Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges
Abstract
:1. Introduction
1.1. Effects of Sulfur
1.1.1. Health
1.1.2. Environment and Climate
1.2. Effects of Sulphur-Containing Fuels on Economy
1.3. Sulphur Content Regulations
Schedule of Sulfur Regulation for Countries
1.4. Existing Technologies for Desulphurization
1.4.1. Hydrodesulfurization (HDS)
1.4.2. Oxidative Desulfurization (ODS)
1.4.3. Adsorption Process
1.4.4. Reactive Adsorption
1.4.5. Physical Adsorption
1.5. Biodesulfurization
1.6. Recent Studies in BDS
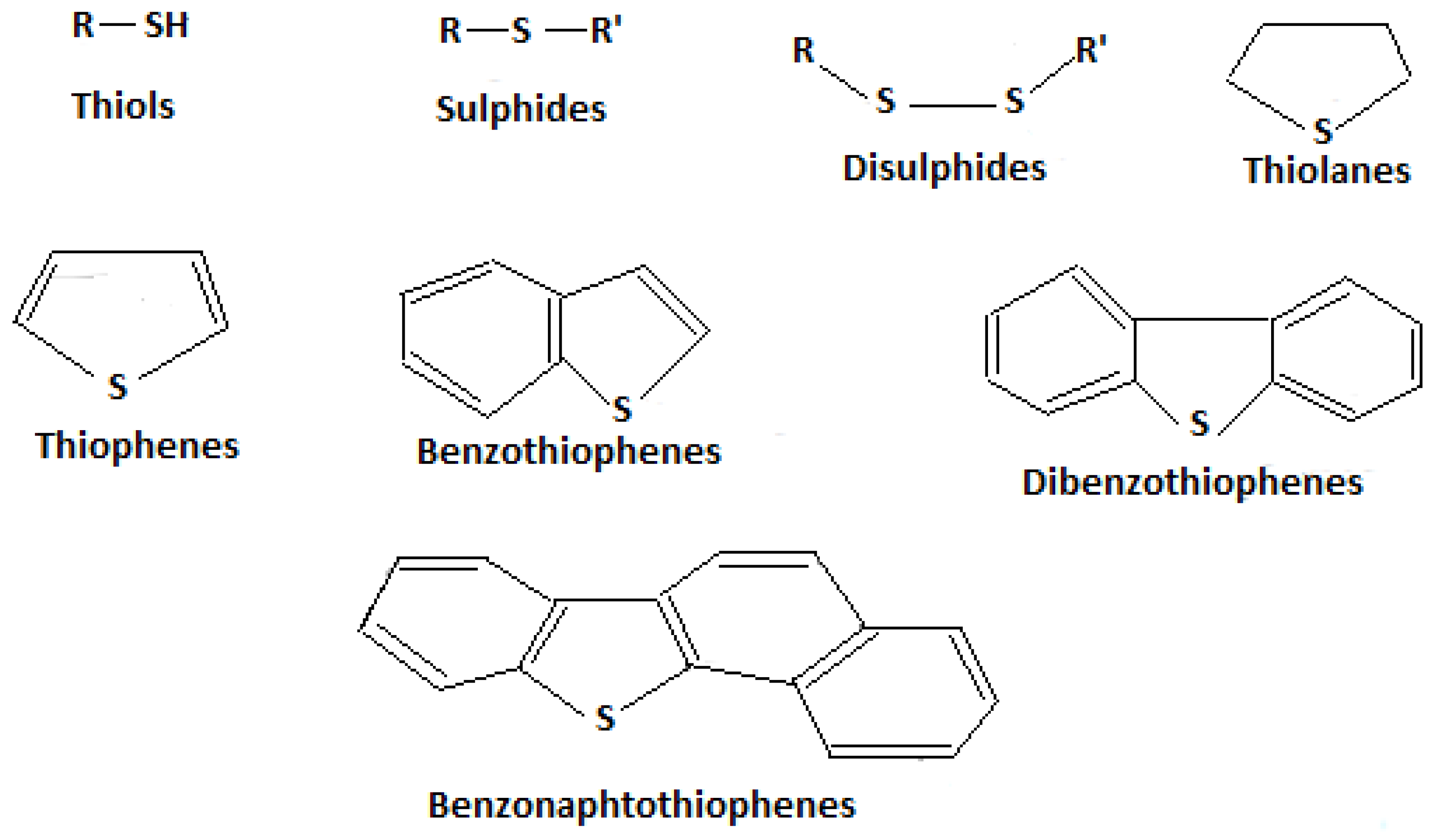
1.7. Sulphur Degradating/Removal Bacterial Strains
Use of Anaerobic and Aerobic Bacteria in Biodesulfurization
2. Issues in Biodesulfurization for Its Industrial Application
3. Conclusions and Future Outlook
- (i)
- In depth investigation into the understanding of the various microbial pathways that are involved in BDS is required towards the optimization and scale-up studies of the process.
- (ii)
- In addition, studies that will explore the combination of BDS with existing methods could be instrumental to achieving a higher reduction in sulphur-containing compounds in the petroleum distillates.
- (iii)
- At the same time, the development of new strains or the modification of sulphur degrading strains via recombinant DNA technology or genetic engineering could be essential to enhancing the degrading performance of the existing bacteria.
Author Contributions
Conflicts of Interest
References
- Kan, H.; Wong, C.M.; Vichit-Vadakan, N.; Qian, Z. The PAPA project teams short term association between sulphur dioxide and daily mortality: The public health and air pollution in Asia (PAPA) study. Environ. Res. 2010, 110, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Bergh, C. Energy Efficiency in the South African Crude Oil Refining Industry: Drivers, Barriers and Opportunities. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 29 May 2012. [Google Scholar]
- Weerasekara, N.S.; García Frutos, F.J.; Cara, J.; Lockwood, F.C. Mathematical modelling of demineralisation of high sulphur coal by bioleaching. Miner. Eng. 2008, 21, 234–240. [Google Scholar] [CrossRef]
- Duissenov, D. Production and Processing of Sour Crude and Natural Gas—Challenges due to Increasing Stringent Regulations; Norwegian University of Science and Technology Faculty of Engineering Science and Technology Department of Petroleum Engineering and Applied Geophysics: Trondheim, Norway, 2013; p. 1. [Google Scholar]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulphur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Chambers, L.; Duffy, M.L. Determination of Total and Speciated Sulfur Content in Petrochemical Samples Using a Pulsed Flame Photometric Detector. J. Chromatogr. Sci. 2003, 41, 528–534. [Google Scholar] [CrossRef]
- Dunleavy, J.K. Sulphur as a Catalyst Poison. Platin. Met. Rev. 2006, 50, 110. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, L.; Zhou, W.; Jia, L.; Hou, B.; Li, D.; Zhao, Y. Overview of catalyst application in petroleum refinery for biomass catalytic pyrolysis and bio-oil upgrading. RSC Adv. 2015, 5, 88287–88297. [Google Scholar] [CrossRef]
- Fang, W.L. Inventory of U.S Greenhouse Gas Emission and Sinks 1990–2003; Clear Air Market Division, United States Environmental Protection Agency: Washington, DC, USA, 2004.
