Development of an Environmental DNA Assay for Prohibited Matter Weed Amazon Frogbit (Limnobium laevigatum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
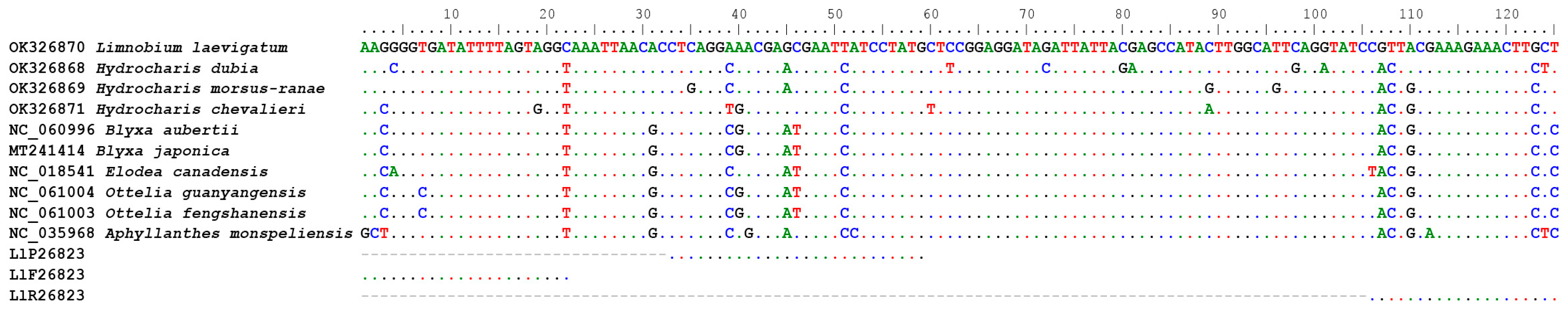
2.2. Assay Development
2.3. Assay Multiplexing with QC1292
2.4. Field Application
- a represents the eDNA concentration of the DNA extraction (copy number/µL),
- b denotes the elution volume of DNA extraction (µL),
- c is the total volume of water filtered for each sample (L), and
- d indicates the proportion of filter extracted.
3. Results
3.1. Assay Development
3.2. Assay Sensitivity
3.3. Field Application
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Brauwer, M.; Chariton, A.; Clarke, L.; Cooper, M.; Dibattista, J.; Furlan, E.; Giblot-Ducray, D.; Gleeson, D.; Harford, A.; Herbert, S.; et al. Environmental DNA Test Validation Guidelines; National eDNA Reference Centre: Canberra, Australia, 2022. [Google Scholar]
- Martellini, A.; Payment, P.; Villemur, R. Use of eukaryotic mitochondrial DNA to differentiate human, bovine, porcine and ovine sources in fecally contaminated surface water. Water Res. 2005, 39, 541–548. [Google Scholar] [CrossRef]
- Wilkes Walburn, J.; Rourke, M.L.; Furlan, E.; DiBattista, J.D.; Broadhurst, M.K.; Fowler, A.M.; Hughes, J.M.; Fielder, S. Robust environmental DNA assay development and validation: A case study with two vulnerable Australian fish. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1225–1231. [Google Scholar] [CrossRef]
- Prabhakaran, G.K.; Sunkara, M.; Raghavan, R.; Umapathy, G. Development of a species-specific qPCR assay for the detection of invasive African sharptooth catfish (Clarias gariepinus) using environmental DNA. Biol. Invasions 2023, 25, 975–982. [Google Scholar] [CrossRef]
- Doi, H.; Takahara, T.; Minamoto, T.; Matsuhashi, S.; Uchii, K.; Yamanaka, H. Droplet Digital Polymerase Chain Reaction (PCR) Outperforms Real-Time PCR in the Detection of Environmental DNA from an Invasive Fish Species. Environ. Sci. Technol. 2015, 49, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Mychek-Londer, J.G.; Balasingham, K.D.; Heath, D.D. Using environmental DNA metabarcoding to map invasive and native invertebrates in two Great Lakes tributaries. Environ. DNA 2020, 2, 283–297. [Google Scholar] [CrossRef]
- McColl-Gausden, E.F.; Weeks, A.R.; Coleman, R.; Song, S.; Tingley, R. Using hierarchical models to compare the sensitivity of metabarcoding and qPCR for eDNA detection. Ecol. Inform. 2023, 75, 102072. [Google Scholar] [CrossRef]
- Yu, Z.; Ito, S.I.; Wong, M.K.; Yoshizawa, S.; Inoue, J.; Itoh, S.; Yukami, R.; Ishikawa, K.; Guo, C.; Ijichi, M.; et al. Comparison of species-specific qPCR and metabarcoding methods to detect small pelagic fish distribution from open ocean environmental DNA. PLoS ONE 2022, 17, e0273670. [Google Scholar] [CrossRef]
