The Burden of Respiratory Disease from Formaldehyde, Damp and Mould in English Housing
Abstract
:1. Introduction
1.1. Formaldehyde
1.2. Damp and Mould
1.3. Study Aim
2. Materials & Methods
2.1. Overview of Estimation Approach
2.2. Study Population
2.3. Exposures
2.3.1. Formaldehyde
2.3.2. Damp and Mould
- Rising damp: where the surveyor has noted the presence of rising damp in at least one of the rooms surveyed during the physical survey. Rising damp occurs when water from the ground rises up into the walls or floors because damp-proof courses in walls or damp-proof membranes in floors are either not present or faulty.
- Penetrating damp: where the surveyor has noted the presence of penetrating damp in at least one of the rooms surveyed during the physical survey. Penetrating damp is caused by leaks from faulty components of the external fabric, e.g., roof covering, gutters etc. or leaks from internal plumbing, e.g., water pipes, radiators etc.
- Condensation or mould: caused by water vapour generated by activities like cooking and bathing condensing on cold surfaces like windows and walls. Virtually all dwellings have some level of condensation. Only serious levels of condensation or mould are considered in the EHS, namely where there are extensive patches of mould growth on walls and ceilings and/or mildew on soft furnishings.
2.4. Epidemiological Relationships
- Study design: Meta-analysis; large/pooled prospective cohort studies
- Age group: Any
- Geography: Any
- Exposure assessment:
- ∘
- Formaldehyde: Residential formaldehyde (airborne), although we did consider meta-analyses that pooled studies from residential and school settings for children
- ∘
- Damp and mould: Residential damp and/or mould assessed via a survey (inspector/surveyor or occupant self-report)
- Effect estimate: Statistically significant and adjusted for confounders
2.5. Health Data
2.6. Attributable Burden of Disease Calculations and Analyses
3. Results
3.1. Formaldehyde Burden of Disease
3.2. Damp and Mould Burden of Disease
4. Discussion
4.1. Formaldehyde
4.2. Damp and Mould
4.3. Inequalities and Trends over Time
4.4. Interpretation and Comparison of Environmental Burden of Disease Estimates
4.5. Uncertainties and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Regional Office for Europe. Environmental Burden of Disease Associated with Inadequate Housing; WHO Regional Office for Europe: Bonn, Germany, 2011; Available online: https://apps.who.int/iris/handle/10665/108587 (accessed on 30 May 2023).
- World Health Organisation. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO European Centre for Environment and Health: Bonn, Germany, 2010; pp. 1–104. Available online: https://www.who.int/publications/i/item/9789289002134 (accessed on 30 May 2023).
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef]
- Dimitroulopoulou, C.; Ashmore, M.R.; Terry, A.C. Use of population exposure frequency distributions to simulate effects of policy interventions on NO2 exposure. Atmos. Environ. 2017, 150, 1–14. [Google Scholar] [CrossRef]
- Chief Medical Officer. Chief Medical Officer’s Annual Report 2022: Air Pollution; Department of Health and Social Care: London, UK, 2022. Available online: https://www.gov.uk/government/publications/chief-medical-officers-annual-report-2022-air-pollution (accessed on 30 May 2023).
- National Academies. Indoor Exposure to Fine Particulate Matter and Practical Mitigation Approaches: Proceedings of a Workshop (2022); National Academy of Sciences, Engineering and Medicine: Washington, DC, USA, 2022; Available online: https://www.nationalacademies.org/our-work/indoor-exposure-to-fine-particulate-matter-and-practical-mitigation-approaches---a-workshop (accessed on 30 May 2023).
- Public Health England. Health Matters: Air Pollution. Available online: https://www.gov.uk/government/publications/health-matters-air-pollution/health-matters-air-pollution (accessed on 20 January 2023).
- RCPCH. The Inside Story: Health Effects of Indoor Air Quality on Children and Young People; Royal College of Paediatrics and Child Health: London, UK, 2020; Available online: https://www.rcpch.ac.uk/resources/inside-story-health-effects-indoor-air-quality-children-young-people (accessed on 30 May 2023).
- UK Health Security Agency. COMEAP: Reports and Statements. Available online: https://www.gov.uk/government/collections/comeap-reports (accessed on 20 January 2023).
- Burke, J.M.; Zufall, M.J.; Ozkaynak, H. A population exposure model for particulate matter: Case study results for PM2.5 in Philadelphia, PA. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 470–489. [Google Scholar] [CrossRef] [Green Version]
- Gariazzo, C.; Lamberti, M.; Hanninen, O.; Silibello, C.; Pelliccioni, A.; Porta, D.; Cecinato, A.; Gherardi, M.; Forastiere, F. Assessment of population exposure to Polycyclic Aromatic Hydrocarbons (PAHs) using integrated models and evaluation of uncertainties. Atmos. Environ. 2015, 101, 235–245. [Google Scholar] [CrossRef]
- Ferguson, L.; Taylor, J.; Symonds, P.; Davies, M.; Dimitroulopoulou, S. Analysis of Inequalities in Personal Exposure to PM2.5: A Modelling Study for the Greater London School-Aged Population. 2023. Available online: http://dx.doi.org/10.2139/ssrn.4435742 (accessed on 10 May 2023). [CrossRef]
- Boulanger, G.; Bayeux, T.; Mandin, C.; Kirchner, S.; Vergriette, B.; Pernelet-Joly, V.; Kopp, P. Socio-economic costs of indoor air pollution: A tentative estimation for some pollutants of health interest in France. Environ. Int. 2017, 104, 14–24. [Google Scholar] [CrossRef]
- Hanninen, O.; Knol, A.B.; Jantunen, M.; Lim, T.A.; Conrad, A.; Rappolder, M.; Carrer, P.; Fanetti, A.C.; Kim, R.; Buekers, J.; et al. Environmental Burden of Disease in Europe: Assessing Nine Risk Factors in Six Countries. Environ. Health Perspect. 2014, 122, 439–446. [Google Scholar] [CrossRef] [Green Version]
- EBoDE Working Group. European Perspectives on Environmental Burden of Disease: Estimates for Nine Stressors in Six European Countries; EBoDE Working Group: Helsinki, Finland, 2011; pp. 1–94. Available online: https://www.julkari.fi/bitstream/handle/10024/79910/b75f6999-e7c4-4550-a939-3bccb19e41c1.pdf (accessed on 30 May 2023).