- Policy Briefs on Global Atmospheric Pollution Forum. Available online: https://www.sei-international.org/gapforum/policy/effectshumanhealth.php (accessed on 4 October 2016).
- Javadli, R.; de Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1-4, 3–19. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Mochida, I. Three-stage deep hydrodesulfurization and decolorization of diesel fuel with CoMo and NiMo catalysts at relatively low pressure. Fuel 1994, 73, 1667–1671. [Google Scholar] [CrossRef]
- Monticello, D.J. Riding the fossil fuel biodesulfurization wave. ChemTech 1998, 28, 38–45. [Google Scholar]
- Sharaf, J. Exhaust Emissions and Its Control Technology for an Internal Combustion Engine. Int. J. Eng. Res. Appl. 2013, 3, 947–960. [Google Scholar]
- Chambliss, S.; Josh Miller, J.; Façanha, C.; Minjares, R.; Blumberg, K. The Impact of Stringent Fuel and Vehicle Standards on Premature mortality and Emissions; ICCT’S Global Transportation Health and Climate Roadmap Series; International Council on Clean Transportation: Washington, DC, USA, 2013; pp. 1–89. [Google Scholar]
- McClellan, R.O.; Hesterberg, T.W.; Wall, J.C. Evaluation of carcinogenic hazard of diesel engine exhaust needs to consider revolutionary changes in diesel technology. Regul. Toxicol. Pharmacol. 2012, 63, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chien, L. Short-term population-based non-linear concentration-response associations between fine particulate matter and respiratory diseases in Taipei (Taiwan): A spatiotemporal analysis. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, L.D.; Escobar, J.D.; Dvonch, J.T.; Sanchez, B.N.; Majersik, J.J.; Brown, D.L.; Smith, M.A.; Morgenstern, L.B. Ambient air pollution and risk of ischemic stroke and TIA. Ann. Neurol. 2008, 64, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Darrow, L.A.; Parker, J.D. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ. Health Perspect. 2008, 116, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Geer, L.A.; Weedon, J.M.L. Ambient air pollution and term birth weight in Texas from 1998 to 2004. J. Air Waste Manag. Assoc. 2012, 62, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z. Control of fossil fuel particulate black carbon and organic matter, possibly the most effective method of slowing global warming. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Air Resource Board. Black Carbon Emissions Reduced by ARB Regulations; Califonia Environmental Protection Agency: Sacramento, CA, USA, 2013; pp. 1–89.
- Koch, T.A.; Krause, K.R.; Manzer, L.E.; Mehdizadeh, M.; Odom, J.M.; Sengupta, S.K. Environmental challenges facing the chemical-industry. New J. Chem. 1996, 20, 163–173. [Google Scholar]
- Whitehurst, D.D.; Isoda, I.; Mochida, I. Present state of art and future challenges in hydrodesulfurization of polyaromatic sulfur compounds. Adv. Catal. 1998, 42, 345–357. [Google Scholar]
- Shiraishi, Y.; Hirai, T.; Komasawa, I. Deep Desulfurization Process for Light Oils by Photochemical Reaction in an Organic Two-Phase Liquid—Liquid Extraction System. Ind. Eng. Chem. Res. 1998, 37, 203–211. [Google Scholar] [CrossRef]
- Parkinson, G. Refiners crack down on sulphur. Chem. Eng. 2000, 107, 45–48. [Google Scholar]
- USEPA. Available online: http://www. epa. Gov/sbrefaldocuments/pnl13f. Pdf (accessed on 25 October 2016).
- Collins, F.M.; Lucy, A.R.; Sharp, C.J. Oxidative desulfurization of oils via hydrogen peroxide and heteropolyanion catalysis. J. Mol. Catal. A Chem. 1997, 117, 397–403. [Google Scholar] [CrossRef]
- Tam, P.S.; Kittrell, J.R.; Eldridge, J.W. Desulfurization of fuel oil by oxidation and extraction. 1. Enhancement of extraction oil yield. Ind. Eng. Chem. Res. 1990, 29, 321–324. [Google Scholar] [CrossRef]
- Bravo, A.H.; Soto, A.R.; Sosa, E.R.; Sánchez, A.P.; Alarcón, J.A.L.; Kahl, J.J.; Ruíz, B.J. Effect of acid rain on building material of the El Tajín archaeological zone in Veracruz, Mexico. Environ. Pollut. 2006, 144, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A. Deep Desulfurization of Diesel Fuel Using a Single Phase Photochemical Microreactor. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 25 October 2010. [Google Scholar]
- UNEPA. Report of the Partnership for Clean Fuels and Vehicles (PCFV). pp. 1–14. Available online: http://www.staging.unep.org/transport/pcfv/PDF/SulphurReport-Brochure.pdf (accessed on 23 November 2017).
- Omidvarborna, H.; Kumar, A.; Kim, D.S. Characterization of particulate matter emitted from transit buses fueled with B20 in idle modes. J. Environ. Chem. Eng. 2014, 2, 2335–2342. [Google Scholar] [CrossRef]
- Cackette, T. Importance of Reducing Emissions from Heavy-Duty Vehicles; California Air Resources Board: Sacramento, CA, USA, 1999; p. 15.
- Gauderman, W.J.; Gilliland, G.F.; Vora, H.; Avol, E.; Stram, D.; McConnell, R.; Thomas, D.; Lurmann, F.; Margolis, H.G.; Rappaport, E.B. Association between Air Pollution and Lung Function Growth in Southern California Children: Results from a second cohort. Am. J. Respir. Crit. Care Med. 2000, 166, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sayyadnejad, M.A.; Ghaffarian, H.R.; Saeidi, M. Removal of hydrogen sulfide by zinc oxide nanoparticles in drilling fluid. Int. J. Environ. Sci. Technol. 2008, 5, 565–569. [Google Scholar] [CrossRef]
- Sekhavatjou, M.S.; Moradi, R.; Alhashemi, A.H.; Hejabi, T. A New Method for Sulphur Components Removal from Sour gas through Application of Zinc and Iron Oxides Nanoparticles. Int. J. Environ. Res. 2014, 8, 273–278. [Google Scholar]
- United States Environmental Protection Agency (UEPA). 2016. Available online: https://www.epa.gov/gasoline-standards/gasoline-sulfur (accessed on 23 November 2017).
- Shell Global. Available online: http://www.shell.com/.../sg-en/shell_for_motorists/fuels/diesel/ulsd_faqs_0914.html?LN=/leftnavs/zzz_lhn4_3_4.html (accessed on 10 October 2016).
