Multi-Endpoint Analysis of Cerium and Gadolinium Effects after Long-Term Exposure to Phaeodactylum tricornutum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Algae Culture and Growth Inhibition Test
2.3. Oxidative Stress Biomarkers and Antioxidant Activity
2.4. Photosynthetic Pigments and Chlorophyll Fluorescence
2.5. Bioaccumulation
2.6. Data Analysis
3. Results
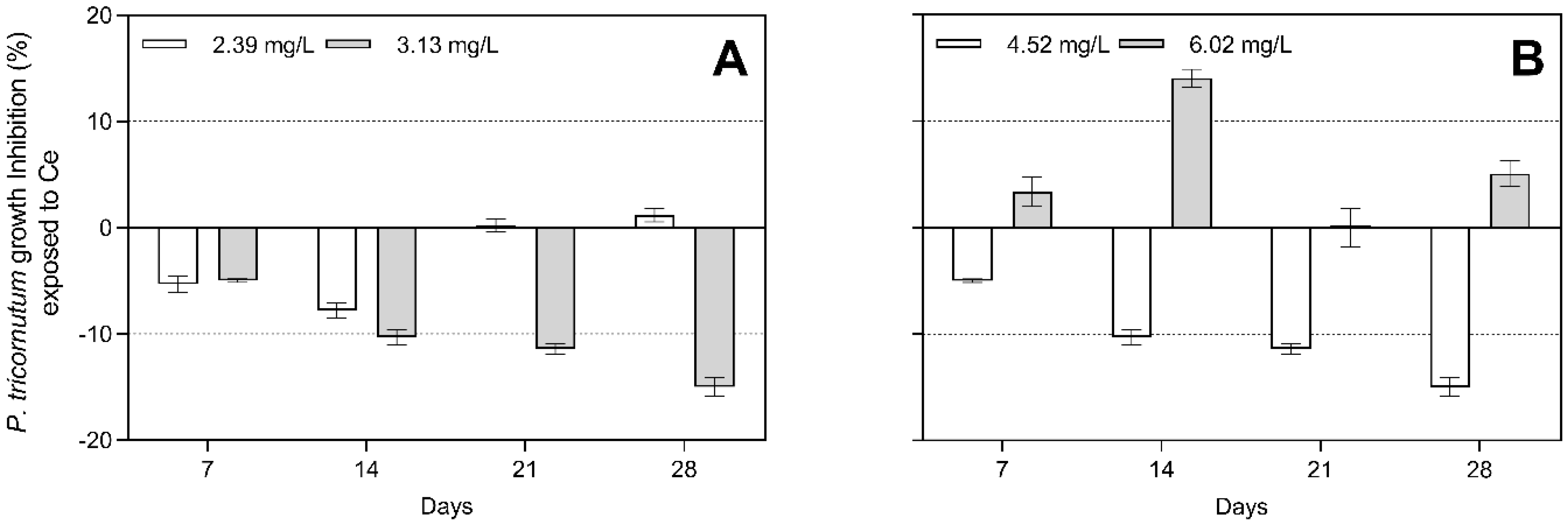
3.1. Algal Growth Inhibitory Effects
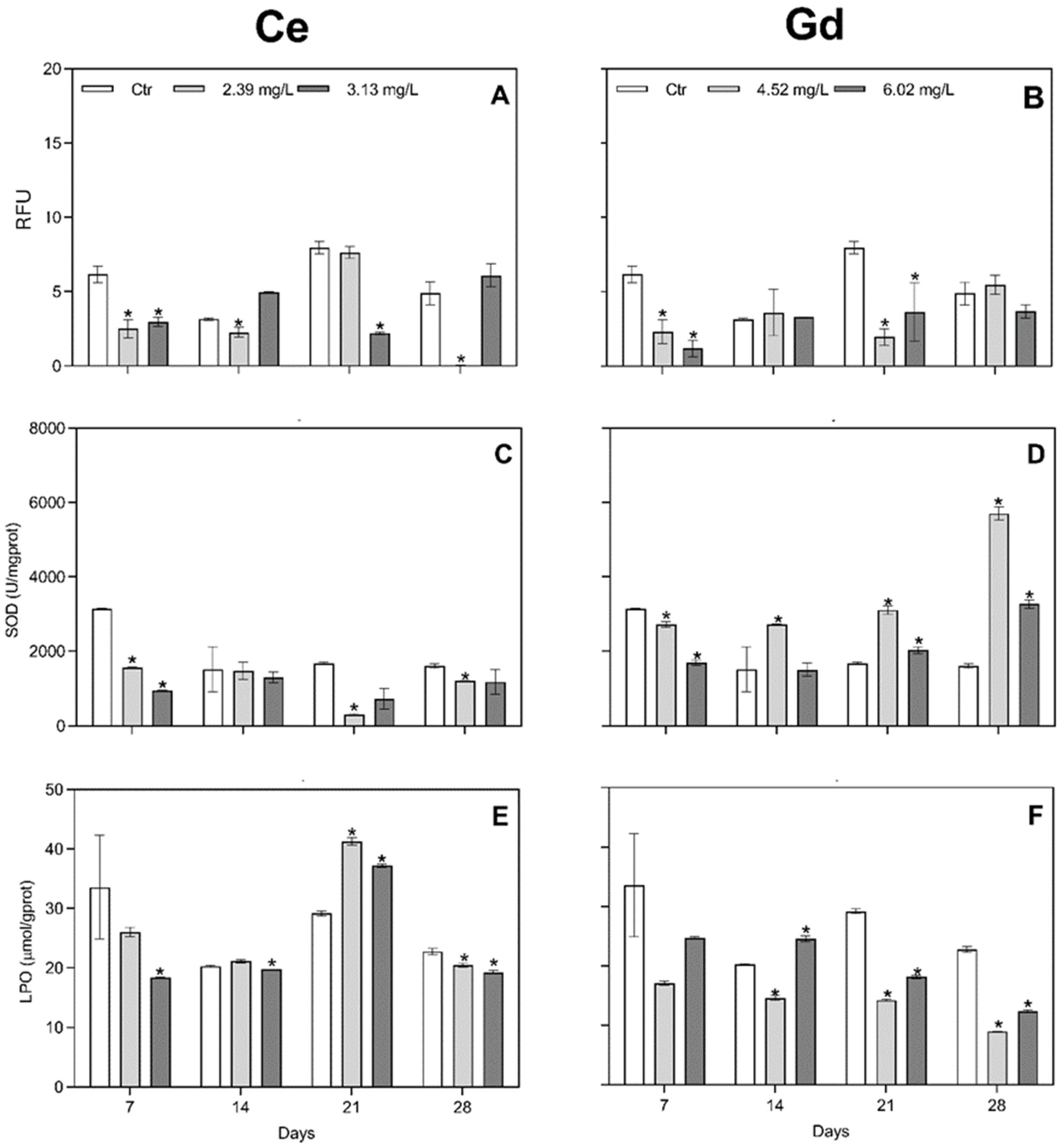
3.2. Oxidative Stress Response
3.3. Photosynthetic Pigments Content
3.4. Chlorophyll Fluorescence
3.5. Bioconcentration Factor (BCF)
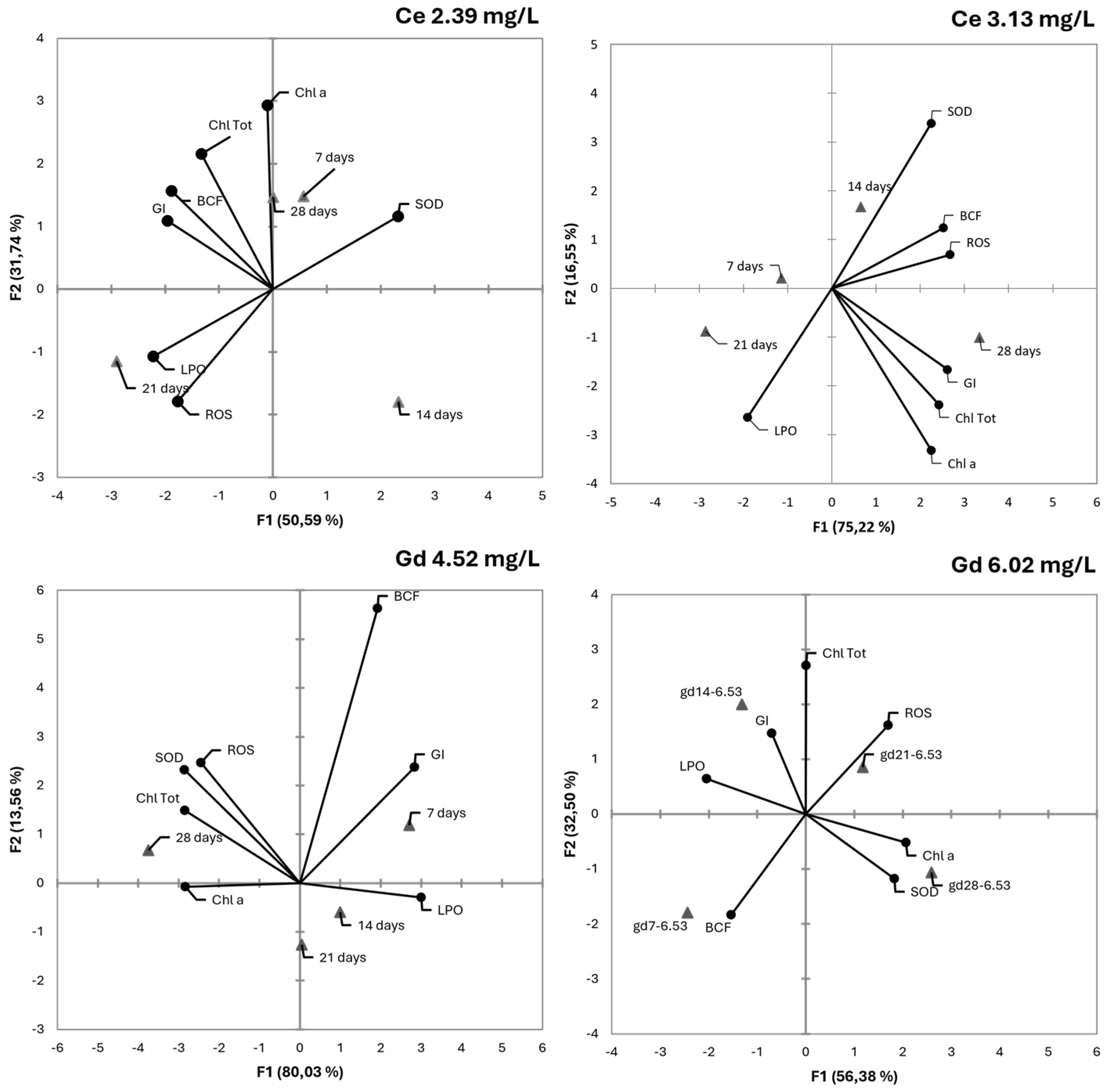
3.6. PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Meng, J.; Guo, L. REE geochemistry of modern eolian dust deposits in Harbin city, Heilongjiang province, China: Implications for provenance. Catena 2014, 123, 70–78. [Google Scholar] [CrossRef]
- Arciszewska, Ż.; Gama, S.; Leśniewska, B.; Malejko, J.; Nalewajko-Sieliwoniuk, E.; Zambrzycka-Szelewa, E.; Godlewska-Żyłkiewicz, B. The translocation pathways of rare earth elements from the environment to the food chain and their impact on human health. Process Saf. Environ. Prot. 2022, 168, 205–223. [Google Scholar] [CrossRef]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Karadaş, C.; Kara, D.; Fisher, A. Determination of rare earth elements in seawater by inductively coupled plasma mass spectrometry with off-line column preconcentration using 2,6-diacetylpyridine functionalized Amberlite XAD-4. Anal. Chim. Acta 2011, 689, 184–189. [Google Scholar] [CrossRef]
- Zhu, Y. Determination of rare earth elements in seawater samples by inductively coupled plasma tandem quadrupole mass spectrometry after coprecipitation with magnesium hydroxide. Talanta 2020, 209, 120536. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Jacobsen, S.B. Rare earth elements in river waters. Earth Planet. Sci. Lett. 1988, 89, 35–47. [Google Scholar] [CrossRef]
- Greaves, M.; Elderfield, H.; Sholkovitz, E. Aeolian sources of rare earth elements to the Western Pacific Ocean. Mar. Chem. 1999, 68, 31–38. [Google Scholar] [CrossRef]
- Nozaki, Y. Rare earth elements and their isotopes in the ocean. Encycl. Ocean. Sci. 2001, 4, 2354–2366. [Google Scholar]
