Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives
Abstract
1. Introduction
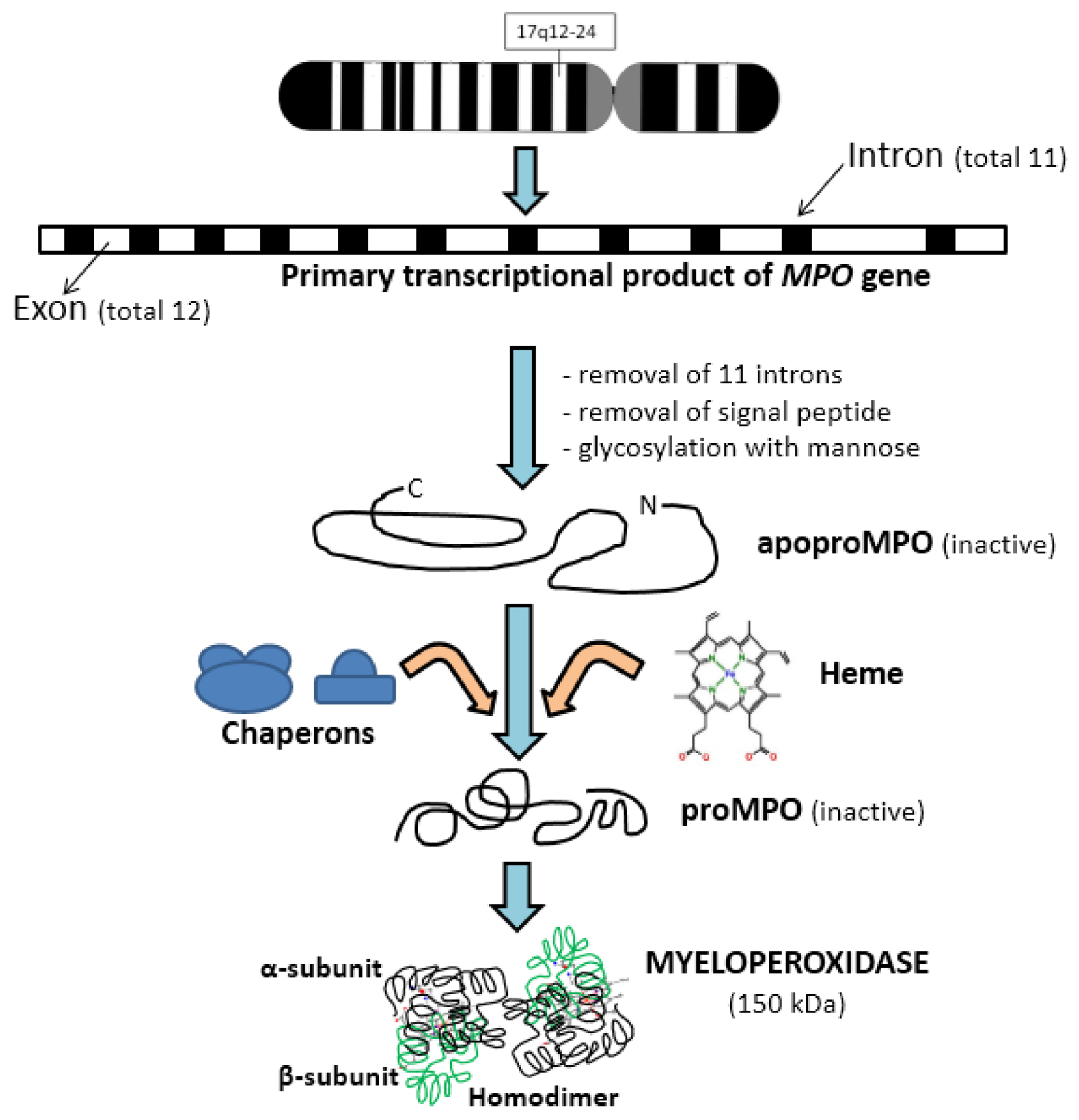
2. Biochemistry of Myeloperoxidase
2.1. Activation and Release of Myeloperoxidase by Neutrophils
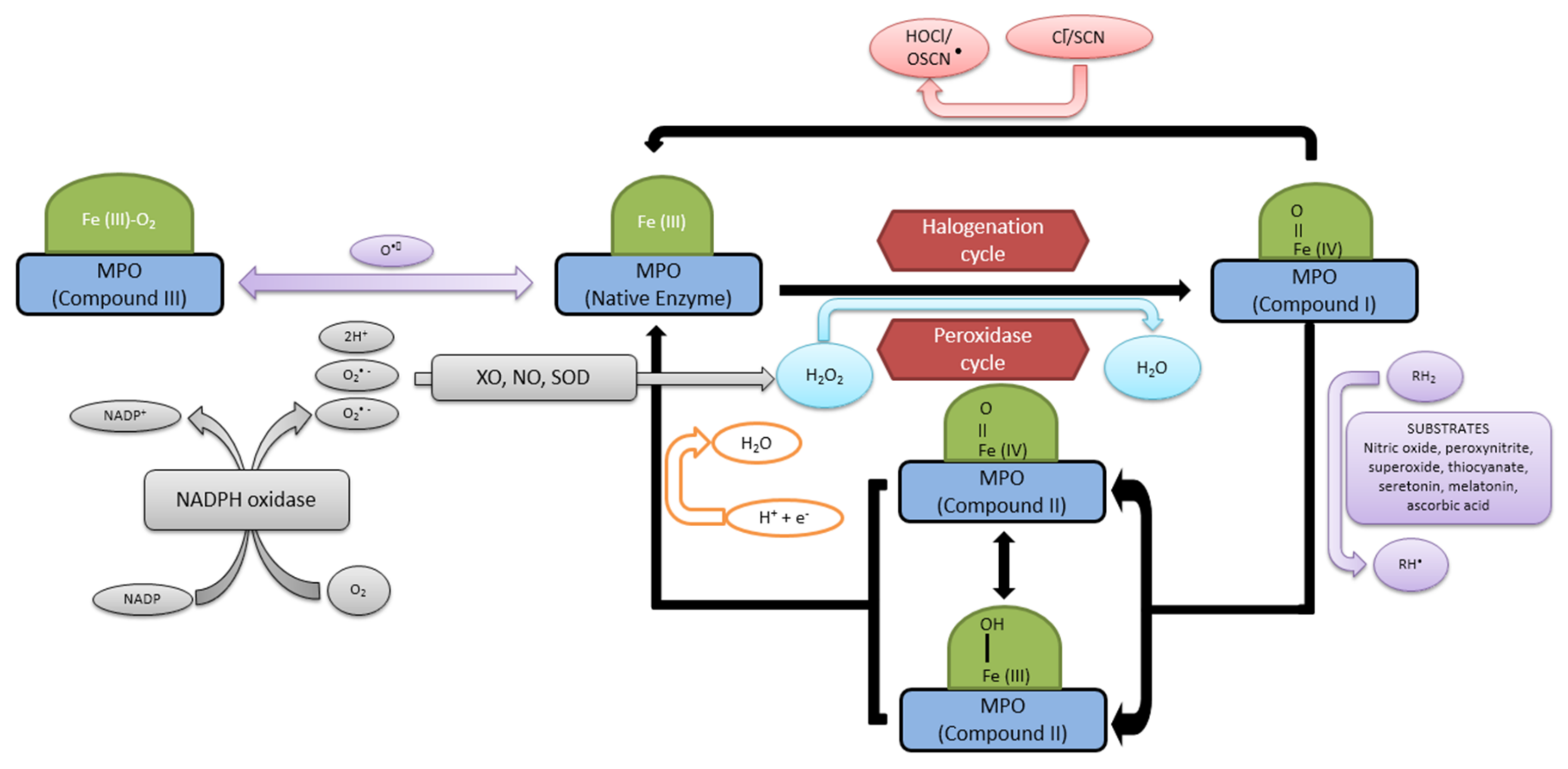
2.2. Reaction Mechanism and Functions of Myeloperoxidase
2.3. Measurement of Myeloperoxidase Activity
2.4. Inhibitors and Activators of Myeloperoxidase
3. Role of Myeloperoxidase in Different Diseases
3.1. Inflammation
3.2. Rheumatoid Arthritis
3.3. Cardiovascular Diseases and Atherosclerosis
3.4. Obesity
3.5. Neurodegenerative Diseases
3.6. Diabetes/Diabetic Retinopathy
3.7. Liver Diseases
3.8. Cancer
3.9. Cystic Fibrosis
4. Myeloperoxidase Deficiency
5. Conclusions
Conflicts of Interest
References
- Khan, A.A.; Rahmani, A.H.; Aldebasi, Y.H.; Aly, S.H. Biochemical and pathological studies on peroxidases –An updated review. Glob. J. Health Sci. 2014, 6, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Q.; Zhang, Y.-Z.; Wu, Y.; Zhang, J.-J.; Li, T.-B.; Jiang, T.; Xiong, X.-M.; Luo, X.-J.; Ma, Q.-L.; Peng, J. Myeloperoxidase-derived hypochlorous acid promotes ox-LDL induced senescence of endothelial cells through a mechanism involving β-catenin signaling in hyperlipidemia. Biochem. Biophys. Res. Commun. 2015, 467, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [PubMed]
- Chen, Y.; Hashiguchi, N.; Yip, L.; Junger, W.G. Hypertonic saline enhances neutrophil elastase release through activation of P2 and A3 receptors. Am. J. Physiol. 2006, 290, C1051–C1059. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanism of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Naegelen, N.; Beaume, N.; Plançon, S.; Schenten, V.; Tschirhart, E.J.; Bréchard, S. Regulation of neutrophil degranulation and cytokine secretion: A novel model approach based on linear fitting. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Kawata, J.; Yamamoto, T.; Ishimaru, Y.; Sakamoto, A.; Ono, T.; Narahara, S.; Sugiuchi, H.; Hirose, E.; Yamaguchi, Y. Mechanism of interferon-γ production by monocytes stimulated with myeloperoxidase and neutrophil extracellular traps. Blood Cells Mol. Dis. 2015, 55, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Gorudko, I.V.; Sokolov, A.V. Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry 2013, 78, 1466–1489. [Google Scholar] [CrossRef] [PubMed]
- Inazawa, J.; Inoue, K.; Nishigaki, H.; Tsuda, S.; Taniwaki, M.; Misawa, S.; Abe, T. Assignment of the human myeloperoxidase gene (MPO) to bands q21.3 → q23 of chromosome 17. Cytogenet. Cell Genet. 1989, 50, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.R.; Austin, G.E.; Chan, W.C.; Conaty, A.L.; Trusler, S.; Trappier, S.; Lindsey, R.B.; Swan, D.C. Chromosomal localization of the human myeloperoxidase gene by in situ hybridization using oligonucleotide probes. Genes Chromosomes Cancer 1990, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Nauseef, W.M. Molecular biology of MPO. In Peroxidases in Chemistry and Biology; Everse, J., Everse, K.E., Grisham, M.B., Eds.; CRC: Boca Raton, FL, USA, 1991; Volume I, pp. 63–81. [Google Scholar]
- Nauseef, W.M.; McCormick, S.J.; Clark, R.A. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J. Biol. Chem. 1995, 270, 4741–4747. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M.; McCormick, S.J.; Goedken, M. Coordinated participation of calreticulin and calnexin in the biosynthesis of myeloperoxidase. J. Biol. Chem. 1998, 273, 7107–7111. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, I.B.; Guzman, G.S.; Bonkovsky, H.L.; Zaki, S.R.; Kinkade, J.M., Jr. The post-translational processing of myeloperoxidase is regulated by the availability of heme. Arch. Biochem. Biophys. 1994, 312, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Krinsky, N.I. The reductive cleavage of myeloperoxidase in half, producing enzymatically active hemi-myeloperoxidase. J. Biol. Chem. 1981, 256, 4211–4218. [Google Scholar] [PubMed]
- Olsen, R.L.; Little, C. Studies on the subunits of human myeloperoxidase. Biochem. J. 1984, 222, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, K.T.; Nemirovskiy, E. Myeloperoxidase isoform activities released by human neutrophils in response to dental and periodontal bacteria. Oral Microbiol. Immunol. 1997, 12, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Havasawa, H.; Lonnerdal, B. Mutations affecting the calcium-binding site of myeloperoxidase and lactoperoxidases. Biochem. Biophys. Res. Commun. 2001, 281, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kamanna, V.S.; Kashyap, M.L. Niacin decreases leukocyte myeloperoxidase: Mechanistic role of redox agents and Src/p38MAP kinase. Atherosclerosis 2014, 235, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W. Myeloperoxidase in human neutrophil host defense. Cell Microbiol. 2014, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y.; Kura, F.; Watanabe, H.; Akagawa, H.; Takano, Y.; Suzuki, K.; Dinauer, M.C.; Maeda, N.; Koyama, H. In vivo role of myeloperoxidase for the host defense. Jpn. J. Infect. Dis. 2004, 57, S15. [Google Scholar] [PubMed]
