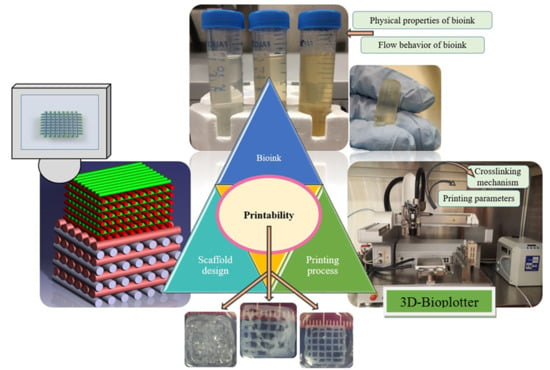
1. Introduction
Extrusion-based bioprinting is an additive manufacturing (AM) technique used for various tissue engineering applications (
Figure 1) [
1]. Many studies have been carried out to create hydrogel scaffolds using this technique [
2,
3]. Generally speaking, computer-aided design (CAD) is used to deposit biomaterials [
4]. However, scaffolds are rarely fabricated exactly according to the CAD model. That is why the printability index is important as an element showing the difference between the scaffold design (typically in a CAD model) and the printed scaffold. Three dimensional (3D) printability of a hydrogel biomaterial is defined as the ability of a hydrogel to form and maintain a reproducible 3D structure with dimensional integrity. Although the range of accuracy for extrusion-based machines is in the order of a micron, there is still a challenge when it comes to shaping the fidelity and printability of scaffolds bio-fabricated using the extrusion-based technique.
The printability can influence other interrelated factors, such as the morphology and mechanical properties of scaffolds. Consequently, it can affect cell response [
5], and it is well-accepted that the mechanical properties of scaffolds can influence cell faith [
6]. Hence, it is important to study elements that can influence printability. Although there are a few studies on the printability of different biomaterials, the real picture and definition of printability remain unclear and there are fundamental questions about how to map the relationships between printability and other interrelated factors such as biomaterial and fabrication. For example, in some studies, the flow behavior of biomaterials was considered to evaluate printability [
7,
8]. In these studies, only the physical and rheological characteristics of materials were investigated [
9]. In another study, the influence of ionic crosslinkers on printability was investigated without considering other factors [
10]. In some studies, only printing parameters were investigated as critical factors influencing printability [
2,
11]. In another study, the gelation properties of materials during the printing process were studied to achieve a mechanically stable structure [
12]. Murphy et al. studied gelation time, swelling, and the printability of various groups of hydrogels [
13]. In another study, analytical methods were implemented to check the printability of materials [
14]. Kyle et al. reported that printability is a matter of rheology, biomaterial composition, nozzle variables, pore and filament dimensions, geometry, and printing angle [
15]. Hence, considering only one of the factors is not a systematic approach to improving printability. As mentioned, different studies have specifically investigated the effect of some factors on printability. However, there is no clear picture of printability considering the interrelated factors influencing printability. In this study, rheological properties, printing parameters, and printing conditions were investigated systematically to map the relationship between these parameters and printability, rather than considering each factor individually. As such, more studies should be performed in this area to define and establish novel approaches to define and measure printability. The key question of this study related to how to measure printability [
15].
Alginate is one of the hydrogels used for the biofabrication of scaffolds used for tissue engineering applications, as reported in numerous studies [
16,
17,
18,
19,
20,
21,
22,
23]. Specifically, one of the approaches to improve the printability of alginate scaffolds fabricated by extrusion-based bioprinting method is to mix alginate with other types of hydrogels [
24]. Gelatin is one of the hydrogels usually mixed with alginate. Gelatin is a natural polymer derived from collagen and it has a cell-friendly environment and this is one of the reasons for mixing alginate with this biomaterial [
12]. Methylcellulose (MC) is another biocompatible hydrocarbon polymer commonly used in scaffold fabrication due to its high hydrophilicity and water absorption, essential for nutrient delivery to the cells [
25]. Hydrogels composed of multiple biomaterials have also been used in scaffold construction. For example, one study analyzed the properties of cell substrates composed of a scaffold containing both gelatin and alginate, and they found these scaffolds to have high water retention rates [
26]. The result suggests that combining different biomaterials may be a way to manipulate the scaffold characteristics and allow for better control in achieving desired scaffold functions.