- Kulaksız, S.; Bau, M. Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet. Sci. Lett. 2007, 260, 361–371. [Google Scholar] [CrossRef]
- Kulaksız, S.; Bau, M. Rare earth elements in the Rhine River, Germany: First case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere. Environ. Int. 2011, 37, 973–979. [Google Scholar] [CrossRef]
- Kulaksız, S.; Bau, M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet. Sci. Lett. 2013, 362, 43–50. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Ort, C.; Keller, J. Detection of anthropogenic gadolinium in treated wastewater in South East Queensland, Australia. Water Res. 2009, 43, 3534–3540. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Freitas, R.; Costa, S.; Cardoso, C.E.; Morais, T.; Moleiro, P.; Matias, A.C.; Pereira, A.F.; Machado, J.; Correia, B.; Pinheiro, D. Toxicological effects of the rare earth element neodymium in Mytilus galloprovincialis. Chemosphere 2020, 244, 125457. [Google Scholar] [CrossRef]
- González, V.; Vignati, D.A.; Pons, M.-N.; Montarges-Pelletier, E.; Bojic, C.; Giamberini, L. Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms. Environ. Pollut. 2015, 199, 139–147. [Google Scholar] [CrossRef]
- Eniso, U. Water quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum Tricornutum. UNI EN ISO 2016, 10253, 2016. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Salbitani, G.; Del Prete, F.; Carfagna, S.; Sansone, G.; Barone, C.M. Enhancement of pigments production by Nannochloropsis oculata cells in response to bicarbonate supply. Sustainability 2021, 13, 11904. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Salbitani, G.; Carillo, P.; Di Martino, C.; Bolinesi, F.; Mangoni, O.; Loreto, F.; Carfagna, S. Microalgae cross-fertilization: Short-term effects of Galdieria phlegrea extract on growth, photosynthesis and enzyme activity of Chlorella sorokiniana cells. J. Appl. Phycol. 2022, 34, 1957–1966. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Neira, P.; Romero-Freire, A.; Basallote, M.D.; Qiu, H.; Cobelo-García, A.; Canovas, C.R. Review of the concentration, bioaccumulation, and effects of lanthanides in marine systems. Front. Mar. Sci. 2022, 9, 920405. [Google Scholar] [CrossRef]