- El Kebir, D.; Jozsef, L.; Pan, W.; Filep, J.G. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ. Res. 2008, 103, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The role of formylated peptides and formyl peptide receptor-1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015, 185, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Germena, G.; Hirsch, E. Dissection of the interplay between class I PI3Ks and Rac13 signaling in phagocytic functions. Sci. World J. 2010, 10, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Rolas, L.; Makhezer, N.; Hadjoudj, S.; El-Benna, J.; Djerdjouri, B.; Elkrief, L.; Moreau, R.; Perianin, A. Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated patients with cirrhosis. Hepatology 2013, 57, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Dinauer, M.C. Disorders of neutrophil function: An overview. Methods Mol. Biol. 2014, 1124, 501–515. [Google Scholar] [PubMed]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Deimann, W. Endogenous peroxidase activity in mononuclear phagocytes. Prog. Histochem. Cytochem. 1984, 15, 1–56. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.L.; Skaff, O.; Senthilmohan, R.; Kettle, A.J.; Davies, M.J. Hypobromous acid and bromamine production by neutrophils and modulation by superoxide. Biochem. J. 2009, 417, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Britigan, B.E.; Ratcliffe, H.R.; Buettner, G.R.; Rosen, G.M. Binding of myeloperoxidase to bacteria: Effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim. Biophys. Acta 1996, 1290, 231–240. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 1996, 64, 3512–3517. [Google Scholar] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [PubMed]
- Flemming, J.; Remmler, J.; Rohring, F.; Arnold, J. (−)-Epicatechin regenerates the chlorinating activity of myeloperoxidase in vitro and in neutrophil granulocytes. J. Inorg. Biochem. 2014, 130, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Araiso, T.; Palcic, M.M.; Dunford, H.B. Compound I of myeloperoxidase. Biochem. Biophys. Res. Commun. 1980, 94, 34–40. [Google Scholar] [CrossRef]
- Andrews, P.C.; Krinsky, N.I. Quantitative determination of myeloperoxidase using tetramethylbenadine as substrate. Anal. Biochem. 1982, 127, 346–350. [Google Scholar] [CrossRef]
- Andrews, P.C.; Krinsky, N.I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J. Biol. Chem. 1982, 257, 13240–13245. [Google Scholar] [PubMed]
- Cai, H.; Griendling, K.K.; Harrison, D.G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003, 24, 471–478. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Rutkowski, R.; Pancewicz, S.A.; Rutkowski, K.; Rutkowska, J. Reactive oxygen and nitrogen species in inflammatory process. Pol. Merkur. Lekarski 2007, 134, 131–136. [Google Scholar]
- Freeman, T.A.; Parvizi, J.; Della-Valle, C.J.; Steinbeck, M.J. Reactive oxygen and nitrogen species induced protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2009, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.M.; Small, A.L.; Mc-Fall-Ngai, M.J. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna–Vibrio mutualism. Proc. Natl. Acad. Sci. USA 1996, 93, 13683–13688. [Google Scholar] [CrossRef] [PubMed]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Reactive oxygen species and oocyte aging: Role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic. Biol. Med. 2008, 44, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, K.T.; Zambon, J.J.; Jones, C.A.; Wilson, M.E. Role of high avidity binding of human neutrophil myeloperoxidase in the killing of Actinobacillus actinomycetemcomatins. Infect. Immun. 1987, 55, 1029–1036. [Google Scholar] [PubMed]
- Haegens, A.; Vernooy, J.H.J.; Heeringa, P.; Mossman, B.T. Wouter, E.F. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008, 31, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zabucchi, G.; Soranzo, M.R.; Menegazzi, R.; Bertoncin, P.; Nardon, E.; Patriarca, P. Uptake of human eosinophil peroxidase and myeloperoxidase by cells involved in the inflammatory process. J. Histochem. Cytochem. 1989, 37, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.J.; Schröder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase attracts neutrophils by physical factors. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Vargunam, M.; Adu, D.; Taylor, C.M.; Michael, J.; Richards, N.; Neuberger, J.; Thompson, R.A. Endothelium myeloperoxidase–antimyeloperoxidase interaction in vasculitis. Nephrol. Dial. Transplant. 1992, 7, 1077–1081. [Google Scholar] [PubMed]