This study aims to present a clear picture of printability, to identify factors that can affect it, and to propose methods to measure 3D printability of hydrogel scaffolds with an alginate matrix. There are some studies on the effect of the flow behavior of biomaterials [
27], ink consistency [
7], and hydrogel mechanical characteristics [
9] on printability. Nevertheless, little attention has been paid to the effect of printing parameters of scaffolds made from a mixture of hydrogels [
2]. Here, the effect of hydrogel composition (alginate, alginate-gelatin, alginate-gelatin-MC, and alginate-MC) on the swelling, mechanical, and degradation properties was tested over time. Then, a systematic study was implemented by characterizing the biomaterial flow behavior, as well as the 2D and 3D printability of hydrogels with different compositions. Finally, a linear regression model was developed to map the relationships between various biomaterial-related and fabricated-related elements affecting printability.
2. Materials and Methods
2.1. Preparation of Hydrogels
Medium viscosity sodium alginate from brown algae (Sigma-Aldrich Canada Ltd., P-code 1001172534, with a molecular weight of 80,000–120,000 g/mol), was used for the preparation of a 3% w/v alginate (Group 1) using distilled water. Gelatin from porcine skin, Type A, Bioreagent, (Sigma-Aldrich Canada Ltd.) was used for the preparation of a 2% w/v alginate and 1% w/v gelatin solution (Group 2). MC, phosphate buffer saline, and calcium chloride were obtained from Sigma-Aldrich Canada (Oakville, ON, Canada). 1.5% w/v alginate, 1% w/v gelatin, and 0.5% w/v MC were mixed together as Group 3. Group 4 consisted of 1.5% w/v alginate and 1.5% w/v MC solution. For bulk gel experiments, the hydrogels were pipetted into molds to a height of 4 mm and incubated with 50 mM calcium chloride at room temperature for cross-linking.
For ease of discussion, the different groups were named herein. Group 1 referred to 3% (w/v) alginate while Group 2 referred to 2% (w/v) alginate and 1% (w/v) gelatin. The third group included 1.5% (w/v) alginate, 1% (w/v) gelatin, and 0.5% (w/v) MC (Group 3). The last group included the 1.5% (w/v) alginate and 1.5% (w/v) methylcellulose (Group 4). To have a uniform solution for printing, we stirred the prepared solutions, centrifuged them, and then kept them in a refrigerator to get rid of bubbles during the preparation procedure to ensure complete hydration. In addition, the solutions were kept in the nozzle for 20 min for a uniform solution with a stable temperature in the printing head before starting the printing process.
2.2. Scaffold Fabrication
A 3D Bioplotter (EnvisionTEC GmbH, Gladbeck, Germany) was used to print scaffolds of 11 × 11 × 11 mm. All groups of hydrogels were deposited using a 200 µm needle inner diameter. Magics13 EnvisionTEC software and Bioplotter RP software were used for the CAD model generation and slicing, respectively. Scaffolds were fabricated layer-by-layer, while hydrogel filaments were deposited into a calcium chloride (CaCl2) bath in a 12-well plate. The filament width, pore sizes, pore area, and the perimeter of the scaffolds were measured using ImageJ® software (National Institute of Health, Gaithersburg, MD, USA). To check the uniformity of the fabricated scaffolds, at least, 3 scaffolds were printed and evaluated in terms of pore size and strand diameter.