- Harper, L.R.; Lawson Handley, L.; Hahn, C.; Boonham, N.; Rees, H.C.; Gough, K.C.; Lewis, E.; Adams, I.P.; Brotherton, P.; Phillips, S.; et al. Needle in a haystack? A comparison of eDNA metabarcoding and targeted qPCR for detection of the great crested newt (Triturus cristatus). Ecol. Evol. 2018, 8, 6330–6341. [Google Scholar] [CrossRef] [PubMed]
- Chandelier, A.; Hulin, J.; San Martin, G.; Debode, F.; Massart, S. Comparison of qPCR and metabarcoding methods as tools for the detection of airborne inoculum of forest fungal pathogens. Phytopathology 2021, 111, 570–581. [Google Scholar] [CrossRef]
- Kress, W.J. Plant DNA barcodes: Applications today and in the future. J. Syst. Evol. 2017, 55, 291–307. [Google Scholar] [CrossRef]
- Anglès d’Auriac, M.B.; Strand, D.A.; Mjelde, M.; Demars, B.O.L.; Thaulow, J. Detection of an invasive aquatic plant in natural water bodies using environmental DNA. PLoS ONE 2019, 14, e0219700. [Google Scholar] [CrossRef]
- Batchelor, K.L.; Bell, K.L.; Campos, M.; Webber, B.L. Can honey bees be used to detect rare plants? Taking an eDNA approach to find the last plants in a weed eradication program. Environ. DNA 2023, 5, 1516–1526. [Google Scholar] [CrossRef]
- Vasar, M.; Davison, J.; Moora, M.; Sepp, S.-K.; Anslan, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; Fabiano, E.C.; et al. Metabarcoding of soil environmental DNA to estimate plant diversity globally. Front. Plant Sci. 2023, 14, 1106617. [Google Scholar] [CrossRef]
- Lennartz, C.; Kurucar, J.; Coppola, S.; Crager, J.; Bobrow, J.; Bortolin, L.; Comolli, J. Geographic source estimation using airborne plant environmental DNA in dust. Sci. Rep. 2021, 11, 16238. [Google Scholar] [CrossRef]
- Jones, L.; Brennan, G.L.; Lowe, A.; Creer, S.; Ford, C.R.; de Vere, N. Shifts in honeybee foraging reveal historical changes in floral resources. Commun. Biol. 2021, 4, 37. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Doi, H.; Fujiwara, A.; Watanabe, S.; Minamoto, T. Evaluation of the environmental DNA method for estimating distribution and biomass of submerged aquatic plants. PLoS ONE 2016, 11, e0156217. [Google Scholar] [CrossRef] [PubMed]
- Gantz, C.A.; Renshaw, M.A.; Erickson, D.; Lodge, D.M.; Egan, S.P. Environmental DNA detection of aquatic invasive plants in lab mesocosm and natural field conditions. Biol. Invasions 2018, 20, 2535–2552. [Google Scholar] [CrossRef]
- Kuehne, L.M.; Ostberg, C.O.; Chase, D.M.; Duda, J.J.; Olden, J.D. Use of environmental DNA to detect the invasive aquatic plants Myriophyllum spicatum and Egeria densa in lakes. Freshw. Sci. 2020, 39, 521–533. [Google Scholar] [CrossRef]
- van de Witte, Y. Limnobium laevigatum (South American Spongeplant); CABI Compendium: Oxford, UK, 2022. [Google Scholar]
- Simon, J.B.; Panetta, F.D.; Kylie, E.G. Progress towards the Eradication of Mikania Vine (Mikania micrantha) and Limnocharis (Limnocharis flava) in Northern Australia. Invasive Plant Sci. Manag. 2008, 1, 296–303. [Google Scholar] [CrossRef]