- Hanninen, O.; Mandin, C.; Wei, L.; Liu, N.; Zhao, Z.; Zhang, Y. Disease Burden of Indoor Air Pollution. In Handbook of Indoor Air Quality; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Rojas-Rueda, D.; Vrijheid, M.; Robinson, O.; Marit, A.G.; Grazuleviciene, R.; Slama, R.; Nieuwenhuijsen, M. Environmental Burden of Childhood Disease in Europe. Int. J. Environ. Res. Public Health 2019, 16, 1084. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.M.; Price, P.N.; Sherman, M.H.; Singer, B.C. A method to estimate the chronic health impact of air pollutants in U.S. residences. Environ. Health Perspect. 2012, 120, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Gruenwald, T.; Seals, B.A.; Knibbs, L.D.; Hosgood, H.D.H. Population Attributable Fraction of Gas Stoves and Childhood Asthma in the United States. Int. J. Environ. Res. Public Health 2023, 20, 75. [Google Scholar] [CrossRef]
- Lam, J.; Koustas, E.; Sutton, P.; Padula, A.M.; Cabana, M.D.; Vesterinen, H.; Griffiths, C.; Dickie, M.; Daniels, N.; Whitaker, E.; et al. Exposure to formaldehyde and asthma outcomes: A systematic review, meta-analysis, and economic assessment. PLoS ONE 2021, 16, e0248258. [Google Scholar] [CrossRef]
- Knibbs, L.D.; Woldeyohannes, S.; Marks, G.B.; Cowie, C.T. Damp housing, gas stoves, and the burden of childhood asthma in Australia. Med. J. Aust. 2018, 208, 299–302. [Google Scholar] [CrossRef]
- Riggs, L.; Keall, M.; Howden-Chapman, P.; Baker, M.G. Environmental burden of disease from unsafe and substandard housing, New Zealand, 2010–2017. Bull. World Health Organ. 2021, 99, 259–270. [Google Scholar] [CrossRef]
- Lee, K.K.; Bing, R.; Kiang, J.; Bashir, S.; Spath, N.; Stelzle, D.; Mortimer, K.; Bularga, A.; Doudesis, D.; Joshi, S.S.; et al. Adverse health effects associated with household air pollution: A systematic review, meta-analysis, and burden estimation study. Lancet Glob. Health 2020, 8, E1427–E1434. [Google Scholar] [CrossRef]
- Pruss-Ustun, A.; Mathers, C.; Corvalan, C.; Woodward, A. Assessing the Environmental Burden of Disease at National and Local Levels: Introduction and Methods; World Health Organisation: Geneva, Switzerland, 2003; Available online: https://www.who.int/publications/i/item/9241546204 (accessed on 30 May 2023).
- Courts and Tribunals Judiciary. Awaab Ishak: Prevention of Future Deaths Report. Available online: https://www.judiciary.uk/prevention-of-future-death-reports/awaab-ishak-prevention-of-future-deaths-report/ (accessed on 4 March 2023).
- DLUHC; DHSC. Response to Prevent Future Deaths Report: Investigation and Inquest into the Death of Awaab Ishak; DLUHC: London, UK, 2023; Available online: https://www.judiciary.uk/wp-content/uploads/2022/11/2022-0365-Response-from-Secretary-of-State-for-Levelling-Up-Housing-and-Communities-and-Department-.pdf (accessed on 30 May 2023).
- Regulator of Social Housing. Regulator of Social Housing Publishes Initial Findings on Damp and Mould in Social Housing. Available online: https://www.gov.uk/government/news/regulator-of-social-housing-publishes-initial-findings-on-damp-and-mould-in-social-housing (accessed on 2 February 2023).
- Health and Safety Executive. UK REACH—RMOA for Formaldehyde and Formaldehyde Releasers (Call for Evidence). Available online: https://consultations.hse.gov.uk/crd-reach/formaldehyde-releasers-rmoa-010/ (accessed on 30 May 2023).
- May, N.; McGilligan, C.; Ucci, M. Health and Moisture in Buildings; UKCMB: London, UK, 2015; Available online: https://ukcmb.org/wp-content/uploads/2019/10/health-and-moisture-in-buildings-report-1.pdf (accessed on 30 May 2023).
- British Lung Foundation. Asthma Statistics. Available online: https://statistics.blf.org.uk/asthma (accessed on 16 February 2023).