- Lloyd, A.C.; Cackette, T.A. Diesel Engines: Environmental Impact and Control. J. Air Waste Manag. Assoc. 2001, 51, 809–847. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.R.; Yim, S.H.; Gilmore, C.K.; Murray, L.T.; Kuhn, S.R.; Tai, A.P.; Yantosca, R.M.; Byun, D.W.; Ngan, F.; Li, X.; et al. Public Health, Climate, and Economic Impacts of Desulfurizing Jet Fuel. Environ. Sci. Technol. 2012, 46, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Marcelis, C. Anaerobic Biodesulfurization of Thiophenes. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Bailey, D.; Plenys, T.; Solomon, G.M.; Campbell, T.R.; Feuer, G.R.; Masters, J.; Tonkonogy, B. Harboring Pollution Strategies to Clean Up U.S. Ports; Natural Resources Defense Council: New York, NY, USA, 2004; pp. 1–97. [Google Scholar]
- Kilbane, J.J. Microbial biocatalyst development to upgrade fossil fuels. Curr. Opin. Biotechnol. 2006, 17, 305–314. [Google Scholar] [CrossRef] [PubMed]
- The Info List—Ultra-Low Sulfur Diesel. Available online: http://www.theinfolist.com/php/SummaryGet.php?FindGo=Ultra-Low%20Sulfur%20Diesel (accessed on 21 November 2016).
- Banerjee, S. The European Fuels Conference. Overview of the Asian Fuel Market. Available online: http://www.efoa.eu/documents/document/20110328145537-2011-03-09_overview_of_the_asian_fuel_ether_market_and_opportunities_for_europe.pdf (accessed on 23 November 2017).
- Overview of Air Pollution from Transportation. Available online: https://www.epa.gov/air-pollution-transportation/learn-about-air-pollution-transportation (accessed on 2 November 2016).
- Fuel Quality in Australia. 2001. Available online: http://www.environment.gov.au/topics/environment-protection/fuel-quality/standards/diesel (accessed on 21 November 2016).
- Diesel Fuel Specifications—Korea. Diesel Fuel EN 590. Available online: https://en590.wordpress.com/ (accessed on 21 November 2016).
- Zietsman, J.; Farzaneh, M.; Storey, J.M.E.; Villa, J.; Ojah, M.; Lee, D.W.; Bella, P. Emissions of Mexican-Domiciled Heavy-Duty Diesel Trucks Using Alternative Fuels; Texas Transportation Institute: College Station, TX, USA, 2007; pp. 4–70. [Google Scholar]
- Kodjak, D. Policies to Reduce Fuel Consumption, Air Pollution, and Carbon Emissions from Vehicles in G20 Nations; The International Council on Clean Transportation: Washington, DC, USA, 2015; pp. 1–22. [Google Scholar]
- AECC Newsletter. 2015. Available online: http://www.aecc.eu/en/content/pdf/AECC%20Newsletter%20May-June%202010.pdf (accessed on 22 November 2016).
- United Nation Environment Programme. 2012. Available online: http://www.unep.org/transport/pcfv/meetings/mauritius50ppm.asp (accessed on 2 November 2016).
- Industry News. Available online: http://www.iol.co.za/motoring/industry-news/sasol-launches-10ppm-clean-diesel-1.1611081#.UpNbXsQW1KJ (accessed on 2 November 2016).
- Shafi, R.; Hutchings, G.J. Hydrodesulfurization of hindered dibenzothiophenes: An overview. Catal. Today 2000, 59, 423–442. [Google Scholar] [CrossRef]
- Startsev, A.N. The Reaction Mechanisms of H2S Decomposition into Hydrogen and Sulfur: Application of Classical and Biological Thermodynamics. J. Thermodyn. Catal. 2017, 8, 2–8. [Google Scholar]
- Bachmann, R.T.; Johnson, A.C.; Edyvean, R.G.J. Biotechnology in the petroleum industry: An overview. Int. Biodeterior. Biodegrad. 2014, 86, 225–237. [Google Scholar]
- Kabe, T.; Ishihara, A.; Tajima, H. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light oil. Ind. Eng. Chem. Res. 1992, 31, 1577–1580. [Google Scholar] [CrossRef]
- Abin-Fuentes, A.; Leung, J.C.; El-Said Mohamed, M.; Wang, D.I.C.; Prather, K.L.J. Rate-limiting step analysis of the microbial desulfurization of dibenzothiophene in a model oil system. Biotechnol. Bioeng. 2014, 111, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, H.; Li, W. Kinetic analysis of biodesulfurization of model oil containing multiple alkyl dibenzothiophenes. Appl. Microbiol. Biotechnol. 2013, 97, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Boulinguiez, M.; Vrinat, M. Hydrodesulfurization of thiophene, dibenzothiophene and gasoil on various Co-Mo/TiO2-Al2O3 catalysis. Catal. Today 1996, 29, 209–213. [Google Scholar] [CrossRef]
- Zeelani, G.G.; Dr., Sundar Lal Pal, S.L. A Review on Desulfurization Techniques of Liquid Fuels. Int. J. Sci. Res. 2016, 5, 2413–2419. [Google Scholar]
- Shong, R.G. Bioprocessing of Crude Oils. Texaco Exploration & Producing Technology Department: Houston, Texas, 77042. Available online: https://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/Volumes/Vol44-1.pdf (accessed on 23 November 2017).