2.3. Testing Hydrogel Construct Swelling Properties
The initial weights of the hydrogel scaffolds were measured after removing them from the crosslinker solution, and the scaffolds were then incubated in 10 mM PBS solution at 37 °C and 5% carbon dioxide. The weights of the samples were measured again after 1 h, 3 h, 12 h, 3 days, 7 days, and 14 days, for any change in mass due to swelling. A Kimwipe was used to eliminate excess or free liquid from the scaffold before weighing each sample. The swelling of the composite scaffolds was calculated using the following equation (Equation (1)):
where
Wt is the hydrogel weight at the specific time, and
W0 is the hydrogel weight at time 0 h.
2.4. Testing the Compressive Strength of the Hydrogel Constructs
The hydrogel scaffolds were tested for compression strength using a compressive testing instrument from BOSE (load cell capacity: 20 Newtons). This device measured the forces required to compress a sample to a series of displacements until a maximum displacement of 2 mm was reached. The area and height of the scaffolds were measured using ImageJ® software before mechanical testing, and the resulting data were used to plot the stress-strain curves for each construct. The elastic modulus was determined by finding the slope of the linear portion of the stress-strain curve.
2.5. Testing Hydrogel Construct Degradation Properties
Scaffolds were freeze-dried and then weighed to determine their initial masses. To obtain the degraded scaffolds, we incubated the samples in 10 mM PBS solution at 37 °C and 5% carbon dioxide for 7, 14, 21, and 28 days. The PBS solution was taken out of the samples and the samples were freeze-dried and weighed again using a digital scale. The hydrogel degradation was calculated using the following equation:
where
WFDt is the freeze-dried hydrogel weight at a given time, and
WFD0 is the freeze-dried hydrogel weight at the time 0.
2.6. Flow Behavior Tests
The flow behaviors of groups 1 to 4 were investigated at 37 °C. A Brookfield Ultra III Rheometer with the CP-41 spindle was used for the testing. The shear rate, shear stress, viscosity, and percentage of torque have been measured at various rotational speeds.
2.7. Printability Studies on Printing Parameters and Condition
Two dimensional (2D) studies were performed to check the printability of the scaffolds by printing lines (scaffolds with two layers). Likewise, follow-up studies were carried out to check the 3D printability of different groups by printing 3D scaffolds. In the flowing subsections, the experimental design on how to evaluate the effect of air pressure, nozzle speed, offset, and pattern selection on printability is discussed.
2.7.1. Air Pressure
For the set of either 2D or 3D studies, air pressure (0.1 to 0.8 bar) and temperature (37, 45, and 55 °C) were subjected to changes while other printing parameters such as nozzle speed and temperature were maintained constant.
2.7.2. Nozzle Speed
For the second set of experiments, nozzle speed was changed, starting from 4 mm/s for several pressures (0.1 to 0.4 bar). For this, the scaffolds crosslinked mechanically and chemically using a cold bed and CaCl2, respectively (except for Group 1, crosslinked chemically). For groups crosslinked physically, the printing temperature was kept at 37 °C while the printing bed temperature was 10 °C (pressure: 0.1–0.5 bar, minimum nozzle speed of 10 mm/s). For all groups crosslinked chemically, the minimum nozzle speed was 4 mm/s (pressure: 0.1 to 0.4 bar) and Group 1 was printed at 24 °C while other groups were printed at 37 °C.
2.7.3. Offset
For the third set of experiments, offset was the variable (−0.08 to 0.08 mm), and the selected temperature was the same as the one for the previous set of experiments (negative values were as per calibration and did not mean any substrate scratching). The offset is the distance from the nozzle to the build platform (
Figure 1). It was analyzed to determine the influence of the offset on the line width. The nozzle speed was maintained at 35 mm/s (pressure: 0.1 and 0.2 bar).
2.7.4. Angular Pattern Printing
In another part of the printability investigation, scaffolds with various angular patterns were printed and evaluated from a printability perspective. For this, scaffolds with angular patterns of 0–25°, 0–45°, and 0–90° were printed. Pressure and nozzle speed were maintained between 0.1 and 0.2 bar and 35 to 40 mm/s, respectively. The temperature was maintained as per
Section 2.7.2.