- Mukherjee, M.; Stoddart, A.; Gupta, R.P.; Nwaru, B.I.; Farr, A.; Heaven, M.; Fitzsimmons, D.; Bandyopadhyay, A.; Aftab, C.; Simpson, C.R.; et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: Analyses of standalone and linked national databases. BMC Med. 2016, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Snell, N.; Strachan, D.; Hubbard, R.; Gibson, J.; Limb, E.; Gupta, R.; Martin, A.; Laffan, M.; Jarrold, I. Burden of lung disease in the UK; findings from the British Lung Foundation’s ‘respiratory health of the nation’ project. Eur. Respir. J. 2016, 48, PA4913. [Google Scholar] [CrossRef]
- Salthammer, T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build. Environ. 2019, 150, 219–232. [Google Scholar] [CrossRef]
- Halios, C.H.; Landeg-Cox, C.; Lowther, S.D.; Middleton, A.; Marczylo, T.; Dimitroulopoulou, S. Chemicals in European residences—Part I: A review of emissions, concentrations and health effects of volatile organic compounds (VOCs). Sci. Total Environ. 2022, 839, 156201. [Google Scholar] [CrossRef]
- Berry, R.; Brown, V.; Coward, S.; Crump, D.; Gavin, M.; GRimes, C.; Higham, D.; Hull, A.; Hunter, C.; Jeffery, I.; et al. Indoor Air Quality in Homes; Parts 1 and 2—The Building Research Establishment Indoor Environment; CRC Ltd.: Watford, UK, 1996. [Google Scholar]
- Raw, G.J.; Coward, S.K.D.; Brown, V.M.; Crump, D.R. Exposure to air pollutants in English homes. J. Expo. Anal. Environ. Epidemiol. 2004, 14, S85–S94. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, F.; Tapia, A.; Lara, S.; Amo-Salas, M. Indoor and outdoor air concentrations of volatile organic compounds and NO2 in schools of urban, industrial and rural areas in Central-Southern Spain. Sci. Total Environ. 2018, 622–623, 222–235. [Google Scholar] [CrossRef]
- Public Health England. Indoor Air Quality Guidelines for Selected Volatile Organic Compounds (VOCs) in the UK; Public Health England: London, UK, 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/831319/VO__statement_Final_12092019_CS__1_.pdf (accessed on 30 May 2023).
- Public Health England. Formaldehyde: Toxicological Overview; Public Health England: London, UK, 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/582279/Formaldehyde__toxicological_overview.pdf (accessed on 30 May 2023).
- Vazquez-Ferreiro, P.; Hueso, F.J.C.; Lopez, B.A.; Diaz-Rey, M.; Martinez-Casal, X.; Barrios, M.A.R. Evaluation of formaldehyde as an ocular irritant: A systematic review and Meta-analysis. Cutan. Ocul. Toxicol. 2019, 38, 169–175. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Chemical Agents and Related Occupations: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100F; International Agency for Research on Cancer: Lyon, France, 2012; ISBN 978-92-832-1323-9. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Chemical-Agents-And-Related-Occupations-2012 (accessed on 30 May 2023).
- Liu, N.R.; Fang, L.; Liu, W.; Kan, H.D.; Zhao, Z.H.; Deng, F.R.; Huang, C.; Zhao, B.; Zeng, X.A.; Sun, Y.X.; et al. Health effects of exposure to indoor formaldehyde in civil buildings: A systematic review and meta-analysis on the literature in the past 40 years. Build. Environ. 2023, 233, 110080. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2017, 91, 35–61. [Google Scholar] [CrossRef] [Green Version]
- Kwak, K.; Paek, D.; Park, J.T. Occupational exposure to formaldehyde and risk of lung cancer: A systematic review and meta-analysis. Am. J. Ind. Med. 2020, 63, 312–327. [Google Scholar] [CrossRef]
- Protano, C.; Buomprisco, G.; Cammalleri, V.; Pocino, R.N.; Marotta, D.; Simonazzi, S.; Cardoni, F.; Petyx, M.; Iavicoli, S.; Vitali, M. The Carcinogenic Effects of Formaldehyde Occupational Exposure: A Systematic Review. Cancers 2022, 14, 165. [Google Scholar] [CrossRef]
- Yu, L.L.; Wang, B.; Cheng, M.; Yang, M.; Gan, S.M.; Fan, L.Y.; Wang, D.M.; Chen, W.H. Association between indoor formaldehyde exposure and asthma: A systematic review and meta-analysis of observational studies. Indoor Air 2020, 30, 682–690. [Google Scholar] [CrossRef]
- McGwin, G.; Lienert, J.; Kennedy, J.I. Formaldehyde Exposure and Asthma in Children: A Systematic Review. Environ. Health Perspect. 2010, 118, 313–317. [Google Scholar] [CrossRef] [Green Version]
- US EPA. IRIS Toxicological Review of Formaldehyde-Inhalation (External Review Draft, 2022); US EPA: Washintgon, DC, USA, 2022. Available online: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=248150#:~:text=In%20April%202022%2C%20EPA%20publicly,of%20inhalation%20exposure%20to%20formaldehyde (accessed on 30 May 2023).
- European Chemicals Agency. Annex XV Restriction Report: Prospoal for a Restriction. Available online: https://echa.europa.eu/documents/10162/13641/rest_formaldehyde_axvreport_en.pdf/2c798a08-591c-eed9-8180-a3c5a0362e37 (accessed on 30 May 2023).