- Sutorius, M.; Mori, C.; Greskowiak, J.; Boettcher, L.; Bunse, C.; Dittmar, T.; Dlugosch, L.; Hintz, N.H.; Simon, M.; Striebel, M. Rare earth element behaviour in seawater under the influence of organic matter cycling during a phytoplankton spring bloom–A mesocosm study. Front. Mar. Sci. 2022, 9, 895723. [Google Scholar] [CrossRef]
- Adebayo, S.B.; Cui, M.; Hong, T.; White, C.D.; Martin, E.E.; Johannesson, K.H. Rare earth elements geochemistry and Nd isotopes in the Mississippi River and Gulf of Mexico mixing zone. Front. Mar. Sci. 2018, 5, 166. [Google Scholar] [CrossRef]
- Akagi, T. Rare earth element (REE)–silicic acid complexes in seawater to explain the incorporation of REEs in opal and the “leftover” REEs in surface water: New interpretation of dissolved REE distribution profiles. Geochim. Et. Cosmochim. Acta 2013, 113, 174–192. [Google Scholar] [CrossRef]
- Siciliano, A.; Sabatino, M.; Paone, A.; Padilla Suarez, E.G.; Toscanesi, M.; Brouziotis, A.A.; Gambino, E.; Saviano, L.; Trifuoggi, M.; Guida, M. A first attempt to evaluate the toxicity to Phaeodactylum tricornutum Bohlin exposed to rare earth elements. Front. Environ. Sci. 2022, 10, 957943. [Google Scholar] [CrossRef]
- Taylor, N.S.; Merrifield, R.; Williams, T.D.; Chipman, J.K.; Lead, J.R.; Viant, M.R. Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 2016, 10, 32–41. [Google Scholar]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological toxicity of lanthanide elements on algae. Chemosphere 2010, 80, 1031–1035. [Google Scholar] [CrossRef]
- Joonas, E.; Aruoja, V.; Olli, K.; Syvertsen-Wiig, G.; Vija, H.; Kahru, A. Potency of (doped) rare earth oxide particles and their constituent metals to inhibit algal growth and induce direct toxic effects. Sci. Total Environ. 2017, 593, 478–486. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Zhang, M.; Zhang, N.; Li, M. Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharm. Sin. B 2013, 3, 20–24. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, X.; Zhao, B.; Yuan, X.; Wang, Y. Effects of rare earth elements on the growth of Cistanche deserticola cells and the production of phenylethanoid glycosides. J. Biotechnol. 2003, 102, 129–134. [Google Scholar] [CrossRef]
