Structural Colors Based on Amorphous Arrays Comprised Solely of Silica Particles
Abstract
:1. Introduction
2. Experimental Setup
2.1. Measurements and Materials
2.2. Synthesis of Spherical Silica Nanoparticles
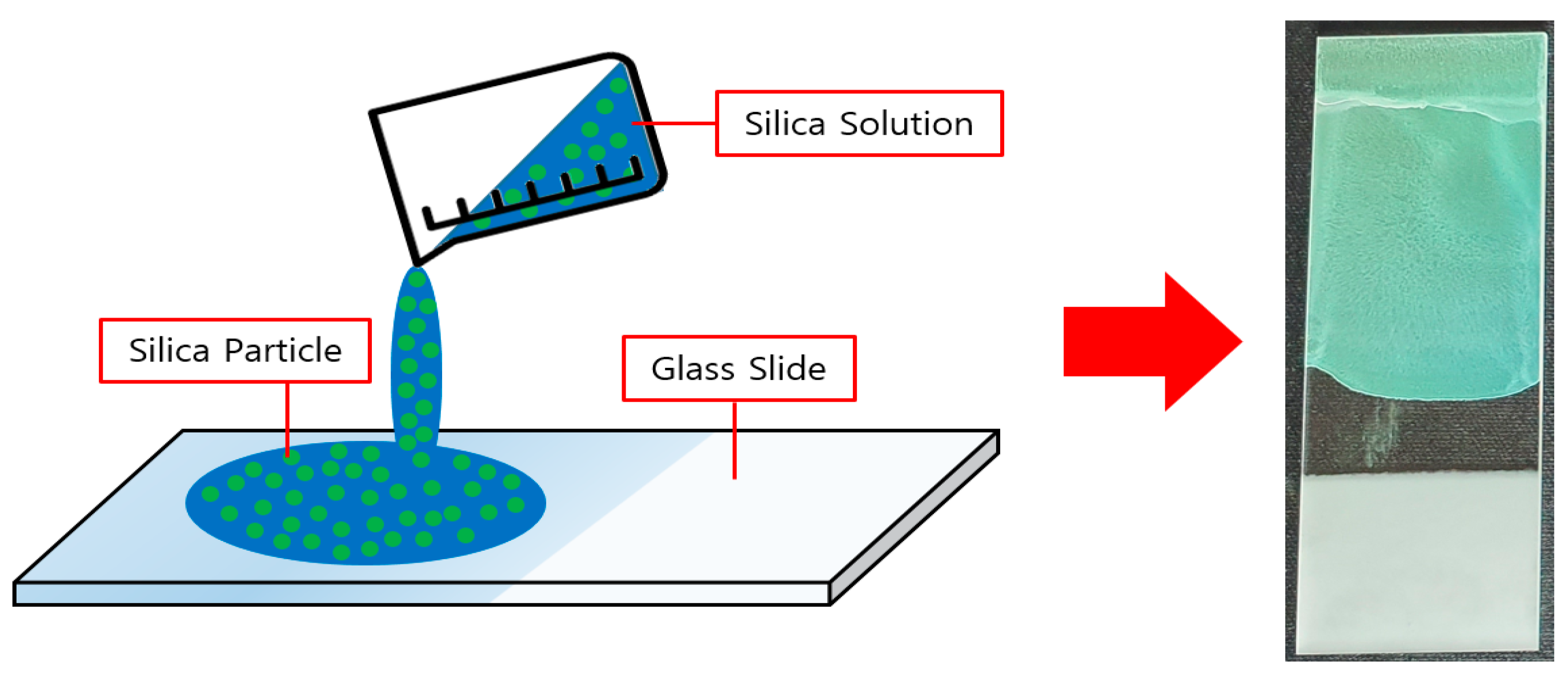
2.3. Coating and Synthesis of Silica Solutions
3. Experimental Results and Discussion
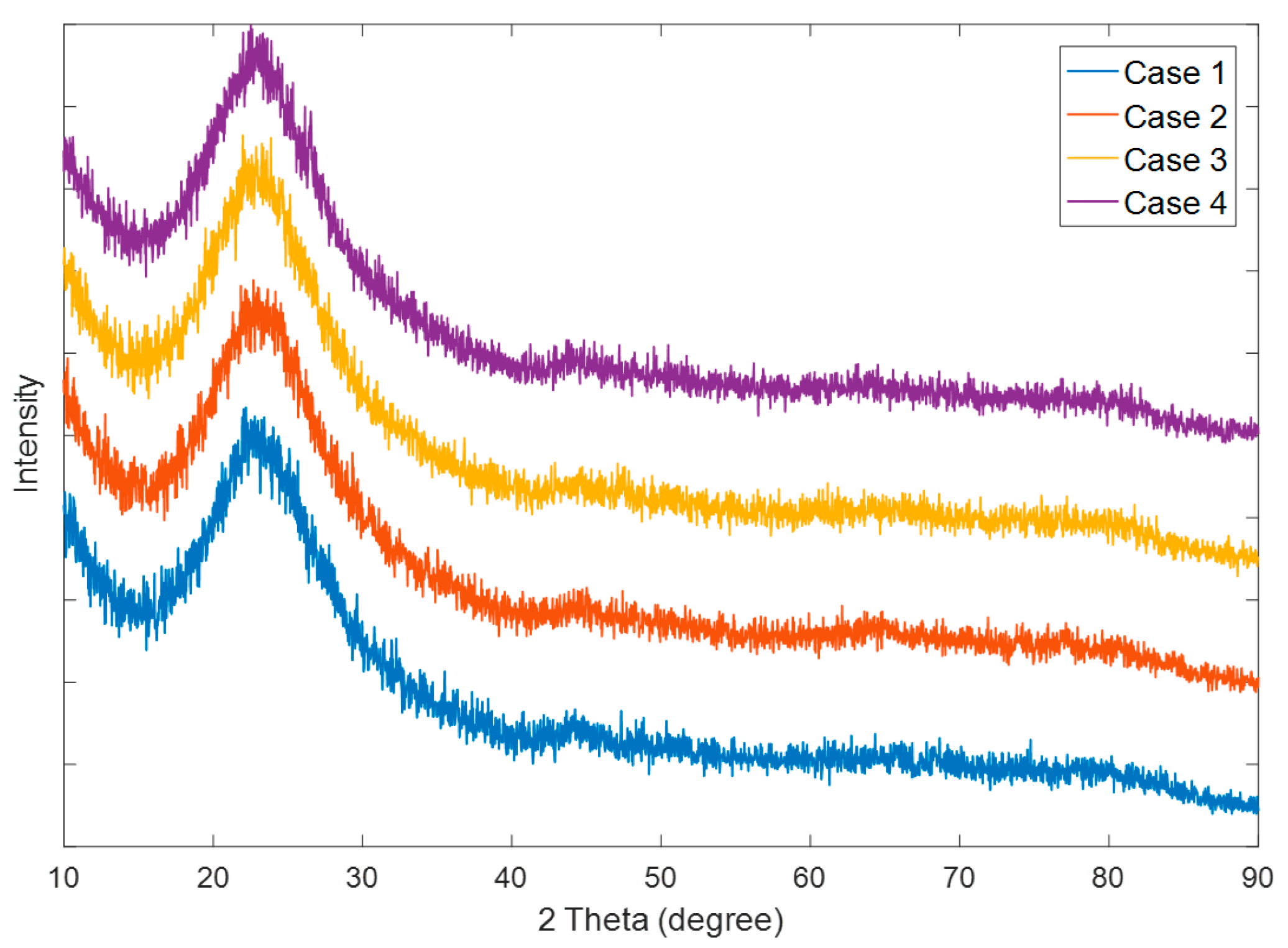
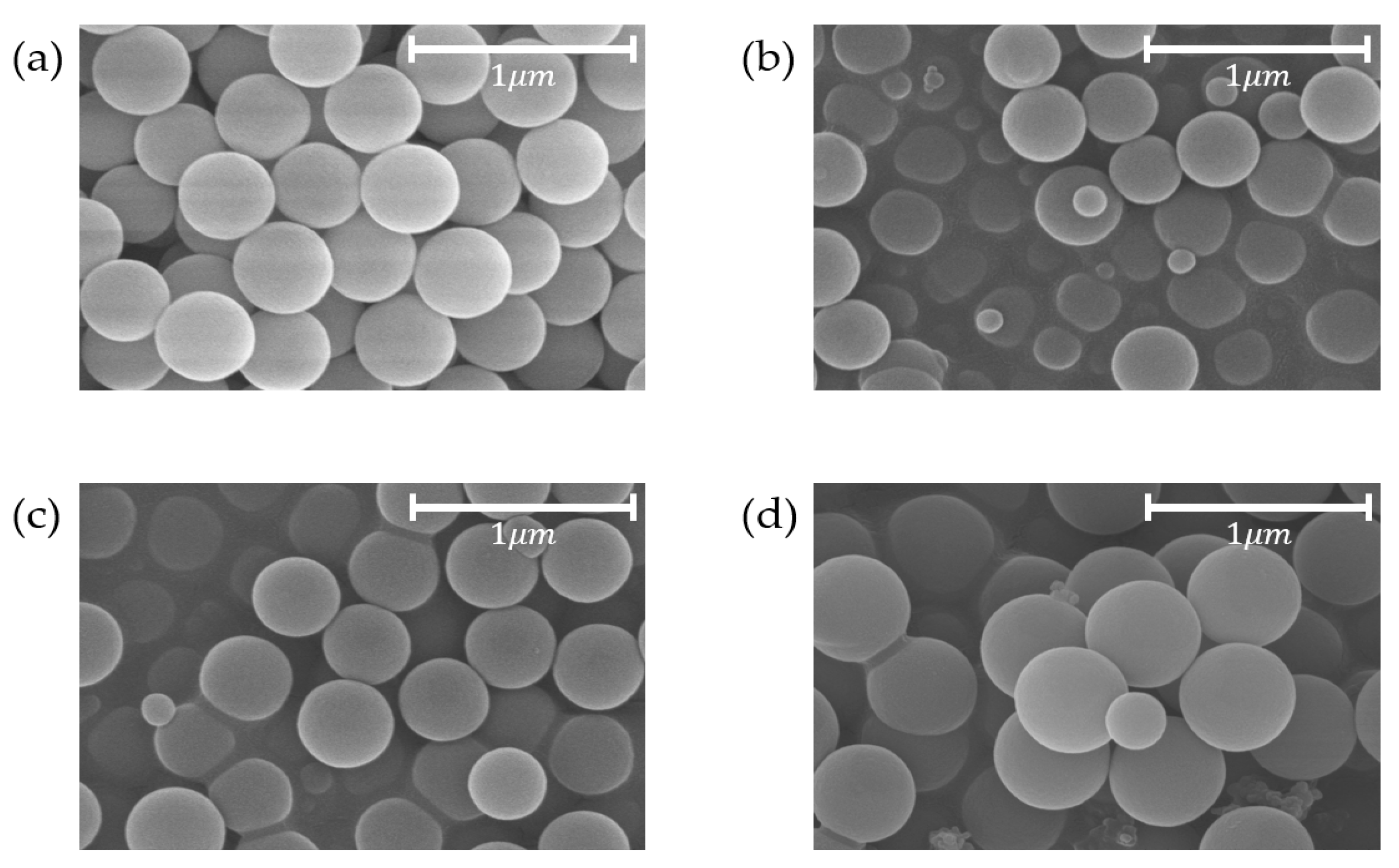
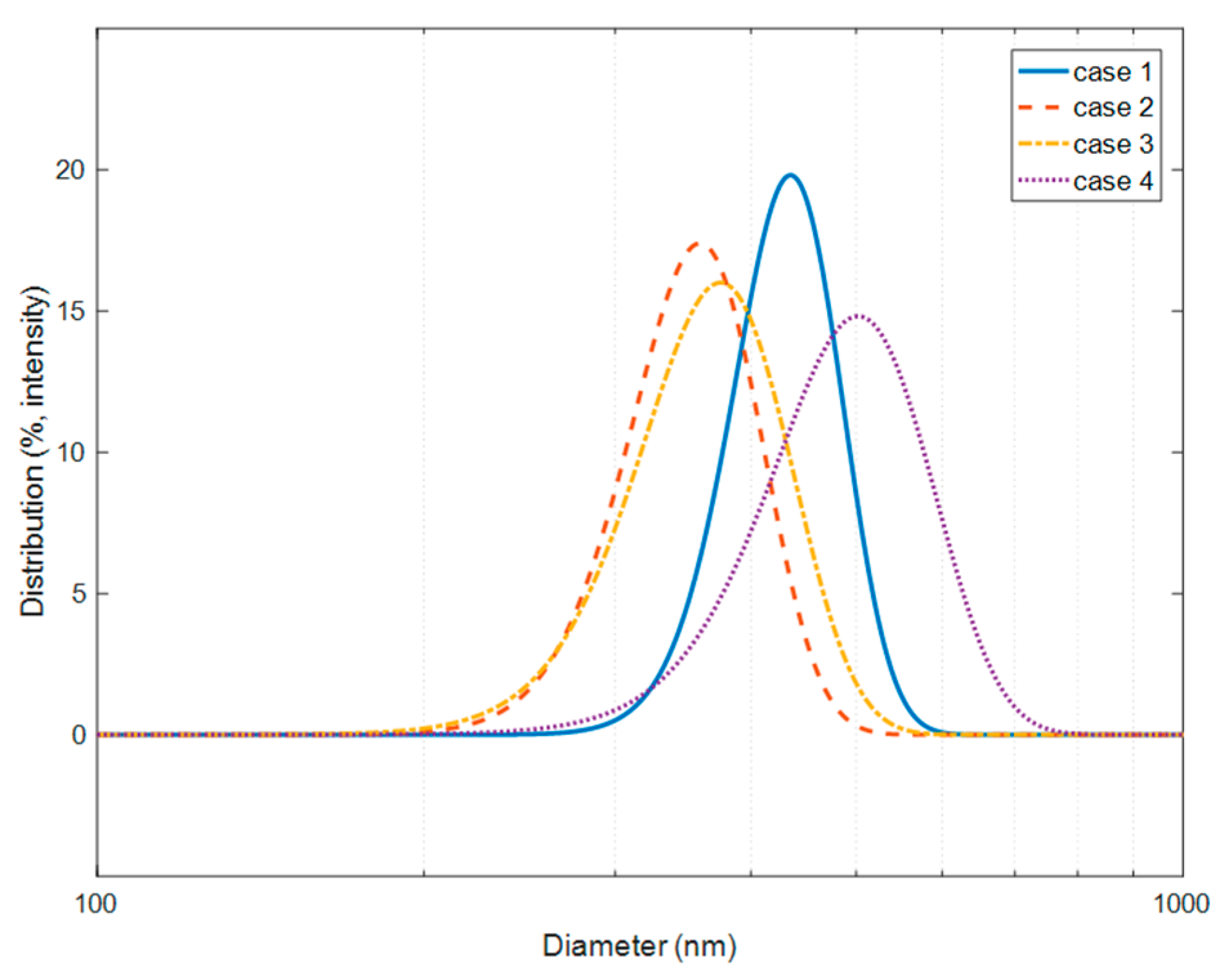
3.1. Spherical Silica Nanoparticles
3.2. Coating of Silica Solutions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kinoshita, S.; Yoshioka, S. Structural colors in nature: The role of regularity and irregularity in the structure. Chem. Phys. Chem. 2005, 6, 1442–1459. [Google Scholar] [CrossRef] [PubMed]
