Similarities in Blood Mononuclear Cell Membrane Phospholipid Profiles during Malignancy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
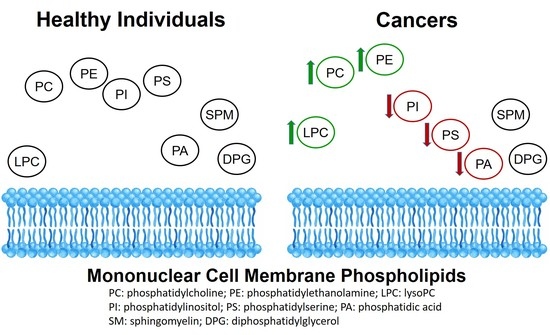
3.1. Phospholipid Levels of Mononuclear Cell Membranes in Diverse Cancer Patients vs. Healthy Individuals
3.2. Phospholipid Content of Mononuclear Cell Membranes in Individual Cancer Patients vs. Healthy Individuals
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etzioni, R.; Urban, N.; Ramsey, S.; McIntosh, M.; Schwartz, S.; Reid, B.; Radich, J.; Anderson, G.; Hartwell, L. The case for early detection. Nat. Rev. Cancer 2003, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Abtin, F.; Brown, K. Computed tomography screening for lung cancer: Has it finally arrived? Implications of the national lung screening trial. J. Clin. Oncol. 2013, 31, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Pace, L.E.; Keating, N.L. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014, 311, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Van Nagell, J.R., Jr.; Hoff, J.T. Transvaginal ultrasonography in ovarian cancer screening: Current perspectives. Int. J. Womens Health 2013, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Boudreau, D.M.; Fishman, P.A.; Elmore, J.G. Cost of breast-related care in the year following false positive screening mammograms. Med. Care 2010, 48, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Croswell, J.M.; Baker, S.G.; Marcus, P.M.; Clapp, J.D.; Kramer, B.S. Cumulative incidence of false-positive test results in lung cancer screening: A randomized trial. Ann. Intern. Med. 2010, 152, 505–512, W176–W180. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haun, J.B.; Castro, C.M.; Wang, R.; Peterson, V.M.; Marinelli, B.S.; Lee, H.; Weissleder, R. Micro-NMR for rapid molecular analysis of human tumor samples. Sci. Transl. Med. 2011, 3, 71ra16. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P. Cancer biomarkers: Can we turn recent failures into success? J. Natl. Cancer Inst. 2010, 102, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.S.; Gambhir, S.S. Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci. Transl. Med. 2011, 3, 109ra116. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.E. Why your new cancer biomarker may never work: Recurrent patterns and remarkable diversity in biomarker failures. Cancer Res. 2012, 72, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Escriba, P.V.; Gonzalez-Ros, J.M.; Goni, F.M.; Kinnunen, P.K.; Vigh, L.; Sanchez-Magraner, L.; Fernandez, A.M.; Busquets, X.; Horvath, I.; Barcelo-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed]
- Escriba, P.V.; Ferrer-Montiel, A.V.; Ferragut, J.A.; Gonzalez-Ros, J.M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: Implications in drug-resistance phenomena. Biochemistry 1990, 29, 7275–7282. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Brodde, A.; Braverman, N.E.; Moser, A.B.; Just, W.W.; Forss-Petter, S.; Brugger, B.; Berger, J. Homeostasis of phospholipids—The level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim. Biophys. Acta 2015, 1851, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.; Riordan, H.D.; Jackson, J.A.; Wong, K.; Miranda-Massari, J.R.; Gonzalez, M.J. Erythrocyte membrane fatty acid composition in cancer patients. P. R. Health Sci. J. 2004, 23, 107–113. [Google Scholar] [PubMed]
- Oremek, G.M.; Sapoutzis, N.; Lorenz, M. Phospholipids, tumour marker and beta-CrossLaps in diagnosis of gastric carcinoma. Anticancer Res. 2003, 23, 859–863. [Google Scholar] [PubMed]