- Howard, G.; Hyde, M.; Bingham, M. Alien Limnobium laevigatum (Humb. & Bonpl. ex Willd.) Heine (Hydrocharitaceae) becoming prevalent in Zimbabwe and Zambia. BioInvasions Rec. 2016, 5, 221–225. [Google Scholar] [CrossRef]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Li, Z.-Z.; Lehtonen, S.; Gichira, A.W.; Martins, K.; Efremov, A.; Wang, Q.-F.; Chen, J.-M. Plastome phylogenomics and historical biogeography of aquatic plant genus Hydrocharis (Hydrocharitaceae). BMC Plant Biol. 2022, 22, 106. [Google Scholar] [CrossRef]
- Anonymous. Multiple Primer Analyzer. Available online: https://www.thermofisher.com/au/en/home/brands/thermo-scientific/molecular-biology/molecular-biology-learning-center/molecular-biology-resource-library/thermo-scientific-web-tools/multiple-primer-analyzer.html (accessed on 4 December 2023).
- Klymus, K.E.; Merkes, C.M.; Allison, M.J.; Goldberg, C.S.; Helbing, C.C.; Hunter, M.E.; Jackson, C.A.; Lance, R.F.; Mangan, A.M.; Monroe, E.M.; et al. Reporting the limits of detection and quantification for environmental DNA assays. Environ. DNA 2020, 2, 271–282. [Google Scholar] [CrossRef]
- Zhu, X.; Bell, K.L.; Rourke, M.L.; Wu, H.; Gopurenko, D. Generic qPCR assays for quality control in environmental DNA research. Environ. DNA 2024, accepted. [Google Scholar]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-González, A.; Thuo, D.N.; Divi, U.; Sparks, K.; Wallenius, T.; Gleeson, D. Detection of Khapra beetle environmental DNA using portable technologies in Australian biosecurity. Front. Insect Sci. 2022, 2, 795379. [Google Scholar] [CrossRef]
- Lunghi, E.; Valle, B.; Guerrieri, A.; Bonin, A.; Cianferoni, F.; Manenti, R.; Ficetola, G.F. Environmental DNA of insects and springtails from caves reveals complex processes of eDNA transfer in soils. Sci. Total Environ. 2022, 826, 154022. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Minamoto, T.; Doi, H. Seasonal change in environmental DNA concentration of a submerged aquatic plant species. Freshw. Sci. 2019, 38, 654–660. [Google Scholar] [CrossRef]
- Kodama, T.; Miyazono, S.; Akamatsu, Y.; Tsuji, S.; Nakao, R. Abundance estimation of riverine macrophyte Egeria densa using environmental DNA: Effects of sampling season and location. Limnology 2022, 23, 299–308. [Google Scholar] [CrossRef]
- Collins, R.A.; Wangensteen, O.S.; O’Gorman, E.J.; Mariani, S.; Sims, D.W.; Genner, M.J. Persistence of environmental DNA in marine systems. Commun. Biol. 2018, 1, 185. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Faiq, M.E.; Li, Z.; Chen, G. Persistence and degradation dynamics of eDNA affected by environmental factors in aquatic ecosystems. Hydrobiologia 2022, 849, 4119–4133. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R.; Jerde, C.L.; Renshaw, M.A.; Chadderton, W.L.; Lodge, D.M. Environmental Conditions Influence eDNA Persistence in Aquatic Systems. Environ. Sci. Technol. 2014, 48, 1819–1827. [Google Scholar] [CrossRef]