- Fudouzi, H. Optical properties caused by periodical array structure with colloidal particles and their applications. Adv. Powder Technol. 2009, 20, 502–508. [Google Scholar] [CrossRef]
- Kriegel, I.; Scotognella, F. Indium tin oxide nanoparticle: Air layers for one-dimensional multilayer photonic structures. Appl. Sci. Basel 2019, 9, 2564. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Zentel, R.; Maka, T.; Romanov, S.G.; Torres, C.M.S. Photonic crystal films with high refractive index contrast. Adv. Mater. 2000, 12, 1499–1503. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, Z.Q. Photonic bandgaps in disordered inverse-opal photonic crystals. Adv. Mater. 2001, 13, 433–436. [Google Scholar] [CrossRef]
- Liu, K.; Schmedake, T.A.; Tsu, R. A comparative study of colloidal silica spheres: Photonic crystals versus Bragg’s law. Phys. Lett. A 2008, 372, 4517–4520. [Google Scholar] [CrossRef]
- Kanamori, Y.; Katsube, H.; Furuta, T.; Hasegawa, S.; Hane, K. Design and fabrication of structural color filters with polymer-based guided-mode resonant gratings by nanoimprint lithography. Jpn. J. Appl. Phys. 2009, 48, 06FH04. [Google Scholar] [CrossRef]
- Mizeikis, V.; Mijulskas, I.; Tomasiunas, R.; Juodkazis, S.; Matsuo, S.; Misawa, H. Optical characteristics of two-dimensional photonic crystals in anodic aluminum oxide films. Jpn. J. Appl. Phys. 2004, 43, 36–43. [Google Scholar] [CrossRef]
- Ko, Y.L.; Tsai, H.P.; Lin, K.Y.; Chen, Y.C.; Yang, H. Reusable macroporous photonic crystal-based ethanol vapor detectors by doctor blade coating. J. Colloid Interface Sci. 2016, 487, 360–369. [Google Scholar] [CrossRef]