- Joint Research Centre; Institute for Health & Consumer Protection; Jäckh, R.; Annys, E.; Heinzow, B.; Witterseh, T.; Glöckner, M.; Kephalopoulos, S.; Coutalides, R.; Lathauwer, D.; et al. Harmonisation Framework for Health Based Evaluation of Indoor Emissions from Construction Products in the European Union Using the EU-LCI Concept; Publications Office: Brussels, Belgium, 2015. [Google Scholar]
- European Comission. EU-LCI Subgroup. Available online: https://single-market-economy.ec.europa.eu/sectors/construction/eu-lci-subgroup_en (accessed on 5 April 2023).
- Richardson, G.; Eick, S.A.; Jones, R.B. How is the indoor environment related to asthma?: Literature review. J. Adv. Nurs. 2005, 52, 328–339. [Google Scholar] [CrossRef]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; on behalf of the Environmental Allergens Workgroup. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Arce, P.; Altamirano-Medina, H.; Berry, J.; Rovas, D.; Sarce, F.; Hodgson, S. Building moisture diagnosis: Processing, assessing and representation of environmental data for root cause analysis of mould growth. In Building Simulation; Tsinghua University Press: Beijing, China, 2020; Volume 13, pp. 999–1008. [Google Scholar] [CrossRef]
- Thomas, A.; Scott, D.; Cole, I.; Higgins, I.; Cavell, L.; Sandoul, T. CIEH Excess Cold Enforcemenet Guidance; Chartered Institute for Environmental Health: London, UK, 2019; Available online: https://www.cieh.org/media/3762/cieh-excess-cold-enforcement-guidance.pdf (accessed on 30 May 2023).
- NICE. Indoor Air Quality at Home; NICE: London, UK, 2020; Available online: https://www.nice.org.uk/guidance/ng149 (accessed on 30 May 2023).
- Mendell, M.J.; Macher, J.M.; Kumagai, K. Measured moisture in buildings and adverse health effects: A review. Indoor Air 2018, 28, 488–499. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521, 305–314. [Google Scholar] [CrossRef]
- Duan, J.F.; Xie, J.; Deng, T.; Xie, X.M.; Liu, H.; Li, B.Z.; Chen, M.Q. Exposure to both formaldehyde and high relative humidity exacerbates allergic asthma by activating the TRPV4-p38 MAPK pathway in Balb/c mice. Environ. Pollut. 2020, 256, 113375. [Google Scholar] [CrossRef]
- Douwes, J. 2 Building dampness and its effect on indoor exposure to biological and non-biological pollutants. In WHO Guidelines for Indoor Air Quality: Dampness and Mould; World Health Organisation: Geneva, Switzerland, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK143945/ (accessed on 30 May 2023).
- Huang, S.D.; Xiong, J.Y.; Zhang, Y.P. The Impact of Relative Humidity on the Emission Behaviour of Formaldehyde in Building Materials. Procedia Eng. 2015, 121, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, S.; Maddalena, R.L.; Russell, M.L.; Apte, M.G. Effect of temperature and humidity on formaldehyde emissions in temporary housing units. J. Air Waste Manag. Assoc. 2011, 61, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Piddington, J.; Nicol, S.; Garrett, H.; Custard, M. The Housing Stock of the United Kingdom; BRE Trust: Garston, UK, 2020. Available online: https://files.bregroup.com/bretrust/The-Housing-Stock-of-the-United-Kingdom_Report_BRE-Trust.pdf (accessed on 30 May 2023).
- DLUHC. English Housing Survey Data on Dwelling Condition and Safety. London, UK. Available online: https://www.gov.uk/government/statistical-data-sets/dwelling-condition-and-safety (accessed on 5 February 2023).
- DLUHC. English Housing Survey 2021 to 2022: Headline Report. London, UK. Available online: https://www.gov.uk/government/statistics/english-housing-survey-2021-to-2022-headline-report/english-housing-survey-2021-to-2022-headline-report#section-2-housing-stock (accessed on 5 February 2023).
- BEIS. Energy Follow Up Survey: Thermal Comfort, Damp and Ventilation: Final Report; BEIS: London, UK, 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018726/efus-thermal.pdf (accessed on 30 May 2023).
- Ferguson, L.; Taylor, J.; Zhou, K.; Shrubsole, C.; Symonds, P.; Davies, M.; Dimitroulopoulou, S. Systemic inequalities in indoor air pollution exposure in London, UK. Build. Cities 2021, 2, 425–448. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. WHO Guidelines for Indoor Air Quality: Dampness and Mould; World Health Organisation: Copenhagen, Denmark, 2009; Available online: https://apps.who.int/iris/handle/10665/164348 (accessed on 30 May 2023).
- May, N.; Carmona, I.; Marincioni, V.; Altamirano-Medina, H. Moisture in New Homes: A Guide for Occupants; UKCMB, London, UK. 2019. Available online: https://www.nhbcfoundation.org/publication/moisture-in-new-homes-a-guide-for-occupants/ (accessed on 30 May 2023).
- Fisk, W.J.; Eliseeva, E.A.; Mendell, M.J. Association of residential dampness and mold with respiratory tract infections and bronchitis: A meta-analysis. Environ. Health 2010, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Fisk, W.J.; Lei-Gomez, Q.; Mendell, M.J. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 2007, 17, 284–296. [Google Scholar] [CrossRef]
- Jaakkola, M.S.; Quansah, R.; Hugg, T.T.; Heikkinen, S.A.; Jaakkola, J.J. Association of indoor dampness and molds with rhinitis risk: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 132, 1099–1110.e18. [Google Scholar] [CrossRef]
- Quansah, R.; Jaakkola, M.S.; Hugg, T.T.; Heikkinen, S.A.; Jaakkola, J.J. Residential dampness and molds and the risk of developing asthma: A systematic review and meta-analysis. PLoS ONE 2012, 7, e47526. [Google Scholar] [CrossRef]
- Mendell, M.J. A Research Agenda on Assessing and Remediating Home Dampness and Mold to Reduce Dampness-Related Health Effects; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2015; Available online: https://escholarship.org/uc/item/5cx8t259 (accessed on 30 May 2023).