2.8. Printability Evaluation
In this study, different evaluations were performed to show methods of how to measure printability. For this, firstly, a standard diameter of strands (
Ds) was calculated and compared with the experimental strand diameter. For this, the following equations were used:
where
,
Q, and
Ds are density, flow rate, and standard strand diameter, respectively. Here, different solutions for groups 1 to 4 were purged for a limited time, and then purged materials were weighted using the Sartorius Scale (model 225d). From Equation (1), the volume can be calculated and then using Equations (2) and (3), the flow rate and standard strand diameter can be calculated, respectively, for different nozzle speeds. For this evaluation, the pressure was maintained between 0.2 to 0.4 bar and the temperature was the same as the one reported in
Section 2.7.2. Based on Equation (3), strand printability was defined as:
where
Dexp. is experimental strand diameter. In another evaluation, pore printability was checked as described in Reference [
8]. The following equation was used for this purpose.
where
and
are the area and perimeter of a pore of a scaffold. In the last evaluation, pore irregularity was defined as:
where
Ix,y is the irregularity of the geometry of scaffolds in different directions of X and Y.
(x,y)th shows the ideal length of a scaffold in the X and Y directions as per CAD design, while
(x,y)exp. represents the experimental lengths in these directions.
2.9. Statistical Significance
Statistical significance was calculated by performing a student’s t-test. For each set of experiments, three replicas were considered and data were presented as a mean ± standard deviation. Significant differences were shown with p-values of p < 0.05 and p < 0.01. Using Minitab® 17.1 software, a linear regression model was developed with two-sided intervals of confidence with 95% value.
4. Discussion
Different methods of scaffold design can be used to manipulate the mechanical properties of hydrogel scaffolds to achieve properties that are best suited for cell support. Various studies have shown that the mechanical properties of scaffolds must be carefully controlled to successfully simulate the extracellular matrix that supports cells in human tissue. This is because there is a dynamic relationship between cell growth and viability and the extracellular matrix [
25,
31,
32,
33,
34]. These experiments have shown that the water retention rate, elasticity, and degradation rate of a hydrogel construct can, to some extent, be controlled by changing the material composition of the scaffold. In this study, hydrogels containing alginate-gelatin showed a higher water retention capacity than the pure alginate gels. One possible explanation for this observation was that pure alginate molecules experienced stronger intermolecular forces with one another compared to when they were part of hybrid hydrogels. Adding gelatin or MC may interfere with the intermolecular forces between adjacent alginate molecules, and as a result, the attraction between the alginate molecules and surrounding water molecules may be stronger than in pure alginate hydrogels. This leads to greater water absorption. Furthermore, the melting point of gelatin is about 35 °C [
35]; therefore, at 37 °C gelatin would be in liquid form, and as a result, there would be gaps in the scaffold which would be replaced by the surrounding water molecules. This result would lead to a higher absorption rate when compared to a pure alginate hydrogel sample. This high water retention capacity allows cells to readily exchange important molecules such as ions and signaling molecules with their environment [
25].
Additionally, hybrid hydrogels containing gelatin had a lower elastic modulus compared to alginate-MC hybrid hydrogels, possibly because of the degradation of gelatin at physiological temperatures. The decomposition of gelatin due to its melting point of around 35 °C would cause the formation of gaps in the construct, and this could compromise its mechanical stability and result in a lower elastic modulus. These results indicated that hydrogels could be constructed with different materials depending on the degree of stiffness necessary for the tissue type that requires regeneration. This outcome allows for better specificity and control in scaffold design and construction. Notably, in future studies, water syneresis can be evaluated to consider the effect of shrinkage.
Group 3 showed the highest percentage of degradation in comparison to the other hydrogels. MC is soluble in water at temperatures lower than 40–50 °C, as mentioned earlier, and it has a melting point of about 35 °C [
35,
36]. The weakened intermolecular interactions of MC and gelatin at physiological temperatures would, therefore, lead to a high rate of degradation of this gel over time. Here, we proposed some compositions with various mechanical and swelling/degradation rates.