- Xiao, F.X.; Pagliaro, M.; Xu, Y.J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J. Photonic crystal-based sensors for detecting alcohol concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, Z.; Ma, C.; Inoue, H.; Yang, G.; Zheng, S.; Chen, D.; Yang, Z.; Masuno, A.; Orava, J.; et al. Mesoscale engineering of photonic glass for tunable luminescence. NPG Asia Mater. 2016, 8, e318. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, E.; O’Dwyer, C. Artificial opal photonic crystals and inverse opal structures—Fundamentals and applications from optics to energy storage. J. Mater. Chem. C 2015, 3, 2109–6143. [Google Scholar] [CrossRef] [Green Version]
- Forster, D.J.; Noh, H.; Liew, S.F.; Saranathan, V.; Schreck, C.F.; Yang, L.; Park, J.G.; O.Prum, R.; Mochrie, S.G.J.; O’Hern, C.S.; et al. Biomimetic isotropic nanostructures for structural coloration. Adv. Mater. 2010, 22, 2939–2944. [Google Scholar] [CrossRef] [Green Version]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Nueangnoraj, K.; Nishhara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of colored pigments with amorphous arrays of black and white colloidal particles. Angew. Chem. 2013, 125, 7402–7406. [Google Scholar] [CrossRef]
- Teshima, M.; Seki, T.; Kawano, R.; Takeuchi, S.; Yoshioka, S.; Takeoka, Y. Preparation of structurally colored, monodisperse spherical assemblies composed of black and white colloidal particles using a micro-flow-focusing device. J. Mater. Chem. C 2014, 3, 769–777. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, B.; Chen, A.; Lui, X.; Shi, L.; Zi, J. Using cuttlefish ink as an additive to produce non-iridescent structural colors of high color visibility. Adv. Mater. 2015, 27, 4719–4724. [Google Scholar] [CrossRef]
- Gokhan, T.; Tugrul, G.; Mustafa, G.; Mustafa, M.D. Non-iridescent structural colors from uniform-sized SiO2 colloids. Photonics Nanostruct. 2017, 29, 22–29. [Google Scholar]
- Qiao, W.; Li, S.; Guo, G.; Han, S.; Ren, S.; Ma, Y. Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin. J. Ind. Eng. Chem. 2015, 21, 1417–1422. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, J.; Yan, L.; Zhang, X. Sol-gel preparation of self-cleaning SiO2-TiO2/SiO2-TiO2 double-layer antireflective coating for solar glass. Results Phys. 2018, 8, 532–536. [Google Scholar] [CrossRef]
- Patel, M.; Mukhopadhyday, I.; Ray, A. Annealing influence over structural and optical properties of sprayed SnS thin films. Opt. Mater. 2013, 35, 1693–1699. [Google Scholar] [CrossRef]
- Mayonado, G.; Mian, S.M.; Robbiano, V.; Cacialli, F. Investigation of the bragg-snell law in photonic crystals. In Proceedings of the 2015 Laboratory Instruction Beyond the First Year, College Park, MD, USA, 22–24 July 2015. [Google Scholar]

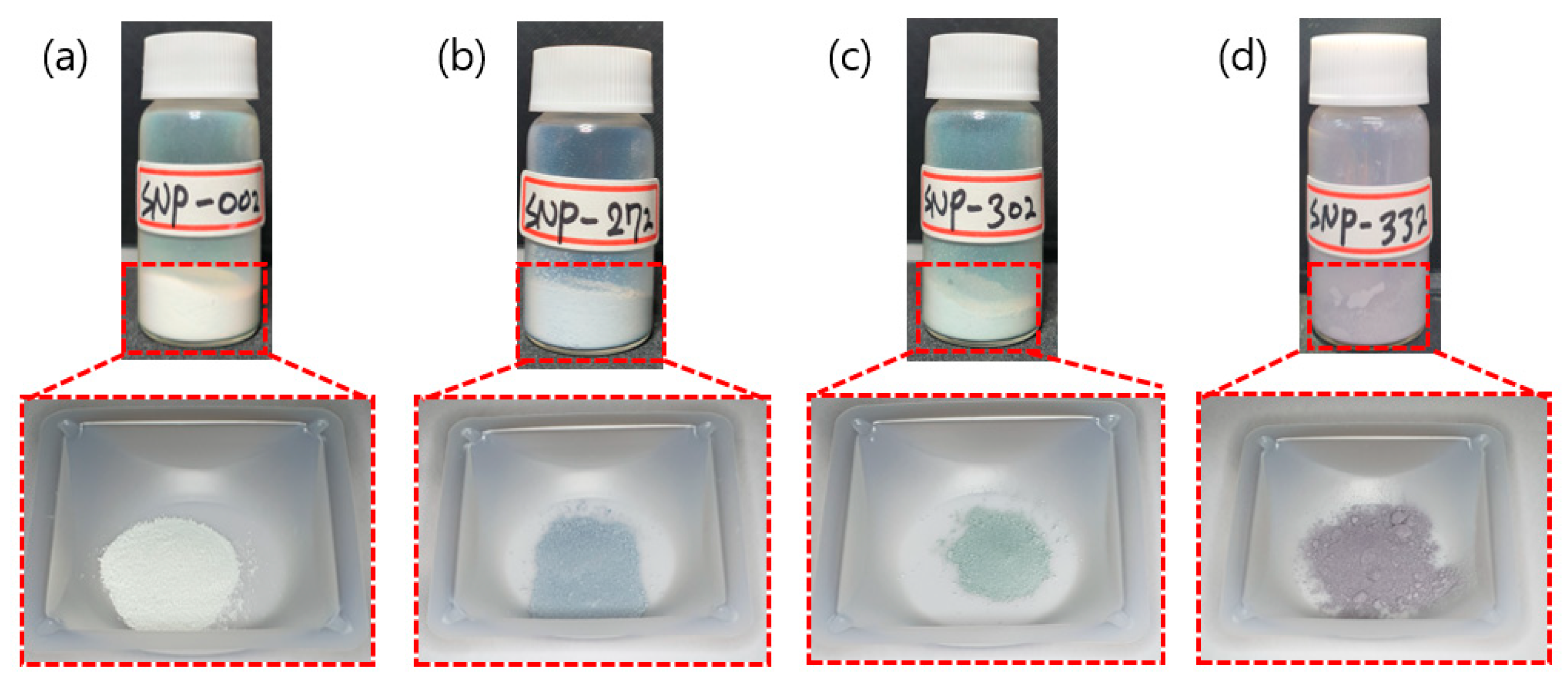
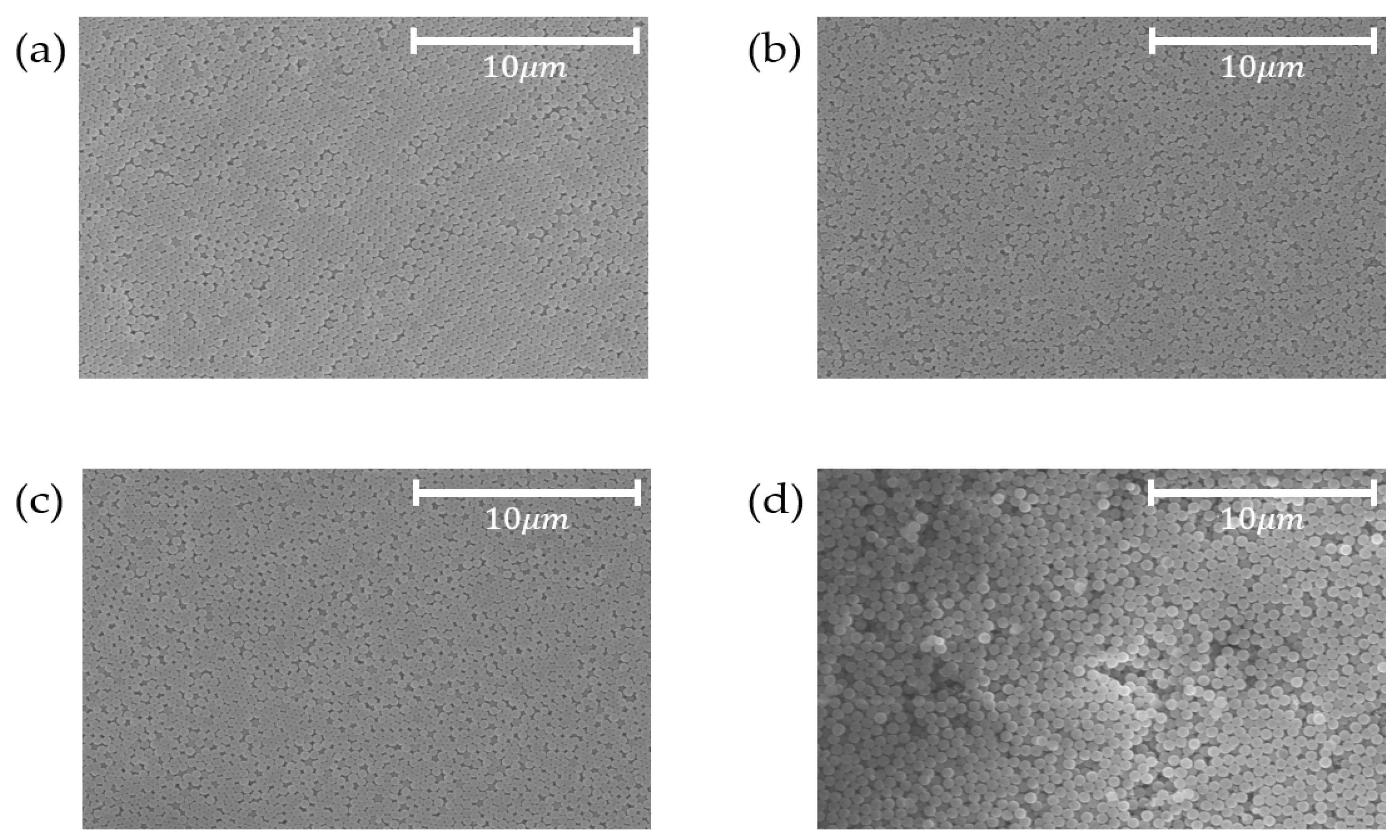
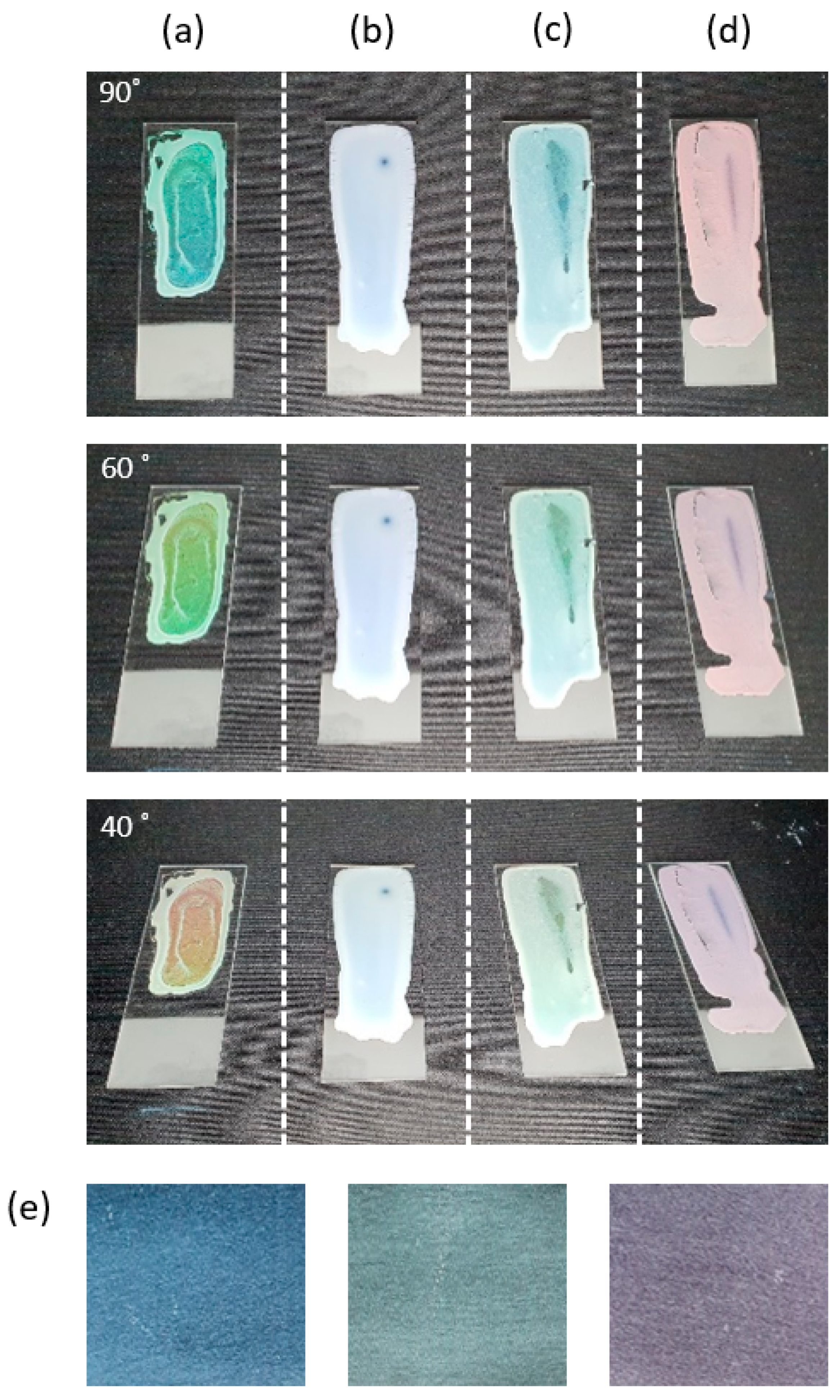
| Sample | Phenol-Formaldehyde Resin [g] | TEOS [mL] | Ethanol [mL] | DI Water [mL] | Ammonia Water [mL] |
|---|---|---|---|---|---|
| Case 1 | - | 20 | 50 | 50 | 6 |
| Case 2 | 0.027 | 20 | 50 | 50 | 6 |
| Case 3 | 0.030 | 20 | 50 | 50 | 6 |
| Case 4 | 0.033 | 20 | 50 | 50 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-S.; Choi, J.-H.; Lee, C.-Y. Structural Colors Based on Amorphous Arrays Comprised Solely of Silica Particles. Appl. Sci. 2020, 10, 420. https://doi.org/10.3390/app10010420
Choi D-S, Choi J-H, Lee C-Y. Structural Colors Based on Amorphous Arrays Comprised Solely of Silica Particles. Applied Sciences. 2020; 10(1):420. https://doi.org/10.3390/app10010420
Chicago/Turabian StyleChoi, Dae-San, Ju-Hwan Choi, and Chang-Yull Lee. 2020. "Structural Colors Based on Amorphous Arrays Comprised Solely of Silica Particles" Applied Sciences 10, no. 1: 420. https://doi.org/10.3390/app10010420