- Tischer, C.; Chen, C.; Heinrich, J. Association of asthma and allergy with domestic mould and mould components in children: A systematic review. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 201–202. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Disability-Adjusted Life Years (DALYs). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158 (accessed on 6 February 2023).
- Haagsma, J.A.; Polinder, S.; Cassini, A.; Colzani, E.; Havelaar, A.H. Review of disability weight studies: Comparison of methodological choices and values. Popul. Health Metr. 2014, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- ONS. Estimates of the Population for the UK, England, Wales, Scotland and Northern Ireland. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 20 January 2023).
- Stamp, S.; Burman, E.; Shrubsole, C.; Chatzidiakou, L.; Mumovic, D.; Davies, M. Seasonal variations and the influence of ventilation rates on IAQ: A case study of five low-energy London apartments. Indoor Built Environ. 2022, 31, 607–623. [Google Scholar] [CrossRef]
- Burman, E.; Stamp, S. Trade-offs between ventilation rates and formaldehyde concentrations in new-build dwellings in the UK. In Proceedings of the AIVC, Ghent, Belgium, 15–16 October 2019; Available online: https://discovery.ucl.ac.uk/id/eprint/10083746/ (accessed on 30 May 2023).
- Gee, I.L.; Watson, A.F.R.; Tavernier, G.; Stewart, L.J.; Fletcher, G.; McL Niven, R. Indoor air quality, environmental tobacco smoke and asthma: A case control study of asthma in a community population. Indoor Built Environ. 2005, 14, 215–219. [Google Scholar] [CrossRef]
- Venn, A.J.; Cooper, M.; Antoniak, M.; Laughlin, C.; Britton, J.; Lewis, S.A. Effects of volatile organic compounds, damp, and other environmental exposures in the home on wheezing illness in children. Thorax 2003, 58, 955–960. [Google Scholar] [CrossRef] [Green Version]
- MHCLG. Ventilation and Indoor Air Quality in New Homes; MHCLG: London, UK, 2019. Available online: https://www.gov.uk/government/publications/ventilation-and-indoor-air-quality-in-new-homes (accessed on 30 May 2023).
- Mohle, G.; Crump, D.; Brown, V.; Hunter, C.; Squire, R.; Mann, H.; Raw, G.J. Development and application of a protocol for the assessment of indoor air quality. Indoor Built. Environ. 2003, 12, 139–149. [Google Scholar] [CrossRef]
- Crump, D.; Dimitroulopoulou, S.; Squire, R.; Ross, D.; Pierce, B.; White, M.; Brown, V.; Coward, S. Ventilation and Indoor Air Quality in New Homes. Pollut. Atmosphérique 2005, 1, 71. Available online: https://www.researchgate.net/publication/228626970_Ventilation_and_indoor_air_quality_in_new_homes (accessed on 30 May 2023).
- Wang, C.M.; Barratt, B.; Carslaw, N.; Doutsi, A.; Dunmore, R.E.; Ward, M.W.; Lewis, A.C. Unexpectedly high concentrations of monoterpenes in a study of UK homes. Environ. Sci.-Process. Impacts 2017, 19, 528–537. [Google Scholar] [CrossRef] [Green Version]
- McGill, G.; Oyedele, L.O.; Keefe, G. Indoor air-quality investigation in code for sustainable homes and passivhaus dwellings. World J. Sci. Technol. Sustain. Dev. 2015, 12, 39–60. [Google Scholar] [CrossRef]
- Mayor of London. The Mayor’s Nursery Air Quality Audit Programme. Available online: https://www.london.gov.uk/programmes-and-strategies/environment-and-climate-change/pollution-and-air-quality/mayors-nursery-air-quality-audit-programme?ac-57396=57394#acc-i-60589 (accessed on 4 April 2023).
- Annesi-Maesano, I.; Hulin, M.; Lavaud, F.; Raherison, C.; Kopferschmitt, C.; de Blay, F.; Charpin, D.A.; Denis, C. Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study. Thorax 2012, 67, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Baloch, R.M.; Maesano, C.N.; Christoffersen, J.; Banerjee, S.; Gabriel, M.; Csobod, E.; Fernandes, E.D.; Annesi-Maesano, I.; Csobod, E.E.; Szuppinger, P.; et al. Indoor air pollution, physical and comfort parameters related to schoolchildren’s health: Data from the European SINPHONIE study. Sci. Total Environ. 2020, 739, 139870. [Google Scholar] [CrossRef]
- DLUHC. English Housing Survey. Available online: https://www.gov.uk/government/collections/english-housing-survey#:~:text=The%20English%20Housing%20Survey%20is,efficiency%20of%20housing%20in%20England (accessed on 5 February 2023).
- MHCLG. English Housing Survey: Headline Report, 2019–20; Ministry of Housing, Communities & Local Government: London, UK, 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/945013/2019-20_EHS_Headline_Report.pdf (accessed on 30 May 2023).
- Morgan, C.; McGill, G.; Sharpe, T.; Devereux, G. Indoor Air Quality in Airtight Homes: A Designer’s Guide; John Gilbert Architects: Glasgow, UK, 2020. Available online: https://static1.squarespace.com/static/5978a800bf629a80c569eef0/t/6159a3651ab568669d832932/1633264501397/HEMAC_Professional_User_Guide_ON_SCREEN.pdf (accessed on 30 May 2023).