- Liu, W.-S.; Laird, J.S.; Ryan, C.G.; Tang, Y.-T.; Qiu, R.-L.; Echevarria, G.; Morel, J.-L.; van Der Ent, A. Rare earth elements, aluminium and silicon distribution in the fern Dicranopteris linearis revealed by μPIXE Maia analysis. Ann. Bot. 2021, 128, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, N.; Le Jean, M.; Berthelot, C.; Chalot, M.; Gross, E.M.; Blaudez, D. Accumulation and fractionation of rare earth elements are conserved traits in the Phytolacca genus. Sci. Rep. 2019, 9, 18458. [Google Scholar] [CrossRef]
- Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of tolerance to lanthanum and cerium in helianthus annuus plant: Effect on growth, mineral nutrition, and secondary metabolism. Plants 2022, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Čížková, M.; Náhlík, V.; Mezricky, D.; Schild, D.; Rucki, M.; Vítová, M. Bio-removal of rare earth elements from hazardous industrial waste of CFL bulbs by the extremophile red alga Galdieria sulphuraria. Front. Microbiol. 2023, 14, 1130848. [Google Scholar] [CrossRef]
- Ashraf, N.; Vítová, M.; Cloetens, P.; Mijovilovich, A.; Bokhari, S.N.H.; Küpper, H. Effect of nanomolar concentrations of lanthanum on Desmodesmus quadricauda cultivated under environmentally relevant conditions. Aquat. Toxicol. 2021, 235, 105818. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Moeris, S.; Vanryckeghem, F.; Demeestere, K.; Huysman, S.; Vanhaecke, L.; De Schamphelaere, K.A. Growth stimulation effects of environmentally realistic contaminant mixtures on a marine diatom. Environ. Toxicol. Chem. 2019, 38, 1313–1322. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghatchouli, N.; Arroussi, H. Overview of the management of heavy metals toxicity by microalgae. J. Appl. Phycol. 2022, 34, 475–488. [Google Scholar] [CrossRef]
- Gauthier, M.; Senhorinho, G.; Scott, J. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Malhotra, N.; Hsu, H.-S.; Liang, S.-T.; Roldan, M.J.M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef]
- Park, S.; Polle, J.E.; Melis, A.; Lee, T.K.; Jin, E. Up-regulation of photoprotection and PSII-repair gene expression by irradiance in the unicellular green alga Dunaliella salina. Mar. Biotechnol. 2006, 8, 120–128. [Google Scholar] [CrossRef]
