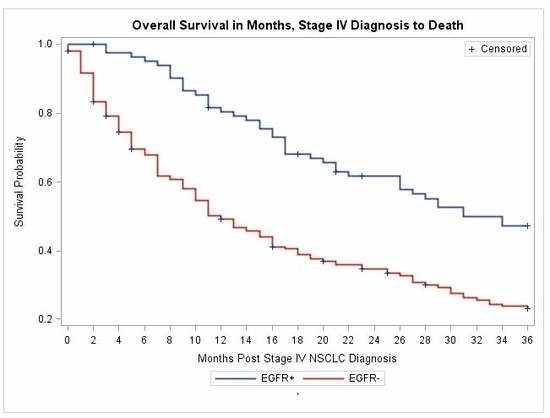
3.3. Treatment with Erlotinib and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Miller, V.A. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: Current knowledge and future directions. J. Clin. Oncol. 2005, 23, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Santamaria, M.; Wollman, D.B. CNS response after erlotinib therapy in a patient with metastatic NSCLC with an EGFR mutation. Nat. Clin. Pract. Oncol. 2007, 4, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Dowell, J.E.; Minna, J.D. EGFR mutations and molecularly targeted therapy: A new era in the treatment of lung cancer. Nat. Clin. Pract. Oncol. 2006, 3, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.A.; Kris, M.G.; Shah, N.; Patel, J.; Azzoli, C.; Gomez, J.; Krug, L.M.; Pao, W.; Rizvi, N.; Pizzo, B.; et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Sima, C.S.; Jackman, D.M.; Sequist, L.V.; Chen, H.; Yang, J.C.; Ji, H.; Waltman, B.; Rosell, R.; Taron, M.; et al. Nomogram to predict the presence of EGFR activating mutation in lung adenocarcinoma. Eur. Respir. J. 2012, 39, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Mok, T.; Yang, J.C.; Wu, Y.L.; Lungershausen, J.; Stammberger, U.; Griebsch, I.; Fonseca, T.; Paz-Ares, L. Afatinib in the treatment of EGFR mutation-positive NSCLC—A network meta-analysis. Lung Cancer 2014, 85, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lilenbaum, R.A.; Horn, L.A. Management of EGFR Mutation-Positive Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14 (Suppl. 5), 672–674. [Google Scholar] [CrossRef] [PubMed]
- Cheema, P.K.; Menjak, I.B.; Winterton-Perks, Z.; Raphael, S.; Cheng, S.Y.; Verma, S.; Muinuddin, A.; Freedman, R.; Toor, N.; Perera, J.; et al. Impact of Reflex EGFR/ALK Testing on Time to Treatment of Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2017, 13, e130–e138. [Google Scholar] [CrossRef] [PubMed]
- Kasymjanova, G.; Small, D.; Cohen, V.; Jagoe, R.T.; Batist, G.; Sateren, W.; Ernst, P.; Pepe, C.; Sakr, L.; Agulnik, J. Lung cancer care trajectory at a Canadian centre: An evaluation of how wait times affect clinical outcomes. Curr. Oncol. 2017, 24, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B.; Brambilla, E.; Travis, W.D. The 2004 World Health Organization classification of lung tumors. Semin. Roentgenol. 