- Boniek, D.; Figueiredo, D.; Batista dos Santos, A.F.; De Resende Stoianoff, M.A. Biodesulfurization: A mini review about the immediate search for the future technology. Clean Technol. Environ. Policy 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Montiel, C.; Quintero, R.; Aburto, J. Petroleum biotechnology: Technology trends from the future. Afr. J. Biotechnol. 2009, 8, 2653–2666. [Google Scholar]
- Zhang, Y.; Wang, D.; Zhang, R.; Zhao, J.; Zheng, Y. ZSM-5-Ln(Pc)2 catalyzed oxygen oxidation of thiophene. Catal. Commun. 2012, 29, 21–23. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, H.; Wang, Z. Desulfurization of gasoline by a new method of electrochemical catalytic oxidation. Fuel 2007, 86, 2747–2753. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as “green” solvents for extractions. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Al-Yacoub, Z.H.; Vedakumar, V.J. Biocatalytic desulfurization of thiophenic compounds and crude oil by newly isolated bacteria. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Hamidi, A. Sulfur Removal of Crude Oil by Ultrasound Assisted Oxidative Method. In Proceedings of the International Conference on Biological, Civil and Environmental Engineering (BCEE-2014), Dubai, United Arab Emirates, 17–18 March 2014. [Google Scholar]
- Bose, D. Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day. World Sci. News 2015, 9, 99–111. [Google Scholar]
- Gary, J.H.; Handwerk, G.E. Petroleum Refining Technology and Economics, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1984. [Google Scholar]
- Medici, L.; Prins, R. The Influence of Chelating Ligands on the Sulfidation of Ni and Mo in NiMo/SiO2 Hydrotreating Catalysts. J. Catal. 1996, 163, 38–49. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; de Beer, V.H.J.; Van Veen, J.A.R.; van Santen, R.A. On the sulfur tolerance of supported Ni (Co)Mo sulfide hydrotreating catalysts. J. Catal. 2003, 215, 353–357. [Google Scholar] [CrossRef]
- Rob van Veen, J.A.R.; Gerkema, E.; van der Kraan, A.M.; Hendriks, P.A.J.M.; Beens, H. A 57Co Mossbauer emission spectrometric study of some supported CoMo hydrodesulfurization catalysts. J. Catal. 1992, 133, 112–123. [Google Scholar] [CrossRef]
- Ohta, Y.; Shimizu, T.; Honma, T.; Yamada, M. Effect of chelating agents on HDS and aromatic hydrogenation over CoMo-and NiW/Al2O3. Stud. Surf. Sci. Catal. 1999, 127, 161–168. [Google Scholar]
- Cattaneo, R.; Rota, F.; Prins, R. An XAFS Study of the Different Influence of Chelating Ligands on the HDN and HDS of γ-Al2O3-Supported NiMo Catalysts. J. Catal. 2001, 199, 318–327. [Google Scholar] [CrossRef]
- Bouwens, S.M.A.M.; van Zon, F.B.M.; van Dijk, M.P.; van der Kraan, A.M.; de Beer, V.H.J.; van Veen, J.A.R.; Koningsberger, D.C. On the structural differences between alumina-supported CoMoS type I and alumina-, silica-, and carbon-supported CoMoS type II phases studied by XAFS, MES, and XPS. J. Catal. 1994, 146, 375–393. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Sun, T.; Jia, J. Recent advances of sodium boronhydride reduction in coal water slurry desulfurization: Integration of chemical and electrochemical reduction. RSC Adv. 2012, 2, 8867–8882. [Google Scholar] [CrossRef]
- Gupta, N.; Roychoudhury, P.K.; Deb, J.K. Biotechnology of desulfurization of diesel: Prospects and challenges. Appl. Microbiol. Biotechnol. 2005, 66, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, F.; Wang, R. Research advances in oxidative desulfurization technologies for the production of low sulfur fuel oils. Pet. Coal 2009, 51, 196–207. [Google Scholar]
- Paniv, P.M.; Pysh’ev, S.V.; Gaivanovich, V.I.; Lazorko, O.I. Current Problems, Nontraditional Technologies, Noncatalytic oxidation desulfurization of the kerosene cut. Chem. Technol. Fuels Oils 2006, 42, 159–166. [Google Scholar] [CrossRef]
- Hirai, T.; Ogawa, K.; Komasawa, I. Desulfurization process for dibenzothiophenes from light oil by photochemical reaction and liquid-liquid extraction. Ind. Eng. Chem. Res. 1996, 35, 586–589. [Google Scholar] [CrossRef]
- Kocal, J.A.; Branvold, T.A. Removal of Sulfur-Containing Compounds from Liquid Hydrocarbon Streams. U.S. Patent 6368495, 9 April 2002. [Google Scholar]
- Chica, A.; Corma, A.; Dómine, M.E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor. J. Catal. 2006, 242, 299–308. [Google Scholar] [CrossRef]
- Wan Abu Bakar, W.; Rusmidah Ali, R.; Abdul Kadir, A.; Wan Mokhtar, W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Sundararaman, S.; Song, C. Catalytic Oxidative Desulfurization of Diesel Fuels Using Air in a Two-Step Approach. Ind. Eng. Chem. Res. 2014, 53, 1890–1899. [Google Scholar] [CrossRef]
- Cheng, S.S. Ultra Clean Fuels via Modified UAOD Process with Room Temperature Ionic Liquid (RTIL) & Solid Catalyst Polishing. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 2008. Available online: http://cee.usc.edu/assets/025/85741.pdf (accessed on 20 October 2016).
- Alavi, S.A.; Hashemi, S.H. A Review on Diesel Fuel Desulfurization by Adsorption Process. In Proceedings of the International Conference on Chemical, Agricultural, and Biological Sciences, Antalya, Turkey, 2–3 May 2014; pp. 33–36. [Google Scholar]
- Velu, S.; Watanabe, S.; Ma, X.; Song, C. Reganarable adsorbents for the adsorptive desulphurization of transportation fuels for fuel cell applications. Chem. Soc. Div. Fuel Chem. 2003, 48, 482–526. [Google Scholar]
- Zhanga, Y.; Yang, Y.; Han, H.; Yang, M.; Wang, L.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance. Appl. Catal. B Environ. 2012, 119, 13–19. [Google Scholar] [CrossRef]
- Gislason, J. Phillips Sulfur removal process nears commercialization. Oil Gas J. 2002, 99, 74–76. [Google Scholar]
- Ma, X.; Velu, S.; Kim, J.H.; Song, C. Deep desulfurization of gasoline by selective adsorption over solid adsorbents and impact of analytical methods on ppm-level sulfur quantification for fuel cell applications. Appl. Catal. B Environ. 2005, 56, 137–147. [Google Scholar] [CrossRef]
- Tawfik, A.; Saleh, G.I.; Danmaliki, T.D.S. Nanocomposites and Hybrid Materials for Adsorptive Desulfurization. Advances in Chemical and Materials Engineering (ACME); IGI Global: Hershey, PA, USA, 2015; pp. 129–153. [Google Scholar]
- Ryzhikov, A.; Bezverkhyy, I.; Bellat, J.P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step. Appl. Catal. B 2008, 84, 766–772. [Google Scholar] [CrossRef]
- Tawara, K.; Nishimura, T.; Iwanami, H.; Nishimoto, T.; Hasuike, T. New hydrodesulfurization catalyst for petroleum-fed fuel cell vehicles and cogenerations. Ind. Eng. Chem. Res. 2001, 40, 2367–2370. [Google Scholar] [CrossRef]
- Srivastav, A.; Srivastava, V.C. Adsorptive desulfurization by activated alumina. J. Hazard. Mater. 2009, 170, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Tawara, K.; Nishimura, T.; Iwanami, H.; Shi, S.G. Ultra-deep hydrodesulfurization of kerosene for fuel cell system. Part 2: Regeneration of sulfur-poisoned nickel catalyst in hydrogen and finding of autoregenerative nickel catalyst. J. Jpn. Petrol. Inst. 2000, 43, 114–120. [Google Scholar] [CrossRef]
- Kim, J.H.; Ma, X.; Zhou, A.; Song, C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today 2006, 111, 74–83. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Ryzhikov, A.; Gadacz, G.; Bellat, J.P. Kinetics of thiophene reactive adsorption on Ni/SiO2 and Ni/ZnO. Catal. Today 2008, 130, 199–205. [Google Scholar] [CrossRef]
- Gawande, P.R.; Dr., Jayant, P.K. A Review on Desulphurization of Liquid Fuel by Adsorption. Int. J. Sci. Res. 2014, 3, 2255–2259. [Google Scholar]
- Moosavi, E.S.; Seyed, A.; Dastgheib, A.S.; Karimzadeh, R. Article Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies 2012, 5, 4233–4250. [Google Scholar] [CrossRef]
- Yang, R.T.; Hernandez-Maldonado, A.J.; Yang, F.H. Desulfurization of transportation fuels with Zeolites under ambient conditions. Science 2003, 301, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, Y.; Chen, Q. Adsorption of N/S-Heteroaromatic Compounds from Fuels by Functionalized MIL-101(Cr) Metal-Organic Frameworks: The Impact of Surface Functional Groups. Energy Fuels 2016, 30, 5593–5600. [Google Scholar] [CrossRef]
- “Incorporating Biotechnology into the Classroom What is Biotechnology?” from the Curricula of the “Incorporating Biotechnology into the High School Classroom through Arizona State University’s BioREACH Program”. Available online: http://www.public.asu.edu/~langland/biotech-intro.html (accessed on 5 November 2016).