- Janani, B.; Raju, L.L.; Thomas, A.M.; Alyemeni, M.N.; Dudin, G.A.; Wijaya, L.; Alsahli, A.A.; Ahmad, P.; Khan, S.S. Impact of bovine serum albumin–A protein corona on toxicity of ZnO NPs in environmental model systems of plant, bacteria, algae and crustaceans. Chemosphere 2021, 270, 128629. [Google Scholar] [CrossRef]
- Falciatore, A.; Jaubert, M.; Bouly, J.-P.; Bailleul, B.; Mock, T. Diatom molecular research comes of age: Model species for studying phytoplankton biology and diversity. Plant Cell 2020, 32, 547–572. [Google Scholar] [CrossRef]
- Gelzinis, A.; Butkus, V.; Songaila, E.; Augulis, R.; Gall, A.; Büchel, C.; Robert, B.; Abramavicius, D.; Zigmantas, D.; Valkunas, L. Mapping energy transfer channels in fucoxanthin–chlorophyll protein complex. Biochim. Et. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 241–247. [Google Scholar] [CrossRef]
- Cheng, J.; Du, X.; Long, H.; Zhang, H.; Ji, X. The effects of exogenous cerium on photosystem II as probed by in vivo chlorophyll fluorescence and lipid production of Scenedesmus obliquus XJ002. Biotechnol. Appl. Biochem. 2021, 68, 1216–1226. [Google Scholar]
- Figueiredo, C.; Grilo, T.F.; Oliveira, R.; Ferreira, I.J.; Gil, F.; Lopes, C.; Brito, P.; Ré, P.; Caetano, M.; Diniz, M. A triple threat: Ocean warming, acidification, and rare earth elements exposure triggers a superior antioxidant response and pigment production in the adaptable Ulva rigida. Environ. Adv. 2022, 8, 100235. [Google Scholar] [CrossRef]
- Goncalves, S.; Almeida, S.F.; Figueira, E.; Kahlert, M. Valve teratologies and Chl c in the freshwater diatom Tabellaria flocculosa as biomarkers for metal contamination. Ecol. Indic. 2019, 101, 476–485. [Google Scholar] [CrossRef]
- Martos de la Fuente, G.C.; Viñegla, B.; Illana Rico, E.; Fernández Ocaña, A.M. Study of the Photosynthesis Response during the Gradual Lack of Water for 14 Olea europaea L. subsp europaea Cultivars and Their Adaptation to Climate Change. Plants 2023, 12, 4136. [Google Scholar] [CrossRef]