2005, 40, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J. Non-small cell lung cancer staging: Proposed revisions to the TNM system. Cancer Imaging 2008, 8, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Wu, Y.L.; Zhang, L. A small step towards personalized medicine for non-small cell lung cancer. Discov. Med. 2009, 8, 227–231. [Google Scholar] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Mok, T.; Yang, J.J.; Lam, K.C. Treating patients with EGFR-sensitizing mutations: First line or second line—is there a difference? J. Clin. Oncol. 2013, 31, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Miller, V.A. EGFR mutations and EGFR tyrosine kinase inhibition in non-small cell lung cancer. Semin. Oncol. Nurs. 2008, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.A.; Riely, G.J.; Zakowski, M.F.; Li, A.R.; Patel, J.D.; Heelan, R.T.; Kris, M.G.; Sandler, A.B.; Carbone, D.P.; Tsao, A.; et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J. Clin. Oncol. 2008, 26, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Everitt, S.; Herschtal, A.; Callahan, J.; Plumridge, N.; Ball, D.; Kron, T.; Schneider-Kolsky, M.; Binns, D.; Hicks, R.J.; MacManus, M.; et al. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer 2010, 116, 5030–5037. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Kestin, L.L.; Grills, I.S.; Battu, M.; Fitch, D.L.; Wong, C.Y.; Margolis, J.H.; Chmielewski, G.W.; Welsh, R.J.; et al. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Ninomiya, H.; Ishikawa, Y.; Matsubara, O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch. Pathol. Lab. Med. 2010, 134, 66–72. [Google Scholar] [PubMed]
- Swanton, C.; Govindan, R. Clinical Implications of Genomic Discoveries in Lung Cancer. N. Engl. J. Med. 2016, 374, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A. Molecular selection trumps clinical selection. J. Clin. Oncol. 2011, 29, 2843–2844. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Brown, C.; Gralla, R.J.; Hirsh, V.; Thongprasert, S.; Tsai, C.M.; Tan, E.H.; Ho, J.C.; Chu da, T.; Zaatar, A.; et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: A meta-analysis. J. Natl. Cancer Inst. 2013, 105, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Mansuet-Lupo, A.; Bobbio, A.; Blons, H.; Becht, E.; Ouakrim, H.; Didelot, A.; Charpentier, M.C.; Bain, S.; Marmey, B.; Bonjour, P.; et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: The experience of a French cohort. CHEST 2014, 146, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Zhao, L.J.; Pang, Q.S.; Yuan, Z.Y.; Li, B.; Wang, P. Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med. Oncol. 2014, 31, 771. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, H.; Serizawa, M.; Koh, Y.; Isaka, M.; Takahashi, T.; Taira, T.; Ono, A.; Maniwa, T.; Takahashi, S.; Mori, K.; et al. Prospective genetic profiling of squamous cell lung cancer and adenosquamous carcinoma in Japanese patients by multitarget assays. BMC Cancer 2014, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Kurishima, K.; Ohara, G.; Kagohashi, K.; Watanabe, H.; Takayashiki, N.; Ishibashi, A.; Satoh, H. Adenosquamous cell lung cancer successfully treated with gefitinib: A case report. Mol. Clin. Oncol. 2014, 2, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.-P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Rossi, S.; D’Argento, E.; Basso, M.; Strippoli, A.; Dadduzio, V.; Cerchiaro, E.; Martini, M.; Cassano, A.; Barone, C. Different EGFR Gene Mutations in Exon 18, 19 and 21 as Prognostic and Predictive Markers in NSCLC: A Single Institution Analysis. Mol. Diagn. Ther. 2016, 20, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Marchioni, A.; Longo, L. EGFR mutations and sensitivity to gefitinib. N. Engl. J. Med. 2004, 351, 1260–1261. [Google Scholar] [PubMed]
- Kobayashi, Y.; Mitsudomi, T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016, 107, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Su, K.Y.; Chen, H.Y.; Li, K.C.; Kuo, M.L.; Yang, J.C.; Chan, W.K.; Ho, B.C.; Chang, G.C.; Shih, J.Y.; Yu, S.L.; et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M. LUX-Lung: Determining the best EGFR inhibitor in NSCLC? Lancet Oncol. 2015, 16, 118–119. [Google Scholar] [CrossRef]
- Rosell, R.; Molina, M.A.; Costa, C.; Simonetti, S.; Gimenez-Capitan, A.; Bertran-Alamillo, J.; Mayo, C.; Moran, T.; Mendez, P.; Cardenal, F.; et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin. Cancer Res. 2011, 17, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
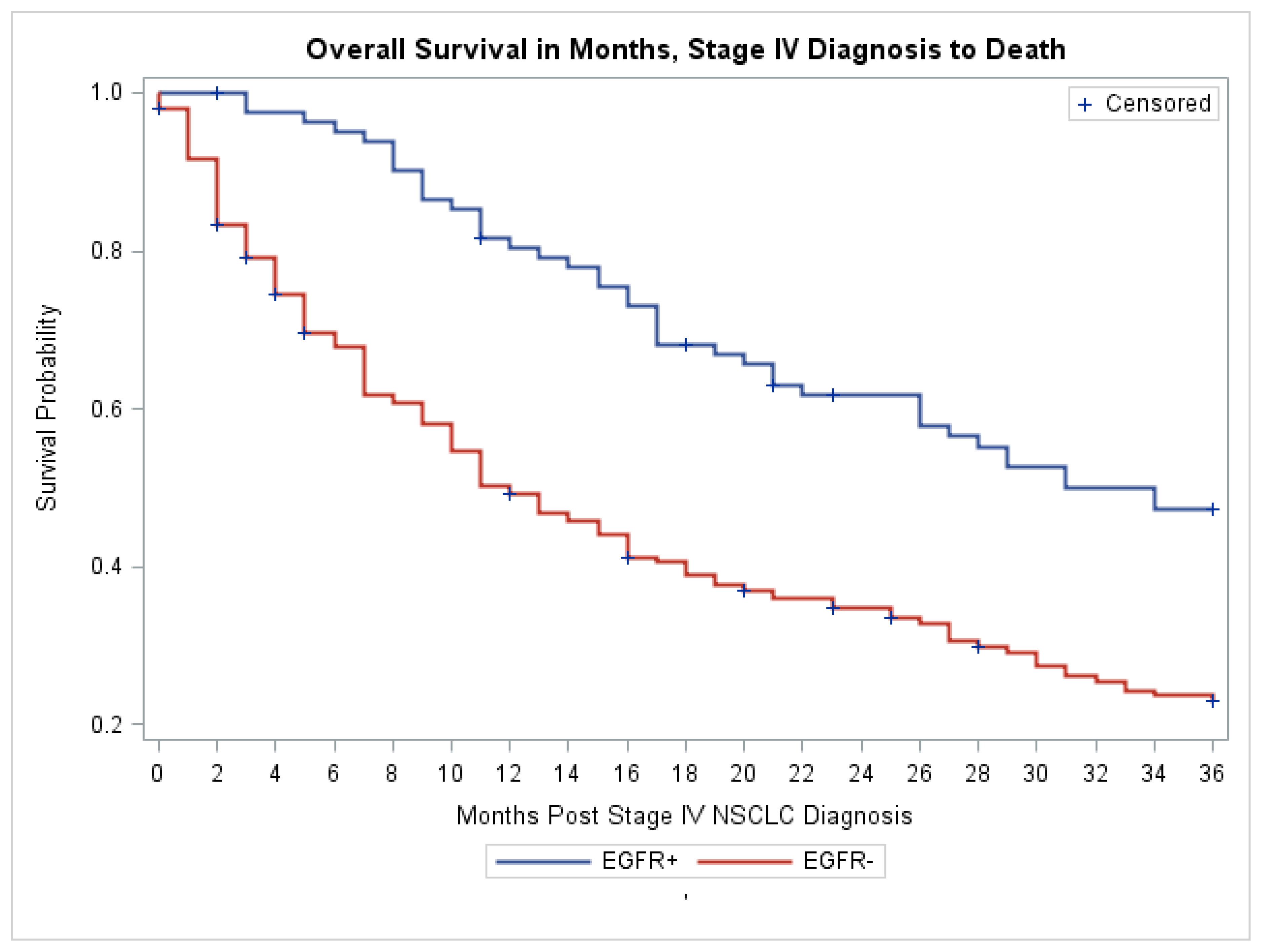
| Clinical Characteristics | EGFR Mutation Negative N = 194 | EGFR Mutation Positive N = 83 | Total N = 277 (%) | p Value |
|---|---|---|---|---|
| Age Median (IQR) | 69.0 (61.0, 76.0) | 64.0 (55.0, 75.0) | 67.0 (60.0, 76.0) | 0.07 * |
| Gender N (%) | ||||
| Male | 89 (73.5) | 32 (26.5) | 121 (43.7) | 0.26 |