- Singh, A.; Singh, B.; Ward, O. Potential applications of bioprocess technology in petroleum industry. Biodegradation 2012, 23, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Chisti, Y. Biotechnology a sustainable alternative to chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K. Exploring microbial diversity for biotechnology: The way forward. Trends Biotechnol. 2010, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Guan, W.; Liu, J.; Lu, L.-J.; Xu, J.-C.; Zhou, Q. Characterization and phylogenetic analysis of biodemulsifier-producing bacteria. Bioresour. Technol. 2010, 101, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ward, O.P.; Singh, A.; Van Hamme, J.D.; Voordouw, G. Petroleum microbiology. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 443–456. [Google Scholar]
- Voordouw, G. Production-related petroleum microbiology: Progress and prospects. Curr. Opin. Biotechnol. 2011, 22, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Silva, T.P.; Arez, B.F.; Paixão, S.M. Enhancement of dibenzothiophene biodesulfurization by Gordonia alkanivorans strain 1B using fructose rich culture media. In Proceedings of the 1st International Congress on Bioenergy, Book of Abstracts, Portalegre, Portugal, 23–25 May 2013; p. 48. [Google Scholar]
- Alves, L.; Salgueiro, R.; Rodrigues, C.; Mesquita, E.; Matos, J.; Girio, F.M. Desulfurization of dibenzothiophene, benzothiophene, and other thiophene analogs by a newly isolated bacterium, Gordonia alkanivorans strain 1B. Appl. Biochem. Biotechnol. 2005, 120, 199–208. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Lange, E.A.; Pienkos, P.T.; Yu, L.Q.; Rouse, M.P.; Lin, Q.; Linguist, L.K. Recent advances in biodesulfurization of diesel fuel. In Proceedings of the 1999 National Petrochemical and Refiners Association, Annual Meeting, NPRA AM-99-27, San Antonio, TX, USA, 21–23 March 1999; pp. 1–26. [Google Scholar]
- Linguist, L.K.; Pacheco, M.A. Enzyme-based diesel desulfurization process offers energy, CO2 advantages. Oil Gas J. 1999, 97, 45–48. [Google Scholar]
- Kaufman, E.N.; Harkins, J.B.; Borole, A.P. Comparison of batchstirred and electrospray reactors for biodesulfurization of dibenzothiophene in crude oil and hydrocarbon feedstocks. Appl. Biochem. Biotechnol. 1998, 73, 127–144. [Google Scholar] [CrossRef]
- Abín-Fuentes, A. Mechanistic Understanding of Microbial Desulfurization. Ph.D. Thesis, Massachusetts Institute of Technology, Department of Chemical Engineering, Cambridge, MA, USA, 2013. [Google Scholar]
- Konishi, J.; Ishii, Y.; Onaka, T. Thermophilic carbon-sulfur-bond-targeted biodesulfurization. Appl. Environ. Microbiol. 1997, 63, 3164–3169. [Google Scholar] [PubMed]
- Borgne, S.L.; Quintero, R. Biotechnological processes for the refining of petroleum. Fuel Process. Technol. 2003, 81, 155–169. [Google Scholar] [CrossRef]
- Soleimani, M.; Bassi, A.; Margaritis, A. Biodesulfurization of refractory organic sulphur compounds in fossil fuels. Biotechnol. Adv. 2007, 25, 570–596. [Google Scholar] [CrossRef] [PubMed]
- Madeira, L.; Santana, V.; Pinto, E. Dibenzothiophene oxidation by horseradish peroxidase in organic media: Effect of the DBT:H2O2 molar ratio and H2O2 addition mode. Chemosphere 2008, 71, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, G.; Ball, A.S.; Rasekh, B.; Kaytash, A. Biodesulphurization potential of a newly isolated bacterium, Gordonia alkanivorans RIPI90A. Enzym. Microb. Technol. 2007, 40, 578–584. [Google Scholar] [CrossRef]
- Alves, L.; Marque, S.; Matos, J.; Tenreiro, R.; Gírio, F.M. Dibenzothiophene Desulfurization by Gordonia alkanivorans Strain 1B Using Recycled Paper Sludge Hydrolyzate. Chemosphere 2008, 70, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.; Leton, P.; Calvo, E.G.; Setti, L. Enhancement of dibenzothiphene biodesulphurization using β-cyclodextrins in oil-to-water media. Fuel 2007, 86, 2632–2636. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.W.J.; Chen, J.M.; Cai, Y.B.; Li, W. Desulfurization of various organic sulphur compounds and the mixture of DBT + 4,6-DMDBT by Mycobacterium sp. ZD-19. Bioresour. Technol. 2008, 99, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Z.; Feng, J.; Cai, X.; Ping, X. Biodesulfurization of DBT in tetradecane and crude oil by a facultative thermophilic bacterium Mycobacterium goodii X7B. J. Biotechnol. 2007, 127, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.; Tinoco, R.; Hernandez, V.; Bremauntz, P.; Duhalt, R.V. Biocatalytic oxidation of fuel as an alternative to biodesulfurization. Fuel Process. Technol. 1998, 57, 101–111. [Google Scholar] [CrossRef]