- Lefkowitz, D.L.; Mills, K.; Morgan, D.; Lefkowitz, S.S. Macrophage activation and immunomodulation by myeloperoxidase. Proc. Soc. Exp. Biol. Med. 1992, 199, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gorudko, I.V.; Sokolov, A.V.; Shamova, E.V.; Grudinina, N.A.; Drozd, E.S.; Shishlo, L.M.; Grigorieva, D.V.; Bushuk, S.B.; Bushuk, B.A.; Chizhik, S.A.; et al. Myeloperoxidase modulates human platelet aggregation via actin cytoskeleton reorganization and store-operated calcium entry. Biol. Open 2013, 2, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, H.; Klinke, A.; Kremserova, S.; Adam, M.; Pekarova, M.; Baldus, S.; Eiserich, J.P.; Kubala, L. Myeloperoxidase induces the priming of platelets. Free Radic. Biol. Med. 2013, 61, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brümmer, J.; Rudolph, V.; Münzel, T.; Heitzer, T.; et al. Myeloperoxidase mediates neutrophil activation by association with CD 11b/CD 18 integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Ageeva, K.V.; Cherkalina, O.S.; Pulina, M.O.; Zakharova, E.T.; Prozorovskii, V.N.; Aksenov, D.V.; Vasilyev, V.B.; Panasenko, O.M. Identification and properties of complexes formed by myeloperoxidase with lipoproteins and ceruloplasmin. Chem. Phys. Lipids 2010, 163, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gorudko, I.V.; Sokolov, A.V.; Shamova, E.V.; Grigorieva, D.V.; Mironova, E.V.; Kudryavtsev, I.V.; Gusev, S.A.; Gusev, A.A.; Chekanov, A.V.; Vasilyev, V.B.; et al. Binding of human myeloperoxidase to red blood cells: Molecular targets and biophysical consequences at the plasma membrane level. Arch. Biochem. Biophy. 2016, 591, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Li, W.; Daehnke, H.L.; Goldstein, J.A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993, 268, 4069–4077. [Google Scholar] [PubMed]
- Marquez, L.A.; Dunford, H.B. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. J. Biol. Chem. 1996, 270, 30434–30440. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Browne, K.; Harris, A.; Hyslop, P.A.; Jackson, J.H.; Quehenberger, O.; Cochrane, C.G. Mechanisms of hypochlorite injury of target cells. J. Clin. Investig. 1990, 85, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed]
- Bozeman, P.M.; Learn, D.B.; Thomas, E.L. Assay of human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. J. Immunol. Methods 1990, 126, 125–133. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Dhiman, M.; Estrada-Franco, J.G.; Pando, J.M.; Ramirez-Aguilar, F.J.; Spratt, H.; Vazquez-Corzo, S.; Perez-Molina, G.; Gallegos-Sandoval, R.; Moreno, R.; Garg, N.J. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin. Vaccine Immunol. 2009, 16, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Sandhu, J.K.; Birnboim, H.C. A myeloperoxidase-specific assay based upon bromide dependent chemiluminiscence of luminol. Anal. Biochem. 1999, 273, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cooray, R.; Petersson, C.G.B.; Gronvik, K.-O. Preparation and characterization of monoclonal antibodies against bovine myeloperoxidase. Vet. Immunol. Immunopathol. 1995, 46, 211–221. [Google Scholar] [CrossRef]
- Mayyas, F.A.; Al-Jarrah, M.I.; Ibrahim, K.S.; Alzoubi, K.H. Level and significance of plasma myeloperoxidase and the neutrophil to lymphocyte ratio in patients with coronary artery disease. Exp. Ther. Med. 2014, 8, 1951–1957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bensalem, S.; Soubhye, J.; Aldib, I.; Bournine, L.; Nguyen, A.T.; Vanhaeverbeek, M.; Rousseau, A.; Boudjeltia, K.Z.; Sarakbi, A.; Kauffmann, J.M.; et al. Inhibition of myeloperoxidase activity by the alkaloids of Peganumharmala L. (Zygophyllaceae). J. Ethnopharmacol. 2014, 154, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Smith, F.; Panizzi, J.R.; Goodwin, D.C.; Panizzi, P. Inactivation of myeloperoxidase by benzoic acid hydrazide. Arch. Biochem. Biophys. 2015, 570, 14–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kettle, A.J.; Gedye, C.A.; Winterbourn, C.C. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem. J. 1997, 321, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Segelmark, M.; Persson, B.; Hellmark, T.; Wieslander, J. Binding and inhibition of myeloperoxidase (MPO): A major function of ceruloplasmin? Clin. Exp. Immunol. 1997, 108, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Qin, S.; Zhang, L.; Kamanna, V.S.; Kashyap, M.L. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009, 202, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Van Antwerpen, P.; Dufrasne, F.; Lequeux, M.; Boudjeltia, K.Z.; Lessgyer, I.; Babar, S.; Moreau, P.; Moguilevsky, N.; Vanhaeverbeek, M.; Ducobu, J.; et al. Inhibition of the myeloperoxidase chlorinating activity by non-steroidal anti-inflammatory drugs: Flufenamic acid and its 5-chloro-derivative directly interact with a recombinant human myeloperoxidase to inhibit the synthesis of hypochlorous acid. Eur. J. Pharmacol. 2007, 570, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, S.; Franck, T.; Van Antwerpen, P.; Boudjeltia, K.Z.; Mouithys-Mickalad, A.; Deby, C.; Moguilevsky, N.; Deby-Dupont, G.; Lamy, M.; Serteyn, D. Resveratrol inhibits the activity of equine neutrophil myeloperoxidase by a direct interaction with the enzyme. J. Agric. Food Chem. 2007, 55, 8080–8087. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Ximenes, V.F.; Regasini, L.O.; Dutra, L.A.; Silva, D.H.; Fonseca, L.M.; Coelho, D.; Machado, S.A.; Bolzani, V.S. 4′-Aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, M.; Carmine, T.C.; Gerber, C.; Niethammer, D.; Bruchelt, G. Evidence for the activation of myeloperoxidase by f-Meth-Leu-Phe prior to its release from neutrophil granulocytes. Biochem. Biphy. Res. Commun. 1997, 232, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; de la Rebiere, G.; Deby-Dupont, G.; Deby, C.; Niesten, A.; Serteyn, D. Activation of equine neutrophils by phorbol myristate acetate or N-formyl-methionyl-leucyl-phenylalanine induces a different response in reactive oxygen species production and release of active myeloperoxidase. Vet. Immunol. Immunopathol. 2009, 130, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Faith, M.; Sukumaran, A.; Pulimood, A.B.; Jacob, M. How reliable an indicator of inflammation is myeloperoxidase activity? Clin. Chim. Acta 2008, 396, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.L.; Lefkowitz, S.S. Microglia and myeloperoxidase: A deadly partnership in neurodegenerative disease. Free Radic. Biol. Med. 2008, 45, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aguilera, C.M.; Gil-Campos, M.; Leis, R.; Bueno, G.; Martinez-Jimenez, M.D.; Valle, M.; Canete, R.; Tojo, R.; Moreno, L.A.; et al. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 2012, 35, 2373–2376. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, L.A.; Hisham, M.F.; Nechev, L.V.; Harris, T.M.; Harris, C.M.; Mamett, L.D. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J. Biol. Chem. 2001, 276, 9066–9070. [Google Scholar] [CrossRef] [PubMed]
- Feyler, A.; Voho, A.; Bouchardy, C.; Kuokkanen, K.; Dayer, P.; Hirvonen, A.; Benhamou, S. Point: myeloperoxidase-463G3A polymorphism and lung cancer risk. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1550–1554. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res. 2011, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Urbancic, M.; Prevodnik, K.V.; Petrovic, D.; Petrovic, G.M. A flow cytometric analysis of vitreous inflammatory cells in patients with proliferative diabetic retinopathy. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.; Marsche, G.; Arnhold, J.; Davies, M.J. Modification of low density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim. Biophys. Acta 2006, 1761, 392–415. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.R.; Jones, B.E. Hepatitis C virus and oxidative stress: A dangerous liaison. Future Virol. 2006, 1, 223–232. [Google Scholar] [CrossRef]
- Altamirano, J.; Miquel, R.; Katoonizadeh, A.; Abraldes, J.G.; Duarte-Rojo, A.; Louvet, A.; Augustin, S.; Mookerjee, R.P.; Michelena, J.; Smyrk, T.C.; et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014, 146, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008, 444, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P. Myeloperoxidase in the neurodegenerative process of Parkinson’s disease. Dtsch. Med. Wochenschr. 2014, 139, 99–102. [Google Scholar] [PubMed]
- Koziol-Montewka, M.; Kolodziejek, A.; Oles, J. Study of myeloperoxidase role in antituberculosis defense in the context of cytokine activation. Inflammation 2004, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Monteseirín, J.; Bonilla, I.; Camacho, J.; Conde, J.; Sobrino, F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: The effect of immunotherapy. J. Allergy Clin. Immunol. 2001, 107, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Fuchs, B.; Arnhold, J.; Arnhold, K. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: Molecular pathways, diagnosis and potential therapeutic strategies. Curr. Med. Chem. 2003, 10, 2123–2145. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, Z.; Marcinkiewicz, J.; Katz, D.R.; Chain, B.M. Neutrophil myeloperoxidase: Soldier and statesman. Arch. Immunol. Ther. Exp. 2012, 60, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Crampette, L.; Mondain, M.; Enander, I.; Jones, I.; Bousquet, J. Myeloperoxidase and interleukin-8 levels in chronic sinusitis. Clin. Exp. Allergy 1997, 27, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Arumugam, G. A biochemical study on the gastroprotective effect of hydroalcoholic extract of Andrographis paniculata in rats. Indian J. Pharmacol. 2011, 43, 402–408. [Google Scholar] [PubMed]