- Kagzi, K.; Hechler, R.M.; Fussmann, G.F.; Cristescu, M.E. Environmental RNA degrades more rapidly than environmental DNA across a broad range of pH conditions. Mol. Ecol. Resour. 2022, 22, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Pochon, X.; Zaiko, A.; Fletcher, L.M.; Laroche, O.; Wood, S.A. Wanted dead or alive? Using metabarcoding of environmental DNA and RNA to distinguish living assemblages for biosecurity applications. PLoS ONE 2017, 12, e0187636. [Google Scholar] [CrossRef]
- Furlan, E.M.; Gleeson, D. Improving reliability in environmental DNA detection surveys through enhanced quality control. Mar. Freshw. Res. 2017, 68, 388–395. [Google Scholar] [CrossRef]
| Label | Direction | Sequence (5′-3′) | Tm (°C) |
|---|---|---|---|
| LlF26823 | Forward | AAGGGGTGATATTTTAGTAGGC | 59.0 |
| LlR26823 | Reverse | AGCAAGTTTCTTTCGTAACG | 58.7 |
| LlP26823 | Probe | [6FAM] CTCAGGAAACGAGCGAATTATCCTATG [BHQ1] | 68.9 |
| Ll26823gBlock | GGGTAGAAAGGGGTGATATTTTAGTAGGCAAATTAACACCTCAGGAAACGAGCG AATTATCCTATGCTCCGGAGGATAGATTATTACGAGCCATACTTGGCATTCAGGTA TCCGTTACGAAAGAAACTTGCTTAAAA | ||
| Locality | Cq ± SE * | eDNA Concentration ± SE in the Sampled Environment ** |
|---|---|---|
| Commercial Road, Oakville | 28.73 ± 1.21 | 4.09 × 105 ± 1.88 × 105 |
| Ogden Road, Oakville | 23.66 ± 0.21 | 1.11 × 106 ± 7.84 × 104 |
| Bellambi Lagoon | 34.63 ± 0.49 | 4.48 × 103 ± 6.35 × 102 |
| Cawley Street, Bellambi | 31.71 ± 0.64 | 9.09 × 104 ± 4,53 × 104 |
| Edyth Street, Corrimal | 31.43 | 1.92 × 104 ± 1.92 × 104 |
| Park Road, Bellambi *** | N/A | N/A |
| Bulahdelah *** | N/A | N/A |
| Rileys Creek, Catherine Field *** | N/A | N/A |
| Lagoon, Catherine Field | 25.06 ± 0.88 | 1.06 × 106 ± 3.37 × 105 |
| Bomaderry | 30.30 ± 0.26 | 3.20 × 105 ± 1.56 × 105 |
| Lake Albert, Wagga Wagga *** | N/A | N/A |
| Assay | Target | Efficiency (%) | LOD (Copies/µL) | LOQ (Copies/µL) |
|---|---|---|---|---|
| Ll26823 | L. laevigatum | 99.06 | 3.66 | 86 |
| Ll26823 + QC1292 * | L. laevigatum and endogenous DNA | 94.54 | 16.50 | 16.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Bell, K.L.; Wu, H.; Gopurenko, D. Development of an Environmental DNA Assay for Prohibited Matter Weed Amazon Frogbit (Limnobium laevigatum). Environments 2024, 11, 66. https://doi.org/10.3390/environments11040066
Zhu X, Bell KL, Wu H, Gopurenko D. Development of an Environmental DNA Assay for Prohibited Matter Weed Amazon Frogbit (Limnobium laevigatum). Environments. 2024; 11(4):66. https://doi.org/10.3390/environments11040066
Chicago/Turabian StyleZhu, Xiaocheng, Karen L. Bell, Hanwen Wu, and David Gopurenko. 2024. "Development of an Environmental DNA Assay for Prohibited Matter Weed Amazon Frogbit (Limnobium laevigatum)" Environments 11, no. 4: 66. https://doi.org/10.3390/environments11040066