- Kirimura, K.; Furuya, T.; Nishii, Y.; Ishii, Y.; Kino, K.; Usami, S. Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon-sulphur bonds by a moderately thermophilic bacterium Bacillus subtilis WU-S2B. J. Biosci. Bioeng. 2001, 91, 262–266. [Google Scholar] [CrossRef]
- Furuya, T.; Kirimura, K.; Kino, K.; Usami, S. Thermophilic biodesulfurization of dibenzothiophene and its derivatives by Mycobacterium phlei WU-F1. FEMS Microbiol. Lett. 2001, 204, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Vossoughi, M.; Kheirolomoom, A.; Tanaka, E.; Katoh, S. Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1. Biochem. Eng. J. 2001, 8, 151–156. [Google Scholar] [CrossRef]
- Oda, S.; Ohta, H. Biodesulfurization of dibenzothiophene with Rhodococcus erythropolis ATCC 53968 and its mutant in an interface bioreactor. J. Biosci. Bioeng. 2002, 94, 474–477. [Google Scholar] [CrossRef]
- Li, F.L.; Xu, P.; Ma, C.Q.; Luo, L.L.; Wang, X.S. Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B. FEMS Microbiol. Lett. 2003, 223, 301–307. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wang, M.D.; Shi, Y. Biodesulfurization of dibenzothiophene and other organic sulphur compounds by a newly isolated Microbacterium strain ZD-M2. FEMS Microbiol. Lett. 2005, 247, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kong, Y.; Yang, J.; Zhang, J.; Shi, D.; Xin, W. Biodesulfurization of dibenzothiophene by immobilized cells of Pseudomonas stutzeri UP-1. Fuel 2005, 84, 1975–1979. [Google Scholar] [CrossRef]
- Gunam, I.B.W.; Yaku, Y.; Hirano, M.; Yamamura, K.; Tomita, F.; Sone, T.; Asano, K. Biodesulfurization of Alkylated Forms of Dibenzothiophene and Benzothiophene by Sphingomonas subarctica T7b. J. Biosci. Bioeng. 2006, 101, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rashtchi, M.; Mohebali, G.H.; Akbarnejad, M.M.; Towfighi, J.; Rasekh, B.; Keytash, A. Analysis of Biodesulphurization of model oil system by the bacterium strain RIPI-22. Biochem. Eng. J. 2006, 29, 169–173. [Google Scholar] [CrossRef]
- Guobin, S.; Huaiying, Z.; Jianmin, X.; Guo, C.; Wangliang, L.; Huizhou, L. Biodesulfurization of hydrodesulfurized diesel oil with Pseudomonas delafieldii R-8 from high density culture. Biochem. Eng. J. 2006, 27, 305–309. [Google Scholar] [CrossRef]
- Grossman, M.J.; Siskin, M.; Ferrughelli, D.T.; Lee, M.K.; Senius, J.D. Method for the Removal of Organic Sulfur from Carbonaceous Materials. U.S. Patent 5910440, 8 June 1999. [Google Scholar]
- Konishi, J.; Ishi, Y.; Okumura, K.; Suzuki, M. High Temperature Desulfurization by Microorganisms. U.S. Patent 5,925,560, 20 July 1999. [Google Scholar]
- Kim, T.S.; Kim, H.Y.; Kim, B.H. Petroleum Desulfurization by DesulfovibrioDesulfuricans M6 using electrochemically supplied reducing equivalent. Biotechnol. Lett. 1990, 12, 757–760. [Google Scholar] [CrossRef]
- Borole, A.P.; Kaufman, E.N.; Grossman, M.J.; Minak-Bernero, V.; Bare, R.; Lee, M.K. Comparison of the emulsion characteristics of Rhodococcus erythropolis and Ecsherichia coli SOXC-5 cells expressing biodesulfurization genes. Biotechnol. Prog. 2002, 18, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen, J.P. Developing Clean Fuels: Novel Techniques for Desulfurization. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2005. [Google Scholar]
- Matsui, T.; Onaka, T.; Maruhashi, K.; Kurane, R. Benzothiophene desulfurization by Gordonia rubropertinctus strain T08. Appl. Microbiol. Biotechnol. 2001, 57, 212–215. [Google Scholar] [PubMed]
- Gün, G.; Yürüm, Y.; Doğanay, G.D. Revisiting the biodesulfurization capability of hyperthermophilic archaeon Sulfolobus solfataricus P2 revealed DBT consumption by the organism in an oil/water two-phase liquid system at high temperatures. Turk. J. Chem. 2015, 39, 255–266. [Google Scholar] [CrossRef]
- Monticello, D.J. Biodesulfurization and the upgrading of petroleum distillates. Curr. Opin. Biotechnol. 2000, 11, 540–546. [Google Scholar] [CrossRef]
- Yu, B.; Ma, C.; Zhou, W.; Zhu, S.; Wang, Y.; Qu, J.; Li, F.; Xu, P. Simultaneous Biodetoxification of S, N, and O Pollutants by Engineering of a Carbazole-Degrading Gene Cassette in a Recombinant Biocatalyst. Appl. Environ. Microbiol. 2006, 72, 7373–7376. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, G.; Ball, A.S. Biocatalytic desulfurization (BDS) of petrodiesel fuels. Microbiology 2008, 154, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.A.; Wirtek, C. Desulfurization and desulfonation: Application of sulphur-controlled gene expression in bacteria. Appl. Microbiol. Biotechnol. 2001, 57, 460–466. [Google Scholar] [PubMed]
- Berthou, F.; Gourmelun, Y.; Dreano, Y.; Friocourt, M.P. Application of gas chromatography on glass capillary columns to the analysis of hydrocarbon pollutants from the Amoco Cadiz oil spill. J. Chromatogr. 1981, 203, 279–292. [Google Scholar] [CrossRef]