- VELUX; RAND Europe. Healthy Homes Barometer 2022: Sustainable Buildings for a Resilient Society; VELUX: Horsholm, Denmark, 2022; Available online: https://www.velux.com/what-we-do/healthy-buildings-focus/healthy-homes-barometer (accessed on 30 May 2023).
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef]
- Wang, J.; Pindus, M.; Janson, C.; Sigsgaard, T.; Kim, J.L.; Holm, M.; Sommar, J.; Orru, H.; Gislason, T.; Johannessen, A.; et al. Dampness and mould at home and at work in the RHINE study: Increased onset and decreased remission of adult respiratory symptoms, asthma and rhinitis. Eur. Respir. J. 2019, 53, 1801921. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 30 May 2023).
- IHME. GBD Results [Data Table]. Available online: https://vizhub.healthdata.org/gbd-results?params=gbd-api-2019-permalink/ee030963fa685db0c2552d0e30bc7365 (accessed on 3 March 2023).
- Counil, E. Contribution of causal factors to disease burden: How to interpret attributable fractions. Breathe 2021, 17, 210086. [Google Scholar] [CrossRef]
- Murray, C.J.; Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S. Comparative quantification of health risks conceptual framework and methodological issues. Popul. Health Metr. 2003, 1, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Oakley-Davis, H.T.; Kinloch Crombie, I.; Tavakoli, M. When can odds ratios mislead? BMJ 1998, 316, 989–991. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA-J. Am. Med. Assoc. 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [Green Version]
- Gowers, A.; Walton, H.; Exley, K.S.; Hurley, F.J. Using epidemiology to estimate the impact and burden of exposure to air pollutants. Philos. Trans. R. Soc. A 2020, 378, 20190321. [Google Scholar] [CrossRef]
- Rumchev, K.B.; Spickett, J.T.; Bulsara, M.K.; Phillips, M.R.; Stick, S.M. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002, 20, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowski, M.; Quackenboss, J.J.; Lebowitz, M.D. Chronic Respiratory Effects of Indoor Formaldehyde Exposure. Environ. Res. 1990, 52, 117–125. [Google Scholar] [CrossRef]
- Garrett, M.H.; Abramson, M.J.; Hooper, B.M.; Rayment, P.R.; Strasser, R.P.; Hooper, M.A. Indoor environmental risk factors for respiratory health in children. Indoor Air 1998, 8, 236–243. [Google Scholar] [CrossRef]
- Battaglia, S.; Benfante, A.; Spatafora, M.; Scichilone, N. Asthma in the elderly: A different disease? Breathe 2016, 12, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Simpson, J.L. The overlap syndrome of asthma and COPD: What are its features and how important is it? Thorax 2009, 64, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Elias, J.A.; Lee, C.G.; Zheng, T.; Ma, B.; Homer, R.J.; Zhu, Z. New insights into the pathogenesis of asthma. J. Clin. Investig. 2003, 111, 291–297. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Song, J.; Kang, J.; Lin, B.; Li, J.; Zhu, Y.; Du, J.; Yang, X.; Xi, Z.; Li, R. Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci. Rep. 2017, 7, 11926. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Duan, J.F.; Song, J.; Luo, C.; Liu, H.; Li, B.Z.; Yang, X.; Yu, W.; Chen, M.Q. Exposure to a combination of formaldehyde and DINP aggravated asthma-like pathology through oxidative stress and NF-kappa B activation. Toxicology 2018, 404, 49–58. [Google Scholar] [CrossRef]
- Murta, G.L.; Campos, K.K.D.; Bandeira, A.C.B.; Diniz, M.F.; Costa, G.D.; Costa, D.C.; Talvani, A.; Lima, W.G.; Bezerra, F.S. Oxidative effects on lung inflammatory response in rats exposed to different concentrations of formaldehyde. Environ. Pollut. 2016, 211, 206–213. [Google Scholar] [CrossRef]
- Wu, Y.; You, H.; Ma, P.; Li, L.; Yuan, Y.; Li, J.; Ye, X.; Liu, X.; Yao, H.; Chen, R.; et al. Role of transient receptor potential ion channels and evoked levels of neuropeptides in a formaldehyde-induced model of asthma in BALB/c mice. PLoS ONE 2013, 8, e62827. [Google Scholar] [CrossRef]
- Cui, Y.; Li, H.M.; Wu, S.H.; Zhao, R.Z.; Du, D.Y.; Ding, Y.; Nie, H.G.; Ji, H.L. Formaldehyde impairs transepithelial sodium transport. Sci. Rep. 2016, 6, 35857. [Google Scholar] [CrossRef]