- Nishida, K.; Ohta, Y.; Ishiguro, I. Contribution of NO synthase to neutrophil infiltration in the gastric mucosal lesions in rats with water immersion restraint stress. FEBS Lett. 1998, 425, 243–248. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, D.; Dembinski, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembinski, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012, 18, 181–187. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kusnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambrozy, T.; Gil, K.; Olszanecki, R.; et al. Essential role of growth hormone and IGF-1 in therapeutic effect of ghrelin in the course of acetic acid-induced colitis. Int. J. Mol. Sci. 2017, 18, 1118. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Galazka, K.; Bonior, J.; Jaworek, J.; Bartus, K.; Gil, K.; et al. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef] [PubMed]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Gosiewski, T.; Bulanda, M.; Kusnierz-Cabala, B.; Galazka, K.; Konturek, P.C. Synergic interaction of Rifaximinand and Mutaflor (Escherichia coli Nissle 1917) in the treatment of acetic acid-induced colitis in rats. Gastroenterol. Res. Pract. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chooklin, S.; Pereyaslov, A.; Bihalskyy, I. Pathogenic role of myeloperoxidase in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 627–631. [Google Scholar] [PubMed]
- Klangprapan, S.; Chaiyarit, P.; Hormdee, D.; Kampichai, A.; Khampitak, T.; Daduang, J.; Tavichakorntrakool, R.; Panijpan, B.; Boonsiri, P. Salivary myeloperoxidase, assessed by 3,3′-diaminobenzidine colorimetry, can differentiate periodontal patients from nonperiodontal subjects. Enzyme Res. 2016, 2016, 7517928. [Google Scholar] [CrossRef] [PubMed]
- Loria, V.; Dato, I.; Graziani, F.; Biasucci, L. Myeloperoxidase: A new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediat. Inflamm. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, D.L. Mechanism responsible for increased vascular permeability in acute inflammation. Inflamm. Res. 1973, 3, 297–306. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train. 2006, 41, 457–465. [Google Scholar] [PubMed]
- Zhang, R.; Brennan, M.-L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Pontsler, A.V.; Marathe, G.K.; Harrison, K.A.; Murphy, R.C.; Hinshaw, J.C.; Prestwich, G.D.; Hilaire, A.S.; Prescott, S.M.; Zimmerman, G.A.; et al. oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor Y ligands and agonists. J. Biol. Chem. 2001, 276, 16015–16023. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L.; Hsu, F.F.; Gaut, J.P.; Crowley, J.R.; Heinecke, J.W. Modification of proteins and lipids by myeloperoxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 88–105. [Google Scholar]
- Van Dalen, C.J.; Winterbourn, C.C.; Senthilmohan, R.; Kettle, A.J. Nitrate as a substrate and inhibitor of myeloperoxidase, implication for nitration and hypochlorous acid production at sites of inflammation. J. Biol. Chem. 2000, 275, 11638–11644. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Wu, W.; Fu, X.; Shen, Z.; Song, W.; Frost, H.; Vadseth, C.; Narine, L.; Lenkiewicz, E.; Borchers, M.T.; et al. A tale of two controversies, defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002, 277, 17415–17427. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.M.S.N.; da Silva, N.P.; Sato, E.I. Increased myeloperoxidase plasma levels in rheumatoid arthritis. Rheumatol. Int. 2012, 32, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Khalilova, I.; Tarr, J.M.; Senthilmohan, R.; Turner, R.; Haigh, R.C.; Winyard, P.G.; Kettle, A.J. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 2012, 51, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gowder, S.J.G. Glutathione peroxidase: A potential marker for the most common diseases and disorders. Recent Patents Biomarkers 2014, 4, 43–52. [Google Scholar] [CrossRef]
- Morgan, P.E.; Sturgess, A.D.; Davies, M.J. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 2005, 52, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.W.; Hallett, M.B. Seeing the wood for the trees: The forgotten role of neutrophils in rheumatoid arthritis. Immunol. Today 1997, 18, 320–324. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edward, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ece, A.; Kelekçi, S.; Hekimoglu, A.; Kocamaz, H.; Balik, H.; Yolbas, I.; Erel, O. Neutrophil activation, protein oxidation and ceruloplasmin levels in children with Henoch–Schönlein purpura. Pediat. Nephrol. 2007, 22, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): An oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003, 278, 28403–28409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brennan, M.L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Ruwende, C.; Eng, C.; Marmur, J.D. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am. J. Cardiol. 2007, 99, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Brennan, M.L.; Philip, K.; Tong, W.; Mann, S.; van Lente, F.; Hazen, S.L. Plasma myeloperoxidase levels in patients with chronic heart failure. Am. J. Cardiol. 2006, 98, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Mehilli, J.; von Beckerath, N.; Schomig, A.; Kastrati, A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur. J. Clin. Investig. 2008, 38, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.M.; Camargo, P.V.; Borges, F.K.; Rossini, A.P.; Polanczyk, C.A. Prognostic value of myeloperoxidase in coronary artery disease: Comparison of unstable and stable angina patients. Coron. Atery Dis. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Penn, M.S.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; McErlean, E.S.; Topol, E.J.; et al. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münze, L.T.; Simoons, M.L.; Hamm, C.W. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Mocatta, T.J.; Pilbrow, A.P.; Cameron, V.A.; Senthilmohan, R.; Frampton, C.M.; Richards, A.M.; Winterbourn, C.C. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 2000, 247, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modification in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [PubMed]