- Yu, B.; Xu, P.; Shi, Q.; Ma, C. Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain. Appl. Environ. Microbiol. 2006, 72, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, D.K. Thermophilic desulfurization of dibenzothiophene and different petroleum oils by Klebsiella sp. 13T. Environ. Sci. Pollut. Res. Int. 2012, 19, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cai, Y.B.; Zhang, W.J.; Li, W. Methoxylation pathway in biodesulfurization of model organosulfur compounds with Mycobacterium sp. Bioresour. Technol. 2009, 100, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Pokethitiyooka, P.; Tangaromsuk, J.; Kruatrachue, M.; Kalambaheti, C.; Borole, A.P. Biological removal of organic sulphur by bacterial strains isolated in Thailand. Sci. Asia 2008, 34, 361–366. [Google Scholar] [CrossRef]
- Yamada, K.O.; Morimoto, M.; Tani, Y. Degradation of dibenzothiophene by sulfate-reducing bacteria cultured in the presence of onlynitrogen gas. J. Biosci. Bioeng. 2001, 91, 91–93. [Google Scholar] [CrossRef]
- Armstrong, S.M.; Sankey, B.; Voordouw, G. Conversion of diben-zothiophene to biphenyl by sulfate reducing bacteria isolated fromoil field production facilities. Biotechnol. Lett. 1995, 17, 1133–1136. [Google Scholar] [CrossRef]
- Kirkwood, K.M.; Ebert, S.; Foght, J.M.; Fedorak, P.M.; Gray, M.R. Bacterial biodegradation of aliphatic sulfides under aerobic carbon- or sulfur-limited growth conditions. J. Appl. Microbiol. 2005, 99, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.M.; Andersson, J.T.; Fedorak, P.M.; Foght, J.M.; Gray, M.R. Sulfur from benzothiophene and alkylbenzothiophenes supports growth of Rhodococcus sp. strain JVH1. Biodegradation 2007, 18, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.M.; Foght, J.M.; Gray, M.R. Selectivity among organic sulphur compounds in one-and two-liquid-phase cultures of Rhodococcus sp. strain JVH1. Biodegradation 2007, 18, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.K.; Chang, J.H.; Chang, Y.K.; Chang, H.N. Desulfurization of dibenzothiophene and diesel oils by a newly isolated Gordona strain, CYKS1. Appl. Environ. Microbiol. 1998, 64, 2327–2331. [Google Scholar] [PubMed]
- Chang, J.H.; Chang, Y.K.; Cho, K.S.; Chang, H.N. Desulfurization of model and diesel oils by resting cells of Gordona sp. Biotechnol. Lett. 2000, 22, 193–196. [Google Scholar] [CrossRef]
- Chang, J.H.; Kim, Y.J.; Lee, B.H.; Cho, K.S.; Rye, H.W.; Chang, Y.K.; Chang, H.N. Production of a desulfurization biocatalyst by two-stage fermentation and its application for the treatment of model and diesel oils. Biotechnol. Prog. 2001, 17, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.J.; Lee, M.K.; Prince, R.C.; Minak-Bernero, V.; George, G.N.; Pickering, I.J. Deep desulfurization of extensively hydrodesulfurized middle distillate oil by Rhodococcus sp. strain ECRD-1. Appl. Environ. Microbiol. 2001, 67, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Folsom, B.R.; Schieche, D.R.; DiGrazia, P.M.; Werner, J.; Palmer, S. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl. Environ. Microbiol. 1999, 65, 4967–4972. [Google Scholar] [PubMed]
- Ishii, Y.; Kozaki, S.; Furuya, T.; Kino, K.; Kirimura, K. Thermophilic biodesulfurization of various heterocyclic sulphur compounds and crude straight-run light gas oil fraction by a newly isolated strain Mycobacterium phlei WU-0103. Curr. Microbiol. 2005, 50, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Guobin, S.; Jianmin, X.; Huaiying, Z.; Huizhou, L. Deep desulfurization of hydrodesulfurized diesel oil by Pseudomonas delafieldii R-8. J. Chem. Technol. Biotechnol. 2005, 80, 420–424. [Google Scholar] [CrossRef]
- Kobayashi, M.; Onaka, T.; Ishii, Y.; Konishi, J.; Takaki, M.; Okada, H.; Ohta, Y.; Koizumi, K.; Suzuki, M. Desulfurization of alkylated forms of both dibenzothiophene and benzothiophene by a single bacterial strain. FEMS Microbiol. Lett. 2000, 187, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Hirata, T.; Izumi, Y. Desulfurization of dibenzothiophene derivatives by whole cells of Rhodococcus erythropolis H-2. FEMS Microbiol. Lett. 1996, 142, 65–70. [Google Scholar] [CrossRef]
- Rashidi, L.; Mohebali, G.; Towfighi darian, J.; Rasekh, B. Biodesulfurization of dibenzothiophene and its alkylated derivatives through the sulphur-specific pathway by the bacterium RIPI-S81. Afr. J. Biotechnol. 2006, 5, 351–356. [Google Scholar]
- Okada, H.; Nomura, N.; Nakahara, T.; Maruhaski, K. Analysis of dibenzothiophene metabolic pathway in Mycobacterium sp. G3. J. Biosci. Bioeng. 2002, 93, 491–497. [Google Scholar] [CrossRef]
- Lee, M.K.; Senius, J.D.; Grossman, M.J. Sulfur-specific microbial desulfurization of sterically hindered analogs of dibenzothiophene. Appl. Environ. Microbiol. 1995, 61, 4362–4366. [Google Scholar] [PubMed]