- Jude, J.; Koziol-White, C.; Scala, J.; Yoo, E.; Jester, W.; Maute, C.; Dalton, P.; Panettieri, R. Formaldehyde Induces Rho-Associated Kinase Activity to Evoke Airway Hyperresponsiveness. Am. J. Respir. Cell Mol. 2016, 55, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Casset, A.; Marchand, C.; Purohit, A.; le Calve, S.; Uring-Lambert, B.; Donnay, C.; Meyer, P.; de Blay, F. Inhaled formaldehyde exposure: Effect on bronchial response to mite allergen in sensitized asthma patients. Allergy 2006, 61, 1344–1350. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Yao, J. Aerosolization of fungal spores in indoor environments. Sci. Total Environ. 2022, 820, 153003. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.A.; Bearman, N.; Thornton, C.R.; Husk, K.; Osborne, N.J. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. J. Allergy Clin. Immun. 2015, 135, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Grinshpun, S.A.; Martuzevicius, D.; Adhikari, A.; Crawford, C.M.; Luo, J.; Reponen, T. Relationship between indoor and outdoor bioaerosols collected with a button inhalable aerosol sampler in urban homes. Indoor Air 2006, 16, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, K.; Larsen, K.; Simkus, M. Volatile metabolites from mold growth on building materials and synthetic media. Chemosphere 2000, 41, 437–446. [Google Scholar] [CrossRef]
- Schiffers, C.; Hristova, M.; Habibovic, A.; Dustin, C.M.; Danyal, K.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens. Am. J. Respir. Cell Mol. 2020, 63, 198–208. [Google Scholar] [CrossRef]
- Gao, F.S.; Cao, T.M.; Gao, Y.Y.; Liu, M.J.; Liu, Y.Q.; Wang, Z. Effects of chronic exposure to Aspergillus fumigatus on epidermal growth factor receptor expression in the airway epithelial cells of asthmatic rats. Exp. Lung Res. 2014, 40, 298–307. [Google Scholar] [CrossRef]
- Zaidman, N.A.; O’Grady, K.E.; Patil, N.; Milavetz, F.; Maniak, P.J.; Kita, H.; O’Grady, S.M. Airway epithelial anion secretion and barrier function following exposure to fungal aeroallergens: Role of oxidative stress. Am. J. Physiol.-Cell Physiol. 2017, 313, C68–C79. [Google Scholar] [CrossRef] [Green Version]
- Stehle, C.; Hernandez, D.C.; Romagnani, C. Innate lymphoid cells in lung infection and immunity. Immunol. Rev. 2018, 286, 102–119. [Google Scholar] [CrossRef]
- Komlosi, Z.I.; Van de Veen, W.; Kovacs, N.; Szucs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Goode, E.-J.; Marczylo, E. A scoping review: What are the cellular mechanisms that drive the allergic inflammatory response to fungal allergens in the lung epithelium? Clin. Transl. Allergy 2023, 13, e12252. [Google Scholar] [CrossRef]
- GBD Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- DLUHC. Building Regulations Approved Documents. Available online: https://www.gov.uk/government/collections/approved-documents (accessed on 5 February 2023).
- Bowers, N.; Smith, C.; Wilkins, T. Energy Efficiency of Housing in England and Wales: 2022; Office of National Statistics: Newport, UK, 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/housing/articles/energyefficiencyofhousinginenglandandwales/2022 (accessed on 30 May 2023).
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC; European Parliament: Strasbourg, France, 2006; p. 849. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006R1907 (accessed on 30 May 2023).
- National Archives. Approved Document F—Ventilation. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20141202115143/http://www.planningportal.gov.uk/buildingregulations/approveddocuments/partf/approved (accessed on 6 May 2023).
- Smith, S.; Morbey, R.; de Lusignan, S.; Pebody, R.G.; Smith, G.E.; Elliot, A.J. Investigating regional variation of respiratory infections in a general practice syndromic surveillance system. J. Public Health 2021, 43, E153–E160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.; Thakur, N. Structural and Social Determinants of Health in Asthma in Developed Economies: A Scoping Review of Literature Published Between 2014 and 2019. Curr. Allergy Asthma Rep. 2020, 20, 5. [Google Scholar] [CrossRef] [Green Version]
- Asthma UK. On the Edge: How Inequality Affects People with Asthma; Asthma UK: London, UK, 2018. Available online: https://www.asthmaandlung.org.uk/sites/default/files/2023-03/auk-health-inequalities-final.pdf (accessed on 30 May 2023).
- NHS. Can Damp and Mould Affect My Health? Available online: https://www.nhs.uk/common-health-questions/lifestyle/can-damp-and-mould-affect-my-health/#:~:text=older%20people,such%20as%20those%20having%20chemotherapy (accessed on 6 May 2023).