| Female | 105 (67.3) | 51 (32.7) | 156 (56.3) | |
| Race/Ethnicity N (%) | ||||
| Asian | 42 (48.8) | 44 (51.2) | 86 (31.1) | <0.0001 |
| White | 140 (79.6) | 36 (20.5) | 176 (63.5) | |
| Black | 12 (80.0) | 3 (20.0) | 15 (5.4) | |
| History of Smoking N (%) | ||||
| Ever-smoker | 130 (87.8) | 18 (12.2) | 148 (53.4) | <0.0001 |
| Never-smoker | 39 (45.4) | 47 (54.7) | 86 (31) | |
| Unknown | 25 (58.1) | 18 (41.9) | 43 (15.5) | |
| Histologic Type N (%) | ||||
| Adenocarcinoma | 163 (67.4) | 79 (32.6) | 242 (87.4) | 0.07 ** |
| Squamous | 19 (90.5) | 2 (9.5) | 21 (7.6) | |
| Adenosquamous | 3 (75.0) | 1 (25.0) | 4 (1.4) | |
| Pure lepedic pattern | 3 (75.0) | 1 (25.0) | 4 (1.4) | |
| Unknown | 6 (100) | 0 (0.0) | 6 (2.2) | |
| Histologic Grade | ||||
| Grade I | 35 (61.4) | 22 (38.6) | 57 (20.6) | <0.0001 |
| Grade II | 59 (63.4) | 34 (36.6) | 93 (33.6) | |
| Grade II–III | 14 (93.3) | 1 (6.7) | 15 (5.4) | |
| Grade III | 86 (88.7) | 11 (11.3) | 97 (35) | |
| Unknown | 0 (0) | 15 (100) | 15 (5.4) | |
| Clinical Characteristics | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Patient Sex | ||||
| Male | REF | |||
| Female | 1.4 (0.8, 2.3) | 0.26 | -- | -- |
| Age at Stage IV diagnosis | ||||
| Increase of 5 years | 0.9 (0.8, 1.0) | 0.08 | -- | -- |
| Race | ||||
| White | REF | |||
| Asian/ Pacific Islander | 4.1 (2.3, 7.1) | <0.01 | 3.4 (1.7, 6.9) | 0.05 |
| Black | 1.0 (0.3, 3.6) | 0.27 | 1.5 (0.3, 8.6) | 0.82 |
| Smoking Status | ||||
| Never smoked | 8.7 (4.6, 17.0) | <0.01 | 8.1 (3.7, 17.5) | <0.01 |
| Ever smoked | REF | |||
| Histology | ||||
| Adenocarcinoma | 3.8 (1.3, 11.0) | 0.02 | 4.9 (1.0, 24.8) | 0.06 |
| Other | REF | |||
| Histology Grade | ||||
| I and II | 5.0 (2.5, 9.8) | <0.01 | 4.7 (2.1, 10.5) | <0.01 |
| II–III and III | REF |
| Ethnicity | Asian | Non-Asian | |||||
|---|---|---|---|---|---|---|---|
| Grade | EGFR Mutation Negative | EGFR Mutation Positive | p Value | Grade | EGFR Mutation Negative | EGFR Mutation Positive | p Value |
| I and II N = 46 (%) | 18 (39.1) | 28 (60.9) | 0.006 | I and II N = 93 (%) | 67 (72.0) | 26 (28.0) | 0.002 |
| II–III and III N = 26 (%) | 19 (73.1) | 7 (26.9) | II–III and III N = 64 (%) | 59 (92.2) | 5 (7.8) | ||
| Grades I and II | Grades II–III and III | ||||||
|---|---|---|---|---|---|---|---|
| Female | EGFR Mutation Negative | EGFR Mutation Positive | p Value | Female | EGFR Mutation Negative | EGFR Mutation Positive | p Value |