- Paixão, S.M.; Silva, T.P.; Arez, B.F.; Alves, K. Advances in the Reduction of the Costs Inherent to Fossil Fuels Biodesulfurization towards its Potential Industrial Application. In Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering; IGI Global: Hershey, PA, USA, 2016; Chapter 13; pp. 390–425. [Google Scholar]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Alves, L.; Paixão, S.M. Enhancement of Dibenzothiophene Desulfurization by Gordonia alkanivorans Strain 1B Using Sugar Beet Molasses as Alternative Carbon Source. Appl. Biochem. Biotechnol. 2014, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Lanzarini, G.; Pifferi, P.G. Immobilized cells for applications in non-conventional systems. In Progress in Biotechnology. Immobilized Cells: Basics and Applications; Wijffels, R.H., Buitelaar, R.M., Bucke, C., Tramper, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 11, pp. 777–784. [Google Scholar]
- Guobin, S.; Huaiying, Z.; Weiquan, C.; Jianmin, X.; Huizhou, L. Improvement of Biodesulfurization Rate by Assembling Nanosorbents on the Surfaces of Microbial Cells. Biophys. J. 2005, 89, L58–L60. [Google Scholar] [CrossRef] [PubMed]
| Policy Type | World-Class Emission Standard |
|---|---|
| Green Freight |
|
| Clean, low-sulphur fuel |
|
| Fuel economy and CO2 standards |
|
| Tailpipe emissions Standards |
|
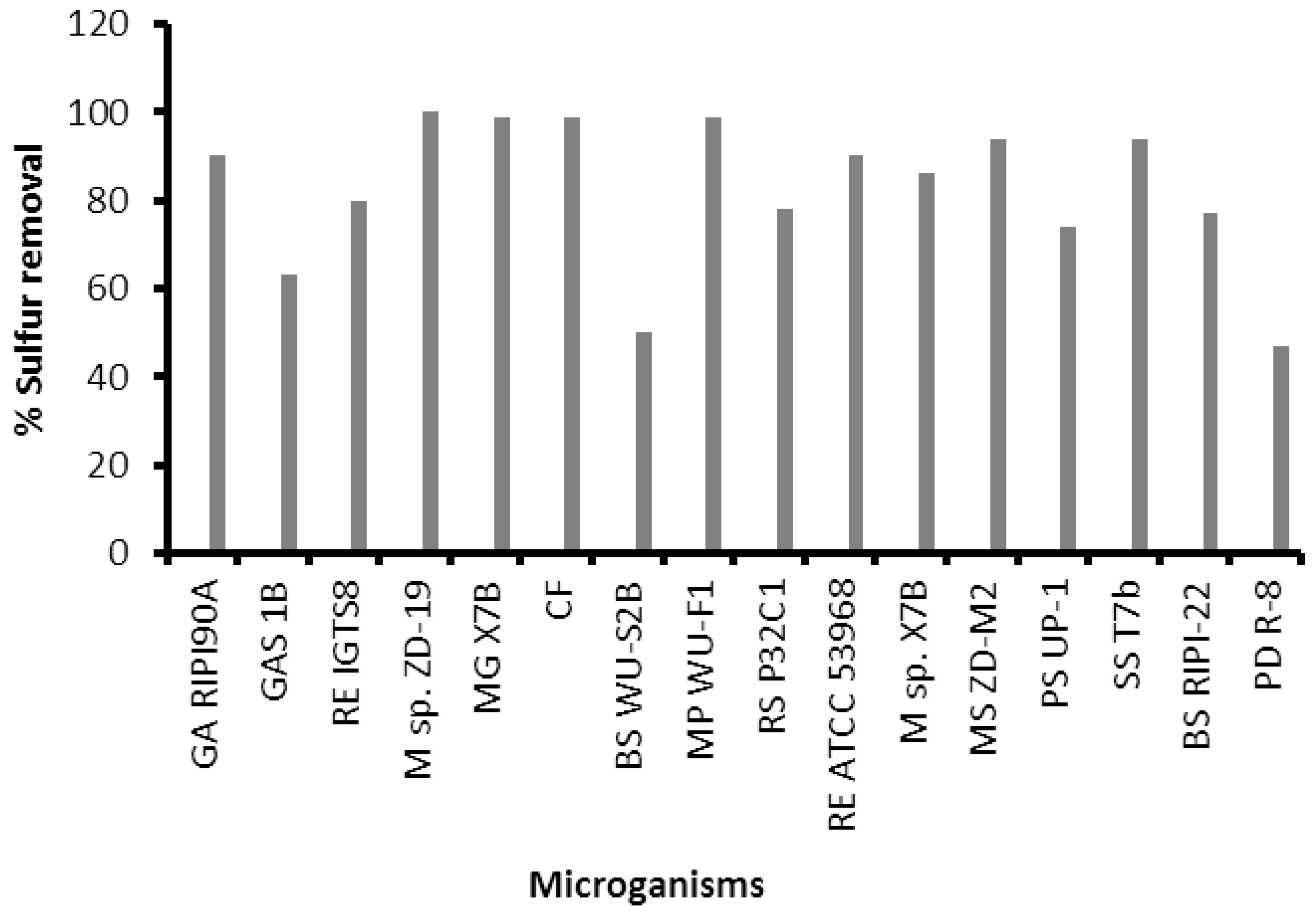
| Bacteria | Optimum Conditions | References | |
|---|---|---|---|
| Sulfur Conc. (ppm) | Temperatures (oC) | ||
| GAR 190A | 100 | 30 | [124] |
| GAS 1B | 100 | 35 | [125] |
| RE IGTS8 | 100 | 30 | [126] |
| M sp ZD-19 | 92 | 30 | [127] |
| MG X7B | 200 | 40 | [128] |
| CF | 1600 | 25 | [129] |
| BS WU-S2B | 100 | 50 | [130] |
| MP WU-F1 | 150 | [131] | |
| RS P32C1 | 1000 | 30 | [132] |
| RE ATCC 53968 | 184 | 30 | [133] |
| M sp. X7B | 535 | 45 | [134] |
| MS ZD-M2 | 36 | 30 | [135] |
| PS UP-1 | 500 | 31 | [136] |
| SS T7b | 280 | 27 | [137] |
| BS RIPI-22 | 100 | 30 | [138] |
| PD R-8 | 591 | 30 | [139] |
| Culture | Oil Fraction | Degree of Desulphurization % | Reference |
|---|---|---|---|
| Gordonia sp. P32C1 (resting cells) | Light diesel fuel after hydrodesulfurization (303 ppm) | 48.5 | [132] |
| Mycobacterium sp. X7B (resting cells) | Diesel fuel after hydrodesulfurization (535 ppm) | 86 | [134] |
| Pseudomonas delafieldii R_8 (growing cells) | Diesel fuel after hydrodesulfurization (591 ppm) | 47 | [139] |
| Gordonia sp. CYKS1 (resting cells) | Middle distillate unit feed (1500 ppm) | 70 | [161] |
| Light gasoil (3000 ppm) | 50 | ||
| Gordonia sp. SYKS1 (resting cells) | Light gasoil (3000 ppm) not subjected to hydrodesulphurization | 35 | [162] |
| Middle distillate unit feed (1500 ppm) | 60 | ||
| Gordonia sp. SYKS1 (resting cells) | Diesel fuel (250 ppm) | 76 | [163] |
| Rhodococcus sp. ECRD_1 (growing cells) | Medium fraction of light gasoil after catalytic cracking (669 ppm) | 92 | [164] |
| Rhodococcus erythropolis I_19 (resting cells) | Medium distillate after hydrodesulfurization (1850 ppm) | 67 | [165] |
| Mycobacterium phlei WU_0103 (growing cells) | Straight-run light gasoil diluted 12 times (1000 ppm) | 52 | [166] |
| Pseudomonas delafieldii R_8 (resting cells) | Diesel fuel after hydrodesulfurization (591 ppm) | 90.5 | [167] |
| Bacterium | Sulphur Substrate(s) | End Product(s) | References |
|---|---|---|---|
| Rhodococcus erythropolis KA2-5-1 | Alkylated DBTs (C2-DBTs and C3-DBTs) | Corresponding hydroxylated Biphenyls | [168] |
| Rhodococcus erythropolis H-2 | 2,8-DMDBT, 4,6-DMDBT | Corresponding hydroxylated biphenyl | [169] |
| 3,4-Benzo-DBT | a-Hydroxy-b-phenylnaphthalene | ||
| Bacterial strain RIPI-S81 | 4-MDBT | 2-Hydroxy-3-methylbiphenyl, | [170] |
| 2-hydroxy-3-methylbiphenyl | |||
| 4,6-DMDBT | 2-Hydroxydimethylbiphenyl | ||
| Mycobacterium sp. G3 | 4,6-Dibutyl DBT, 4,6-dipentyl DBT; 4,6-DMDBT, 4,6-DEDBT | C–S bond cleavage products | [171] |
| Paenibacillus sp. A11-2 | Methyl, ethyl, dimethyl, trimethyl and | Corresponding hydroxylated | [120] |
| propyl DBTs | Biphenyls | [141] | |
| Rhodococcus sp. ECRD-1 | 4,6-DEDBT | Hydroxydimethylbiphenyl | [172] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges. Environments 2017, 4, 85. https://doi.org/10.3390/environments4040085
Sadare OO, Obazu F, Daramola MO. Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges. Environments. 2017; 4(4):85. https://doi.org/10.3390/environments4040085
Chicago/Turabian StyleSadare, Olawumi O., Franklin Obazu, and Michael Olawale Daramola. 2017. "Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges" Environments 4, no. 4: 85. https://doi.org/10.3390/environments4040085