Our results also showed different flow behaviors from Newtonian to non-Newtonian ones. Group 1, having the highest viscosity, showed good printability discussed later on. It meant that the more viscous the biomaterial, the more appropriate printability could be achieved. However, high viscous biomaterials may not printable. Referring back to the mentioned general rule, we recommend an appropriate range of viscosity from 300 to 30,000 cps (0.3–30 PaS). Group 4 was not in the recommended range, which agreed with our printability results so that a poor printability was observed for this group. Notably, as we use lower viscous biomaterials; likewise, higher speeds should be implemented. However, it may cause difficulties in printing, such as sudden direction changes in the edges of scaffolds.
From the air pressure perspective, Group 1 behaved like a highly viscous biomaterial. However, it is recommended to mix alginate with other biomaterials to achieve synergistic properties. However, adding gelatin or MC to alginate reduced the viscosity of the final solutions, as discussed previously on the variation of viscosity observed in different groups. Therefore, compared to Group 1, for other groups, a lower amount of pressure is required to dominate the surface tension of biomaterials. Studying the effect of pressure on
Ds can clarify an appropriate range of pressure that is suitable for printing. Owing to several groups with different viscosities, pressures between 0.1 to 0.2 showed good results, and at either lower or higher pressures, the biomaterials were not printable or had an unstable printing condition due to the application of high pressure. Referring to the changes in viscosity among the different groups, the lower viscous groups were printable at relatively higher nozzle speeds. It meant that due to their relatively lower viscosities (e.g., Groups 3 and 4), the biomaterials flowed easily and at the same pressure required to increase the nozzle speed to prevent extra deposition of biomaterials. As mentioned earlier, Group 1 was printable at speeds around 10 mm/s while the starting point of speed for groups 3 and 4 was more than 18 mm/s (
Figure 6).
From a 2D strand and pore printability point of view, Group 1 had acceptable strand printability for speeds ranging from 4 to 8 mm/s, whereas pore printability showed acceptable results for speeds between 4 and 14 mm/s. However, Group 2 did not have acceptable strand printability for speeds less than 8 mm/s. This result might be due to a lower viscosity compared to Group 1, and so higher speeds were required to reach acceptable printability. In addition, speeds higher than 14 mm/s showed poor strand printability for Group 2 whereas, for all speeds in between 6 and 22 mm/s, the pore printability was acceptable. It meant that using some speeds; we may achieve acceptable pore printability while strand printability may not be acceptable. Hence, both pore and strand printability should be considered together to find a suitable nozzle printing speed rather than considering either pore or strand printability separately. It should be noted that the criteria used only accounted for pore shape. In this case, it meant that for the cited parameters, the scaffolds still presented perfectly square pores but with different sizes from the design. For Group 3, speeds more than 30 mm/s showed an appropriate range of pore and strand printability. For the last group, all speeds between 18 and 24 mm/s showed acceptable printability.
Using an offset of −0.02 to 0.08 mm, we observed strand diameter between 200 and 300 µm for groups 1 and 4. At the offset less than −0.02 mm, a significant change in strand diameter was observed. For Group 3, strand diameters of more than 300 µm were observed and this may have been due to having low viscosity resulting in quick biomaterial flow leading to a relatively large strand diameter. Group 4 showed a decrease in strand diameter by increasing the offset. The offset should be selected carefully because having a large space between the printing bed and needle leads to non-continuous printing. Meanwhile, having a small offset may lead to squeezing of the biomaterial and prevention of the proper flow of biomaterial during the deposition.
Regarding angle printing, at acute angles, angle printability was acceptable while, for example, at 90, poor printability was observed in terms of angle. These results were interpreted as meaning that changing the needle direction by having a sharp angle of more than 90 may lead to poor angle printability due to a sudden change of the direction of the nozzle. Such a change in direction may cause stretching of the strands and results in modulating the strand diameter.