- Fiancette, R.; Vincent-Fabert, C.; Guerin, E.; Trimoreau, F.; Denizot, Y. Lipid mediators and human leukemic blasts. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L.; Derua, R.; Munck, S.; Van Veldhoven, P.P.; Waltregny, D.; Daniels, V.W.; Machiels, J.; et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010, 70, 8117–8126. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Floegel, A.; Sookthai, D.; Johnson, T.; Rolle-Kampczyk, U.; Otto, W.; von Bergen, M.; Boeing, H.; Kaaks, R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Raynor, A.; Jantscheff, P.; Ross, T.; Schlesinger, M.; Wilde, M.; Haasis, S.; Dreckmann, T.; Bendas, G.; Massing, U. Saturated and mono-unsaturated lysophosphatidylcholine metabolism in tumour cells: A potential therapeutic target for preventing metastases. Lipids Health Dis. 2015, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Z.; Tan, S.; Li, X.; Hou, Y.; Cao, G.; Li, K.; Kou, J.; Lou, G. Identification of phosphatidylcholine and lysophosphatidylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumour Biol. 2016, 37, 5485–5492. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, Z.; Milosevic, M.; Cvetkovic, B.; Masnikosa, R.; Arsic, A.; Petrovic, S.; Vucic, V. Plasma phospholipid changes are associated with response to chemotherapy in non-Hodgkin lymphoma patients. Leuk. Res. 2017, 54, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.; Galle, P.R. Immune evasion by tumours: Involvement of the CD95 (APO-1/Fas) system and its clinical implications. Mol. Med. Today 1998, 4, 63–68. [Google Scholar] [CrossRef]
- Koncarevic, S.; Lossner, C.; Kuhn, K.; Prinz, T.; Pike, I.; Zucht, H.D. In-depth profiling of the peripheral blood mononuclear cells proteome for clinical blood proteomics. Int. J. Proteom. 2014, 2014, 129259. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.B.; Kuntz, M.M.; Kim, Y.T.; Weksler, M.E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J. Clin. Investig. 1979, 64, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ernster, I.R.Z.R.; Lindberg, O.A. Method for the determination of tracer phosphate in biological material. Acta Chem. Scand. 1950, 4, 942–945. [Google Scholar] [CrossRef]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Keith, B.; Simon, M.C. HIF2alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Du, G. Dysregulated lipid metabolism in cancer. World J. Biol. Chem. 2012, 3, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, M.P.; Bornschlegl, S.; Park, S.S.; Gastineau, D.A.; Roberts, L.R.; Dietz, A.B.; Hallemeier, C.L. Comprehensive assessment of circulating immune cell populations in response to stereotactic body radiation therapy in patients with liver cancer. Adv. Radiat. Oncol. 2017, 2, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Andoh, Y.; Okazaki, S.; Ueoka, R. Molecular dynamics study of lipid bilayers modeling the plasma membranes of normal murine thymocytes and leukemic GRSL cells. Biochim. Biophys. Acta 2013, 1828, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bozza, P.T.; Viola, J.P. Lipid droplets in inflammation and cancer. Prostagland. Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.; Jakubzig, B.; Grundmann, M.; Massing, U.; Kostenis, E.; Schlesinger, M.; Bendas, G. The molecular mechanism by which saturated lysophosphatidylcholine attenuates the metastatic capacity of melanoma cells. FEBS Open Bio 2016, 6, 1297–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podo, F.; Paris, L.; Cecchetti, S.; Spadaro, F.; Abalsamo, L.; Ramoni, C.; Ricci, A.; Pisanu, M.E.; Sardanelli, F.; Canese, R.; et al. Activation of Phosphatidylcholine-Specific Phospholipase C in Breast and Ovarian Cancer: Impact on MRS-Detected Choline Metabolic Profile and Perspectives for Targeted Therapy. Front. Oncol. 2016, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Kuliszkiewicz-Janus, M.; Tuz, M.A.; Kielbinski, M.; Jazwiec, B.; Niedoba, J.; Baczynski, S. 31P MRS analysis of the phospholipid composition of the peripheral blood mononuclear cells (PBMC) and bone marrow mononuclear cells (BMMC) of patients with acute leukemia (AL). Cell. Mol. Biol. Lett. 2009, 14, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balgoma, D.; Astudillo, A.M.; Perez-Chacon, G.; Montero, O.; Balboa, M.A.; Balsinde, J. Markers of monocyte activation revealed by lipidomic profiling of arachidonic acid-containing phospholipids. J. Immunol. 2010, 184, 3857–3865. [Google Scholar] [CrossRef] [PubMed]