- Delporte, C.; Boudjeltia, K.Z.; Noyon, C.; Furtmuller, P.G.; Nuyens, V.; Slomianny, M.-C.; Madhoun, P.; Desmet, J.-M.; Raynal, P.; Dufour, D.; et al. Impact of myeloperoxidase–LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J. Lipid Res. 2014, 55, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.J.; Stocker, R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem. J. 1993, 290, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, Z.; Zhang, W.; Zhou, J.; Wu, Y.; Zhang, M.; Zhu, H.; Zou, M.H. Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes 2014, 63, 4172–4185. [Google Scholar] [CrossRef] [PubMed]
- Zur, B.; Look, M.; Holdenrieder, S.; Stoffel-Wagner, B. Elevated plasma myeloperoxidase concentration in adults with obesity. Clin. Chim. Acta 2011, 412, 1891–1892. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.L.; Petruceli, E.; Belo, V.A.; Andrade-Fernandes, C.M.; Caetano Russi, C.V.; Bosco, A.A.; Tanus-Santos, J.E.; Sandrim, V.C. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin. Biochem. 2012, 45, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Goldberg, I.J. Myeloperoxidase: A therapeutic target for preventing insulin resistance and the metabolic sequelae of obesity? Diabetes 2014, 63, 4001–4003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet 1999, 353, 1649–1652. [Google Scholar] [CrossRef]
- Ford, E.S. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of U.S. adults. Am. J. Epidemiol. 2002, 155, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, K.; Yamane, K.; Hanafusa, M.; Mori, H.; Mito, K.; Okubo, M.; Hara, H.; Kohno, N. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care 2004, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Borato, D.C.; Parabocz, G.C.; Riba, J.T.; Netto, H.P.; Erdmann, F.C.; Wiecheteck, L.D.; Manente, F.A.; Mello, L.R.; Bello, C.; dos Santos, F.A. Biomarkers in obesity: Serum myeloperoxidase and traditional cardiac risk parameters. Exp. Clin. Endocrinol. Diabetes 2016, 124, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Katyal, A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci. Biobehav. Rev. 2016, 68, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Johnson, B.D.; Sheps, D.S.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; Rutledge, T.; Shaw, L.J.; Sopko, G.; Bairey Merz, C.N. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia. J. Am. Coll. Cardiol. 2007, 50, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Selek, S.; Altindag, A.; Saracoglu, G.; Aksoy, N. Oxidative markers of myeloperoxidase and catalase and their diagnostic performance in bipolar disorder. J. Affect. Dis. 2015, 181, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Kaji, Y.; Usui, T.; Ishida, S.; Yamashiro, K.; Moore, T.C.; Moore, J.; Yamamoto, Y.; Yamamoto, H.; Adamis, A.P. Inhibition of diabetic leukostasis and bloodretinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Investig. Ophthalmol. Vis. Sci. 2007, 48, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Urbancic, M.; Stunf, S.; Milutinovic, A.Z.; Petrovic, D.; Petrovic, M.G. Epiretinal membrane inflammatory cell density might reflect the activity of proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8576–8582. [Google Scholar]
- Miyamoto, K.; Ogura, Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin. Ophthalmol. 1999, 14, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol. 2001, 158, 147–152. [Google Scholar] [CrossRef]
- Rarok, A.A.; Limburg, P.C.; Kallenberg, C.G. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J. Leukoc. Biol. 2003, 74, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Kamesh, L.; Savage, C.O. Translating basic science into patient therapy for ANCA-associated small vessel vasculitis. Clin. Sci. 2005, 108, 101–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K.; Jaeschke, H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol. Pathol. 2007, 35, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Ho, Y.S.; Fisher, M.A.; Lawson, J.A.; Farhood, A. Glutathione peroxidase deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: Importance of an intracellular oxidant stress. Hepatology 1999, 29, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Poli, G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol. Aspects Med. 2000, 21, 49–98. [Google Scholar] [CrossRef]
