Mechanism of Corrosion in Porcelain Insulators and Its Effect on the Lifetime
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
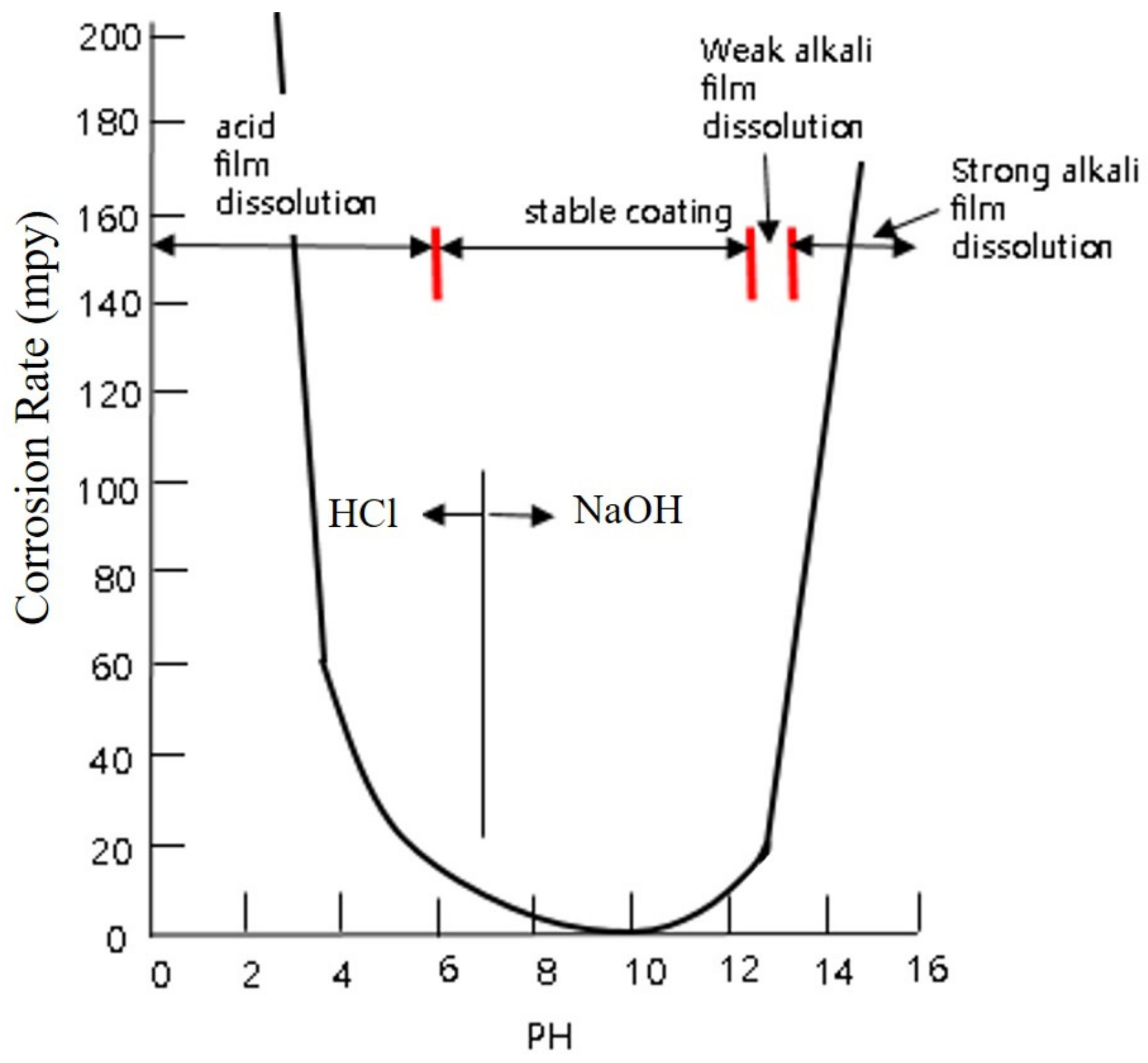
3.1. Galvanic Corrosion
3.2. Electrolytic Corrosion
3.3. Crevice Corrosion
3.4. Effects of Corrosion
3.5. Geographical Dependence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, I.H.; Kim, T.K.; Yoon, Y.B.; Nguyen, T.; Yi, J. A Study on the Life-Time Assessment Ways and Various Failure Types of 154 kV Porcelain Insulators Installed in South Korea. Trans. Electr. Electron. Mater. 2018, 19, 188–194. [Google Scholar] [CrossRef]
- Luo, L.; Wang, L.; Mei, H.; Guan, Z. Corrosion mechanism and process of hardware of disc suspension type Insulators on ± 800 kV transmission lines. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 339–349. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, B.; Hu, Q.; Yin, F. Effect of ultrasonic fog on AC flashover voltage of polluted porcelain and glass insulators. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 429–434. [Google Scholar] [CrossRef]
- Luo, L.; Wang, L.; Guan, Z. Influence of pin corrosion on mechanical characteristic of UHVDC disc suspension insulators and solutions. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2242–2251. [Google Scholar]
- Yang, Z.; Jiang, X.; Zhang, Z.; Zhang, D. Study on the influence rules of soluble contaminants on flashover voltage of disc suspension insulators. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3523–3530. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, X.; Zhang, Z.; Zhang, D.; Liu, Y. Electrical property of different types insulator string under typical pollution constituents. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 170–175. [Google Scholar]
- Graedel, T.E. Corrosion Mechanisms for Zinc exposed to the atmosphere. J. Electrochem. Soc. 1989, 136, 193C–203C. [Google Scholar] [CrossRef]
- Baker, A.C.; Farzaneh, M.; Gorur, R.S.; Gubanski, S.M.; Hill, R.J. Insulator Selection for AC Overhead Lines with Respect to Contamination. IEEE Trans. Power Deliv. 2009, 24, 1633–1641. [Google Scholar] [CrossRef]
- Montoya, G.; Ramirez, I.; Montoya, J.I. Corelation among ESDD and NSDD and Leakage Current in Insulators. IEE Proc. Gener. Transm. Distrib. 2004, 151, 334–340. [Google Scholar] [CrossRef]