- Wu, Y.X.; Duan, J.F.; Li, B.Z.; Liu, H.; Chen, M.Q. Exposure to formaldehyde at low temperatures aggravates allergic asthma involved in transient receptor potential ion channel. Environ. Toxicol. Pharmacol. 2020, 80, 103469. [Google Scholar] [CrossRef] [PubMed]
- Morantes, G.; Jones, B.; Sherman, M.; Molina, C. A preliminary assessment of the health impacts of indoor air contaminants determined using the DALY metric. Int. J. Vent. 2023, 1–10. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20. [Google Scholar] [CrossRef] [Green Version]



| Exposure | Health Outcome | Source | Type of Study | Number of Studies/ Cohorts | ERF (e.g., RR, OR) [95% CI] | ERF Lower | ERF Upper | Ages (yrs) |
|---|---|---|---|---|---|---|---|---|
| Main (primary) estimates | ||||||||
| Formaldehyde (μg/m3) | Asthma | Liu et al., 2023 [42] | Meta-analysis | 22 | 1.20 [1.11–1.31] per 10 μg/m3 Meta-analysis by Lam et al., 2021 [20] (n = 9 studies) produced the same central effect estimate | 50 μg/m3 * (primary) 20 μg/m3 (sensitivity); 60 μg/m3 (sensitivity) | 100 μg/m3 ** | 0–14 |
| Damp and/or mould (% of dwellings) | Asthma | Quansah et al., 2012 [73] | Meta-analysis | 16 | 1.50 [1.25–1.80] | - | - | 0–14 |
| Damp and/or mould (% of dwellings) | Asthma | Wang et al., 2019 [96] | Longitudinal cohort study | Large cohort pooling data across 5 Scandinavian countries | 1.43 [1.12–1.83] | - | - | 15–49 |
| Damp and/or mould (% of dwellings) | LRI *** | Fisk et al., 2010 [70] | Meta-analysis | 15 | 1.50 [1.32–1.70] Respiratory infections excluding common cold and nonspecific upper respiratory infections *** | - | - | 0–14 15–49 |
| Secondary estimates | ||||||||
| Damp and/or mould (% of dwellings) | Allergic Rhinitis | Jaakkola et al., 2013 [72] **** | Meta-analysis | 19 ***** | 1.43 [1.34–1.53] | - | - | 0–14 |
| Damp and/or mould (% of dwellings) | Allergic Rhinitis | Wang et al., 2019 [96] | Longitudinal cohort study | Large cohort pooling data across 5 Scandinavian countries | 1.28 [1.08–1.52] | - | - | 15-49 |
| Damp and/or mould (% of dwellings) | Bronchitis | Fisk et al., 2010 [70] | Meta-analysis | 13 | 1.45 [1.32–1.59] Acute or chronic bronchitis | - | - | 0–14 15–49 |
| Health Outcome | Age Group (Yrs) | Exposure Distribution | PAF [95% CI] | Disease Incidence [95% CI] | Disease Incidence per 100,000 People [95% CI] | DALYs Lost [95% CI] | DALYs Lost per 100,000 People [95% CI] |
|---|---|---|---|---|---|---|---|
| Formaldehyde | μg/m3 | ||||||
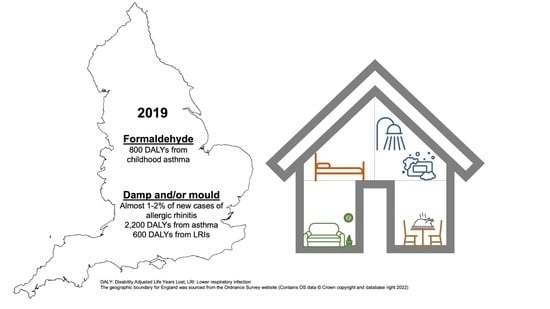
| Asthma * | 0–14 | GM: 22.8 GSD: 2.0 ** | 0.025 [0.013–0.039] | 4038 [1423–9184] | 40 [14–91] | 777 [246–2021] | 8 [2–20] |
| Damp and/or mould | % of dwellings (EHS) | ||||||
| Asthma | 0–14 | 4.2% | 0.021 [0.010–0.033] | 3389 [1120–7654] | 34 [11–76] | 652 [193–1684] | 6 [2–17] |
| LRI | 0–14 | 4.2% | 0.021 [0.013–0.029] | 3902 [1998–6624] | 39 [20–66] | 193 [104–314] | 2 [1–3] |
| Asthma | 15–49 | 3.4% | 0.014 [0.004–0.027] | 1632 [330–4086] | 6 [1–16] | 1520 [274–4299] | 6 [1–17] |
| LRI | 15–49 | 3.4% | 0.017 [0.011–0.023] | 4554 [2588–7239] | 18 [10–28] | 397 [247–571] | 2 [1–2] |
| Health Outcome | Source of Exposure Information | % of Dwellings with Damp and/or Mould * | PAF | Disease Incidence (per 100,000 People) | DALY Rate (per 100,000 People) |
|---|---|---|---|---|---|
| Children (0–14) | |||||
| Asthma | Healthy Homes Barometer Report [94]—Western Europe | 14% | 0.065 | 106 | 20 |
| LRI | Healthy Homes Barometer Report [94]—Western Europe | 14% | 0.065 | 122 | 6 |
| Asthma | Energy Follow Up Survey [66]—England | 39% | 0.163 | 265 | 51 |
| LRI | Energy Follow Up Survey [66]—England | 39% | 0.163 | 305 | 15 |
| Adults (15–49) | |||||
| Asthma | Healthy Homes Barometer Report [94]—Western Europe | 14% | 0.057 | 25 | 24 |
| LRI | Healthy Homes Barometer Report [94]—Western Europe | 14% | 0.065 | 70 | 6 |
| Asthma | Energy Follow Up Survey [66]—England | 27% | 0.104 | 46 | 43 |
| LRI | Energy Follow Up Survey [66]—England | 27% | 0.119 | 127 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, S.N.; Lam, H.C.Y.; Goode, E.-J.; Marczylo, E.L.; Exley, K.S.; Dimitroulopoulou, S. The Burden of Respiratory Disease from Formaldehyde, Damp and Mould in English Housing. Environments 2023, 10, 136. https://doi.org/10.3390/environments10080136
Clark SN, Lam HCY, Goode E-J, Marczylo EL, Exley KS, Dimitroulopoulou S. The Burden of Respiratory Disease from Formaldehyde, Damp and Mould in English Housing. Environments. 2023; 10(8):136. https://doi.org/10.3390/environments10080136
Chicago/Turabian StyleClark, Sierra N., Holly C. Y. Lam, Emma-Jane Goode, Emma L. Marczylo, Karen S. Exley, and Sani Dimitroulopoulou. 2023. "The Burden of Respiratory Disease from Formaldehyde, Damp and Mould in English Housing" Environments 10, no. 8: 136. https://doi.org/10.3390/environments10080136