- Jallet, D.; Caballero, M.A.; Gallina, A.A.; Youngblood, M.; Peers, G. Photosynthetic physiology and biomass partitioning in the model diatom Phaeodactylum tricornutum grown in a sinusoidal light regime. Algal Res. 2016, 18, 51–60. [Google Scholar] [CrossRef]
- Lepetit, B.; Gélin, G.; Lepetit, M.; Sturm, S.; Vugrinec, S.; Rogato, A.; Kroth, P.G.; Falciatore, A.; Lavaud, J. The diatom Phaeodactylum tricornutum adjusts nonphotochemical fluorescence quenching capacity in response to dynamic light via fine-tuned L hcx and xanthophyll cycle pigment synthesis. New Phytol. 2017, 214, 205–218. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Čížková, M.; Mezricky, P.; Mezricky, D.; Rucki, M.; Zachleder, V.; Vítová, M. Bioaccumulation of rare earth elements from waste luminophores in the red algae, Galdieria phlegrea. Waste Biomass Valorization 2021, 12, 3137–3146. [Google Scholar] [CrossRef]
- Hao, S.; Xiaorong, W.; Liansheng, W.; Lemei, D.; Zhong, L.; Yijun, C. Bioconcentration of rare earth elements lanthanum, gadolinium and yttrium in algae (Chlorella vulgarize Beijerinck): Influence of chemical species. Chemosphere 1997, 34, 1753–1760. [Google Scholar] [CrossRef]
- El-Akl, P.; Smith, S.; Wilkinson, K.J. Linking the chemical speciation of cerium to its bioavailability in water for a freshwater alga. Environ. Toxicol. Chem. 2015, 34, 1711–1719. [Google Scholar] [CrossRef]
- Yang, X.; Yin, D.; Sun, H.; Wang, X.; Dai, L.; Chen, Y.; Cao, M. Distribution and bioavailability of rare earth elements in aquatic microcosm. Chemosphere 1999, 39, 2443–2450. [Google Scholar] [CrossRef]
- Khan, A.M.; Bakar, N.K.A.; Bakar, A.F.A.; Ashraf, M.A. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: A review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef]
- Costa, M.; Henriques, B.; Pinto, J.; Fabre, E.; Dias, M.; Soares, J.; Carvalho, L.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Influence of toxic elements on the simultaneous uptake of rare earth elements from contaminated waters by estuarine macroalgae. Chemosphere 2020, 252, 126562. [Google Scholar] [CrossRef] [PubMed]