In agreement with the 2D printability results, 3D printability studies showed that all printed scaffolds had acceptable pore printability while strand printability was not acceptable (most values were more than 1 ± 0.1). As mentioned, the criteria used only accounted for pore shape. Hence, both pore and strand printability should be considered. Surprisingly, strand printability has been neglected in the literature and our results showed that while having acceptable pore printability, scaffolds can also have poor strand printability. One possible solution to address poor strand printability is to modulate the nozzle speed at constant pressures to get closer to the theoretical values. However, using such an approach, other interrelated factors, such as poor printability, can be affected. Except for Group 4, all the groups showed less than 10 percent irregularity in the
X and
Y directions. For Groups 1 and 2, scaffolds with 15 layers were printed but Groups 3 and 4 showed poor printability for scaffolds made from more than five layers. Referring back to
Section 3.2, Groups 3 and 4 with relatively lower viscosities showed poor printability while other groups showed better printability. The viscosity of biomaterials can significantly influence the printability of the bioplotted scaffolds, such that high viscosity biomaterials need higher pressure and low viscous materials require less pressure to be extruded.
Overall, many factors are causing a deviancy between experimental and theoretical values, including pressure, nozzle speed, and offset. These are interrelated elements from a printability perspective and, thus, modulating one of them can affect the other elements. That said, all elements should be selected carefully to avoid, on the one hand, any strand coiling owing to over-extrusion of an extruded strand, and on the other hand, using a low pressure that cannot dominate the surface tension of the biomaterial. To create a clear picture of printability and significantly effective elements, we developed the following linear regression models (R
2 more than 85%) to map the relationship between the studied parameters including nozzle speed, pore, and strand printability for 2D printing (
Table 4), based on the results presented in
Table 2.
It is worthwhile to cite the fact that a high concentration of alginate is not an appropriate environment for cells [
37]. High concentrations of alginate can interrupt diffusion mass transfer mechanisms and lead to low cell viability. Inhabitation of cell migration/proliferation is another reason to avoid high concentration alginate. That being said, the present study showed that we could mix other cell-friendly hydrogels such as gelatin to have a low concentration of alginate along with improving the printability of such low concentration alginate.
It should be noted that this study investigated factors that affected printability. However, it should be noted that cell viability is a vital factor to be considered alongside other mentioned factors. It means that factors affecting printability such as pressure can directly affect cell viability and that is why it is important to consider cell viability. Readers are encouraged to check a recent study on printability and cell viability for more information [
29].
5. Conclusions
Hydrogels are valuable concerning their ability to serve as an appropriate environment for cells due to their ease of preparation and similarity to the extracellular matrix of many human tissues. The extrusion-based bioprinting method is used widely to create hydrogel scaffolds for different tissue engineering applications. In this regard, it is recommended to mix hydrogels to achieve synergistic properties. Here, we examined the swelling, as well as degradation, rate, and mechanical properties (elastic moduli) of hydrogels with various compositions of alginate, gelatin, and MC. The results showed that composite hydrogels had better water absorption ability compared to a pure alginate hydrogel. Additionally, all combinations of hydrogels showed a decreasing pattern of elastic modulus with time, while alginate-MC combination gels showed the highest elastic moduli. After evaluating scaffolds from the mechanical perspective, more experiments were conducted to investigate the hydrogel printability. The results showed that biomaterial-related elements, e.g., viscosity, and fabrication-related ones, e.g., air pressure, nozzle speed, offset, and selected angular pattern, could influence the printing quality. Modulating these parameters, we improved the printability of different groups of hydrogels, including alginate, gelatin, and MC. Conducting research studies on printability can open the door for further improvements in the fabrication of hydrogel scaffolds using the extrusion-based technique. To conclude, taking biomaterial- and fabrication-related elements, printability can be improved and accordingly, scaffolds can be specialized depending on which tissue requires regenerative tissue therapy.