- Todor, I.N.; Lukyanova, N.Y.; Chekhun, V.F. The lipid content of cisplatin- and doxorubicin-resistant MCF-7 human breast cancer cells. Exp. Oncol. 2012, 34, 97–100. [Google Scholar] [PubMed]
- Sun, B.; Zhang, X.; Talathi, S.; Cummings, B.S. Inhibition of Ca2+-independent phospholipase A2 decreases prostate cancer cell growth by p53-dependent and independent mechanisms. J. Pharmacol. Exp. Ther. 2008, 326, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wilkins, P.; Hu, W.; Murthy, K.S.; Chen, J.; Lee, Z.; Oyesanya, R.; Wu, J.; Barbour, S.E.; Fang, X. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem. J. 2007, 406, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Voelker, D.R. Phosphatidylserine decarboxylase. Biochim. Biophys. Acta 1997, 1348, 236–244. [Google Scholar] [CrossRef]
- Cui, Z.; Vance, J.E.; Chen, M.H.; Voelker, D.R.; Vance, D.E. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J. Biol. Chem. 1993, 268, 16655–16663. [Google Scholar] [PubMed]
- Iida, Y.; Sunami, E.; Yamashita, H.; Hiyoshi, M.; Ishihara, S.; Yamaguchi, H.; Inoue, A.; Makide, K.; Tsuno, N.H.; Aoki, J.; et al. Phosphatidylserine-specific phospholipase A1 (PS-PLA1) expression in colorectal cancer correlates with tumor invasion and hematogenous metastasis. Anticancer Res. 2015, 35, 1459–1464. [Google Scholar] [PubMed]
- Taylor, L.A.; Arends, J.; Hodina, A.K.; Unger, C.; Massing, U. Plasma lyso-phosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status. Lipids Health Dis. 2007, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, P.J.; Barlow, C.K.; Mellett, N.A.; Mundra, P.A.; Bonham, M.P.; Larsen, A.; Cameron-Smith, D.; Sinclair, A.; Nestel, P.J.; Wong, G. Postprandial Plasma Phospholipids in Men Are Influenced by the Source of Dietary Fat. J. Nutr. 2015, 145, 2012–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuno, K.; Hazama, S.; Yoshino, S.; Yoshida, S.; Oka, M. Increased prevalence of regulatory T-cells in the peripheral blood of patients with gastrointestinal cancer. Anticancer Res. 2009, 29, 1527–1532. [Google Scholar] [PubMed]
- Dong, H.P.; Holth, A.; Kleinberg, L.; Ruud, M.G.; Elstrand, M.B.; Trope, C.G.; Davidson, B.; Risberg, B. Evaluation of cell surface expression of phosphatidylserine in ovarian carcinoma effusions using the annexin-V/7-AAD assay: Clinical relevance and comparison with other apoptosis parameters. Am. J. Clin. Pathol. 2009, 132, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Grapov, D.; DeFelice, B.C.; Taylor, S.; Kim, K.; Kelly, K.; Wikoff, W.R.; Pass, H.; Rom, W.N.; Fiehn, O.; et al. Serum phosphatidylethanolamine levels distinguish benign from malignant solitary pulmonary nodules and represent a potential diagnostic biomarker for lung cancer. Cancer Biomark. 2016, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakobyan, G.; Davtyan, H.; Harutyunyan, K.; Alexanyan, K.; Amirkhanyan, Y.; Gharibyan, A.L.; Asatryan, L.; Tadevosyan, Y. Similarities in Blood Mononuclear Cell Membrane Phospholipid Profiles during Malignancy. Med. Sci. 2018, 6, 105. https://doi.org/10.3390/medsci6040105
Hakobyan G, Davtyan H, Harutyunyan K, Alexanyan K, Amirkhanyan Y, Gharibyan AL, Asatryan L, Tadevosyan Y. Similarities in Blood Mononuclear Cell Membrane Phospholipid Profiles during Malignancy. Medical Sciences. 2018; 6(4):105. https://doi.org/10.3390/medsci6040105
Chicago/Turabian StyleHakobyan, Gohar, Hasmik Davtyan, Kristine Harutyunyan, Knarik Alexanyan, Yelizaveta Amirkhanyan, Anna L. Gharibyan, Liana Asatryan, and Yuri Tadevosyan. 2018. "Similarities in Blood Mononuclear Cell Membrane Phospholipid Profiles during Malignancy" Medical Sciences 6, no. 4: 105. https://doi.org/10.3390/medsci6040105