| Asian N = 23 (%) | 9 (39.1) | 14 (60.9) | 0.01 | Asian N = 16 (%) | 11 (66.8) | 5 (31.3) | 0.09 * |
| Non-Asian N = 55 (%) | 38 (69.1) | 17 (30.9) | Non-Asian N = 36 (%) | 33 (91.7) | 3 (8.3) | ||
| Male | Grades I and II | p Value | Male | Grades II–III and III | p Value | ||
| Asian N = 23 (%) | 9 (39.1) | 14 (60.9) | 0.004 | Asian N = 10 (%) | 8 (80.0) | 2 (20.0) | 0.28 * |
| Non-Asian N = 38 (%) | 29 (76.3) | 9 (23.7) | Non-Asian N = 28 (%) | 26 (92.9) | 2 (7.1) | ||
| Never-Smokers | Ever-Smokers | Smoking History Unknown | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | EGFR Mutation Negative | EGFR Mutation Positive | p Value | Grade | EGFR Mutation Negative | EGFR Mutation Positive | p Value | Grade | EGFR Mutation Negative | EGFR Mutation Positive | p Value |
| I and II N = 52 (%) | 21 (40.4) | 31 (59.6) | 0.10 * | I and II N = 65 (%) | 54 (83.1) | 11 (16.9) | 0.005 ** | I and II N = 22 (%) | 10 (45.5) | 12 (54.5) | 0.07 ** |
| II–III and III N = 21 (%) | 13 (61.9) | 8 (38.1) | II–III and III N = 56 (%) | 55 (98.2) | 1 (1.8) | II–III and III N = 13 (%) | 10 (76.9) | 3 (23.1) | |||
| EGFR Mutation Positive N = 83 | EGFR Mutation Negative N = 194 | p Value | |
|---|---|---|---|
| Number of patients received erlotinib | 78 (94%) | 42 (21.6%) | <0.0001 ** |
| Median length of exposure (month) [Quartile1, Quartile 3] | 11 [5, 33] | 1 [0, 5] | <0.0001 ** |
| Overall Survival | |||
| 12-month | 81.65% | 49.04% | <0.0001 * |
| 24-month | 61.67% | 34.04% | |
| 36-month | 47.24% | 22.96% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, M.; Lyon, L.; Barbero, E.; Wong, J.; Suga, J.M.; Sam, D.; Pan, M. Histologic Grade Is Predictive of Incidence of Epidermal Growth Factor Receptor Mutations in Metastatic Lung Adenocarcinoma. Med. Sci. 2017, 5, 34. https://doi.org/10.3390/medsci5040034
Levy M, Lyon L, Barbero E, Wong J, Suga JM, Sam D, Pan M. Histologic Grade Is Predictive of Incidence of Epidermal Growth Factor Receptor Mutations in Metastatic Lung Adenocarcinoma. Medical Sciences. 2017; 5(4):34. https://doi.org/10.3390/medsci5040034
Chicago/Turabian StyleLevy, Michelle, Liisa Lyon, Erika Barbero, John Wong, Jennifer Marie Suga, Danny Sam, and Minggui Pan. 2017. "Histologic Grade Is Predictive of Incidence of Epidermal Growth Factor Receptor Mutations in Metastatic Lung Adenocarcinoma" Medical Sciences 5, no. 4: 34. https://doi.org/10.3390/medsci5040034