- Roetheli, B.E.; Cox, G.L.; Literal, W.B. Effect of pH on the corrosion products and corrosion rate of zinc in oxygenated aqueous solutions. Met. Alloys 1932, 3, 73–76. [Google Scholar]
- Cabtree, I.M.; Mackey, K.J.; Kito, K.; Naito, K.; Watanabe, A.; Irie, T. Studies on electrolytic corrosion of hardware of dc line insulators. IEEE Trans. Power Appar. Syst. 1985, 104, 645–654. [Google Scholar] [CrossRef]
- Cherney, E.A.; Baker, A.C.; Kuffel, J.; Lodi, Z.; Phillips, A.; Powell, D.G.; Stewart, G.A. Evaluation of and replacement strategies for aged high voltage porcelain suspension-type insulators. IEEE Trans. Power Deliv. 2014, 29, 275–282. [Google Scholar] [CrossRef]
- Daiming, Y.; Fanghui, Y.; Hongwei, M.; Liming, W. In-Situ Monitoring of Electrolytic Corrosion on the Caps of HVDC Insulators. IEEE Sens. J. 2018, 18, 8569–8577. [Google Scholar]
- Luo, L.; Wang, L.; Guan, Z.; Mei, H.; Zhang, F.; Li, R.; Li, L. Research on the electrolytic corrosion problem of porcelain insulator’s hardware on UHVDC transmission line. In Proceedings of the 2014 IEEE Electrical Insulation Conference (EIC), Philadelphia, PA, USA, 8–11 June 2014. [Google Scholar]
- PosMac 3.0. Available online: https://www.posco.co.kr/homepage/docs/eng5/dn/product/info/posmac.pdf (accessed on 15 September 2018).







| Years in Service | Region | Pollution Level | Number of Samples |
|---|---|---|---|
| 38 | Daejeon | A | 176 |
| 38 | Ulsan | B | 147 |
| 38 | Busan | C | 147 |
| 38 | Gunsan | C | 120 |
| 40 | Gangneung | A | 273 |
| 42 | Gyeongnam | C | 200 |
| 47 | Gunsan | C | 120 |
| 48 | Dae-gu | A | 441 |
| 49 | Namyangju | clean area | 200 |
| 51 | Namyangju | clean area | 200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Lee, Y.-J.; Sanyal, S.; Woo, J.-W.; Choi, I.-H.; Yi, J. Mechanism of Corrosion in Porcelain Insulators and Its Effect on the Lifetime. Appl. Sci. 2020, 10, 423. https://doi.org/10.3390/app10010423
Kim T, Lee Y-J, Sanyal S, Woo J-W, Choi I-H, Yi J. Mechanism of Corrosion in Porcelain Insulators and Its Effect on the Lifetime. Applied Sciences. 2020; 10(1):423. https://doi.org/10.3390/app10010423
Chicago/Turabian StyleKim, Taeyong, Youn-Jung Lee, Simpy Sanyal, Jung-Wook Woo, In-Hyuk Choi, and Junsin Yi. 2020. "Mechanism of Corrosion in Porcelain Insulators and Its Effect on the Lifetime" Applied Sciences 10, no. 1: 423. https://doi.org/10.3390/app10010423