- Qiang, T.; Xiao-Rong, W.; Li-Qing, T.; Le-Mei, D. Bioaccumulation of the rare earth elements lanthanum, gadolinium and yttrium in carp (Cyprinus carpio). Environ. Pollut. 1994, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Guo, C.; Xue, C.; Ma, C.; Mu, H.; Sun, L. Toxic Effects of Two Representative Rare Earth Elements (La and Gd) on Danio rerio Based on Transcriptome Analysis. Toxics 2022, 10, 519. [Google Scholar] [CrossRef]
- Lachaux, N.; Catrouillet, C.; Marsac, R.; Poirier, L.; Pain-Devin, S.; Gross, E.M.; Giamberini, L. Implications of speciation on rare earth element toxicity: A focus on organic matter influence in Daphnia magna standard test. Environ. Pollut. 2022, 307, 119554. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Battuello, M.; Nurra, N.; Sartor, R.M.; Benedetto, A.; Pessani, D.; Abete, M.C. A first report of rare earth elements in northwestern Mediterranean seaweeds. Mar. Pollut. Bull. 2017, 122, 236–242. [Google Scholar] [CrossRef] [PubMed]


| Bioconcentration | Factor (BFC) | |||
|---|---|---|---|---|
| Exposure Time (d) | Ce 2.39 mg/L | Ce 3.13 mg/L | Gd 4.52 mg/L | Gd 6.02 mg/L |
| 7 | 2.76 | 149 | 2.29 | 8.40 |
| 14 | 1.99 | 7.17 | 1.37 | 3.48 |
| 21 | 3.50 | 4.32 | 1.11 | 1.20 |
| 28 | 3.80 | 8.44 | 1.30 | 3.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Spampinato, M.; Salbitani, G.; Guida, M.; Carfagna, S.; Brouziotis, A.A.; Trifuoggi, M.; Bossa, R.; Saviano, L.; Padilla Suarez, E.G.; et al. Multi-Endpoint Analysis of Cerium and Gadolinium Effects after Long-Term Exposure to Phaeodactylum tricornutum. Environments 2024, 11, 58. https://doi.org/10.3390/environments11030058
Siciliano A, Spampinato M, Salbitani G, Guida M, Carfagna S, Brouziotis AA, Trifuoggi M, Bossa R, Saviano L, Padilla Suarez EG, et al. Multi-Endpoint Analysis of Cerium and Gadolinium Effects after Long-Term Exposure to Phaeodactylum tricornutum. Environments. 2024; 11(3):58. https://doi.org/10.3390/environments11030058
Chicago/Turabian StyleSiciliano, Antonietta, Marisa Spampinato, Giovanna Salbitani, Marco Guida, Simona Carfagna, Antonios Apostolos Brouziotis, Marco Trifuoggi, Rosanna Bossa, Lorenzo Saviano, Edith Guadalupe Padilla Suarez, and et al. 2024. "Multi-Endpoint Analysis of Cerium and Gadolinium Effects after Long-Term Exposure to Phaeodactylum tricornutum" Environments 11, no. 3: 58. https://doi.org/10.3390/environments11030058