- Eash, K.J.; Means, J.M.; White, D.W.; Link, D.C. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 2009, 113, 4711–4719. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Delarche, C.; Paradis, V.; Mathurin, P.; Grenier, A.; Crestani, B.; Dehoux, M.; Thabut, D.; Gougerot-Pocidalo, M.-A.; Thierry Poynard, T.; et al. Polymorphonuclear neutrophils are a source of hepatocyte growth factor in patients with severe alcoholic hepatitis. J. Hepatol. 2002, 36, 342–348. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, A.K.; Narasimhan, R.L.; Bhalla, A.; Sharma, N.; Sharma, R. Granulocyte colony stimulating factor in severe alcoholic hepatitis: A randomized pilot study. Am. J. Gastroenterol. 2014, 109, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Mookerjee, R.P.; Hodges, S.; Wright, G.A.; Davies, N.A.; Jalan, R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 2008, 48, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. Role of C5 activation products in sepsis. Sci. World J. 2010, 10, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Markwick, L.J.; Riva, A.; Ryan, J.M.; Cooksley, H.; Palma, E.; Tranah, T.H.; Manakkat Vijay, G.K.; Vergis, N.; Thursz, M.; Evans, A.; et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 2015, 148, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Juttner, B.; Younes, A.; Weissig, A.; Ahrens, J.; Becker, T.; Scheinichen, D. Reduced post-operative neutrophil activation in liver transplant recipients suffering from post-hepatitic cirrhosis. Clin. Transplant. 2009, 23, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, M.; Reichert, E.; Fitzpatrick, F.A. Influence of myeloperoxidase on colon tumor occurrence in inflamed versus non-inflamed colons of ApcMin/+ mice. Redox Biol. 2015, 6, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, C.; Feng, J.; Xu, Q. Ding Association between the myeloperoxidase gene polymorphisms and the susceptibility to prostate cancer: A case-control study in a Chinese population. Actas Urol. Esp. 2013, 37, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hoy, A.; Tregouet, D.; Leininger-Muller, B.; Poirier, O.; Maurice, M.; Sass, C.; Siest, G.; Tiret, L.; Visvikis, S. Serum myeloperoxidase concentration in a healthy population: Biological variations, familial resemblance and new genetic polymorphisms. Eur. J. Hum. Genet. 2001, 9, 780–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nauseef, W.M.; Brigham, S.; Cogley, M. Hereditary myeloperoxidase deficiency due to a missense mutation of arginine 569 to trypophan. J. Biol. Chem. 1994, 269, 1212–1216. [Google Scholar] [PubMed]
- Trush, M.A.; Seed, J.L.; Kensler, T.W. Oxidant-dependent metabolic activation of polycyclic aromatic hydrocarbons by phorbol ester stimulated human polymorphonuclear leukocytes: Possible link between inflammation and cancer. Proc. Natl. Acad. Sci. USA 1985, 82, 5194–5198. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, D.A.; French, R.C.; Ross, D.; Smith, M.T. Metabolic activation of diethylstilbestrol by stimulated human leukocytes. Cancer Lett. 1987, 35, 79–86. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, K.; Uchida, K.; Kolenc, D.; Hlupic, L.; Zarkovic, N. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic. Res. 2006, 40, 543–552. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Coussens, L.M. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, B.S.; de Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef] [PubMed]
- Jesneck, J.L.; Mukherjee, S.; Yurkovetsky, Z.; Clyde, M.; Marks, J.R.; Lokshin, A.E.; Lo, J.Y. Do serum biomarkers really measure breast cancer? BMC Cancer 2009, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tamimi, R.M.; Hankinson, S.E.; Hunter, D.J.; Han, J. A prospective study of genetic polymorphism in MPO, antioxidant status, and breast cancer risk. Breast Cancer Res. Treat. 2009, 113, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Jilani, M.; Vincent, T.; Faraji, H.; Giles, F.J.; Estey, E.; Kantarjian, H.P.; Albitar, M. Clinical relevance of circulating myeloperoxidase (MPO) in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Blood 2004, 104, 1073. [Google Scholar]
- Lynch, S.V.; Bruce, K.D. The cystic fibrosis airway microbiome. Cold Spring Harb. Perspect. Med. 2013, 3, a009738. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Wagner, B.D.; Anthony, M.M.; Emmett, P.; Zemanick, E.T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Conor, P.; Murray, C.P.; Stick, S.M. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinraunch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Patriarca, P.; Solero, G.P.; Baralle, F.E.; Romano, M. Genetic studies on myeloperoxidase deficiency in Italy. Jpn. J. Infect. Dis. 2004, 57, S10e2. [Google Scholar]
- Rudolph, T.K.; Wipper, S.; Reiter, B.; Rudolph, V.; Coym, A.; Detter, C.; Lau, D.; Klinke, A.; Friedrichs, K.; Rau, T.; et al. Myeloperoxidase deficiency preserves vasomotor function in humans. Eur. Heart J. 2012, 33, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Nunoi, H.; Kohi, F.; Kajiwara, H.; Suzuki, K. Prevalence of inherited myeloperoxidase deficiency in Japan. Microbiol. Immunol. 2003, 47, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.Y.; Kameoka, Y.; Persad, A.S.; Koi, F.; Yamagoe, S.; Hashimoto, K.; Suzuki, K. Novel missense mutation found in a Japanese patient with myeloperoxidase deficiency. Gene 2004, 327, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Persad, A.S.; Kameoka, Y.; Kanda, S.; Niho, Y.; Suzuki, K. Arginine to cysteine mutation (R499C) found in a Japanese patient with complete myeloperoxidase deficiency. Gene Expr. 2006, 13, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, E.; Huitema, M.G.; Mulder, A.H.; Heeringa, P.; van Goor, H.; Tervaert, J.W.; Weening, J.J.; Kallenberg, C.G. Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int. 1994, 45, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Wibke, B.T.; Allen, P.M. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001, 167, 1601–1608. [Google Scholar] [CrossRef]
- Singbartl, K.; Bockhorn, S.G.; Zarbock, A.; Schmolke, M.; Van Aken, H. T cell modulates neutrophils-dependent acute renal failure during endotoxemia: Critical role of CD28. J. Am. Soc. Nephrol. 2005, 16, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 1998, 76, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.M.; McKinney, E.F.; Smith, K.G. Emerging concepts in the pathogenesis of antineutrophil cytoplasmic antibody-associated vasculitis. Curr. Opin. Rheumatol. 2015, 27, 197–203. [Google Scholar] [CrossRef] [PubMed]
| No. | Name of Disease | Brief Etiology and Possible Role of MPO | Reference |
|---|---|---|---|
| 1 | CVD and atherosclerosis | Raised level of MPO causes RBCs deformability, accumulation of cholesterol and its esters, ruptures in atherosclerotic plaque | [8,60] |
| 2 | Obesity | Neutrophil infiltration and activation of MPO in adipose tissue | [84,85] |
| 3 | Neurodegenerative diseases | Release of neurotoxic mediators by many factors spearheaded by MPO from neurons, astrocytes, microglia cells | [11] |
| 4 | Cancer | MPO-derived ROS/RNS react with major biomolecules causing mutagenesis, gene polymorphism, SNPs, acrolein-protein adduct formation | [86,87,88] |
| 5 | Diabetes/diabetic retinopathy | Neutrophil activation and the release of MPO in vessels and retina, upregulation of leukocyte adhesion molecules, and increased production of anti-MPO antibodies | [89,90] |
| 6 | Renal diseases | MPO-initiated HOCl-modified proteins in glomerular peripheral basement membranes | [91] |
| 7 | Liver diseases | Neutrophil infiltration, hepatic fibrosis by activation of Kupffer cells cause production of oxidants, impaired signaling events | [92,93] |
| 8 | Lung injury | Activation and expression of proinflammatory cytokines and mediators by MPO | [5] |
| 9 | Cystic fibrosis | Bacterial infiltration, especially Pseudomonas aeruginosa and infiltrating neutrophils | [94] |
| 10 | Multiple sclerosis | MPO-generated ROS cause axonal damage by proteolytic enzymes and cytotoxic oxidants by activated immune cells and glia | [95] |
| 11 | Alzheimer’s disease | Increased production of oxidants like advanced glycation end products, o,o′-dityrosine, lipid oxidation products, protein carbonyls, oxidized DNA, and 3-nitrotyrosine in neuronal tissues proposed by increased expression of MPO | [96] |
| 12 | Parkinson’s disease | Upregulation of MPO and its byproduct, 3-chlorotyrosine, in ventral midbrain | [97] |
| 13 | Tuberculosis | Enhanced MPO expression along with TNF-α and IL-12 activation | [98] |
| 14 | Asthma | Excessive MPO release from neutrophils in lower respiratory tract cells | [99] |
| 15 | Rheumatoid arthritis | Inflamed synovium intervened by lymphocytes and neutrophils leads to the release of proinflammatory mediators | [100,101] |
| 16 | Chronic sinusitis | Enhanced level of MPO and IL-8 in sinuses | [102] |
| 17 | Peptic ulcer | Free radicals formation initiated by MPO | [103] |
| 18 | Gastric ulcer | Neutrophil infiltration and the release of MPO into gastric mucosal tissue | [104] |
| 19 | Duodenal ulcer | MPO and other pro-inflammatory agents | [105] |
| 20 | Colitis | Increased activity of MPO and pro-inflammatory mediators like IL-1β and TNF-α | [106,107,108] |
| 21 | Pancreatitis | Increased MPO activity causes increased ROS that leads to this disease | [109] |
| 22 | Chronic periodontitis | Increased MPO activity in gingival crevicular fluid | [110] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. https://doi.org/10.3390/medsci6020033
Khan AA, Alsahli MA, Rahmani AH. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Medical Sciences. 2018; 6(2):33. https://doi.org/10.3390/medsci6020033
Chicago/Turabian StyleKhan, Amjad A., Mohammed A. Alsahli, and Arshad H. Rahmani. 2018. "Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives" Medical Sciences 6, no. 2: 33. https://doi.org/10.3390/medsci6020033
APA StyleKhan, A. A., Alsahli, M. A., & Rahmani, A. H. (2018). Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Medical Sciences, 6(2), 33. https://doi.org/10.3390/medsci6020033





