Influence of Living and Dead Roots of Gansu Poplar on Water Infiltration and Distribution in Soil
Abstract
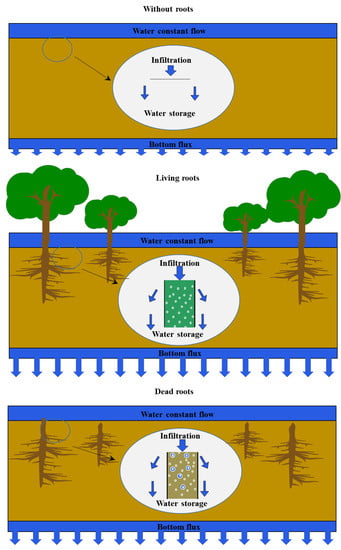
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Materials
2.2. Volumes of Flow and Water Flux at the Bottom Outlet
2.3. SEM
3. Results and Discussion
3.1. Volumes of Water Distribution in Soil
3.2. Temporal Variation in Water Flux at the Bottom Outlets
3.3. Electron Micrographs of Root Macropores
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scanlan, C.A. Processes and Effects of Root-Induced Changes to Soil Hydraulic Properties; University of Western Australia: Perth, Australia, 2009. [Google Scholar]
- Cheng, J.; Zhang, H.; Wang, W.; Zhang, Y.; Chen, Y. Changes in preferential flow path distribution and its affecting factors in southwest China. Soil Sci. 2011, 176, 652–660. [Google Scholar] [CrossRef]
- Bogner, C.; Borken, W.; Huwe, B. Impact of preferential flow on soil chemistry of a podzol. Geoderma 2012, 175–176, 37–46. [Google Scholar] [CrossRef]
- Thompson, S.E.; Harman, C.J.; Heine, P.; Katul, G.G. Vegetation-infiltration relationships across climatic and soil type gradients. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Bartens, J.; Day, S.D.; Harris, J.R.; Dove, J.E.; Wynn, T.M. Can urban tree roots improve infiltration through compacted subsoils for stormwater management? J. Environ. Qual. 2008, 37, 2048–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Sharma, S.D. Influence of forest tree species on reclamation of semiarid sodic soils. Soil Use Manag. 2010, 26, 445–454. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Guo, Q.; Lian, J.; Chen, L. Increase and spatial variation in soil infiltration rates associated with fibrous and tap tree roots. Water 2019, 11, 1700. [Google Scholar] [CrossRef] [Green Version]
- Bramley, H.; Hutson, J.; Tyerman, S.D. Floodwater infiltration through root channels on a sodic clay floodplain and the influence on a local tree species Eucalyptus largiflorens. Plant Soil 2003, 253, 275–286. [Google Scholar] [CrossRef]
- Baker, S.W. Pore size distribution—A factor to be considered in infiltration studies. J. Hydrol. 1979, 41, 279–290. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J. The importance of plant root characteristics in controlling concentrated flow erosion rates. Earth Surf. Process. Landf. 2003, 28, 371–384. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Meersmans, J.; Serlet, L. Cover crops and their erosion-reducing effects during concentrated flow erosion. Catena 2011, 85, 237–244. [Google Scholar] [CrossRef]
- Materechera, S.A.; Dexter, A.R.; Alston, A.M. Penetration of very strong soils by seedling roots of different plant species. Plant Soil 1991, 135, 31–41. [Google Scholar] [CrossRef]
- Fischer, C.; Tischer, J.; Roscher, C.; Eisenhauer, N.; Ravenek, J.; Gleixner, G.; Attinger, S.; Jensen, B.; de Kroon, H.; Mommer, L.; et al. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil 2015, 397, 1–16. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Knapen, A.; Barberá, G.G.; Navarro, J.A. Root characteristics of representative mediterranean plant species and their erosion-reducing potential during concentrated runoff. Plant Soil 2007, 294, 169–183. [Google Scholar] [CrossRef]
- Childs, E.C.; Collis-George, N. The permeability of porous materials. R. Soc. Lond. Ser. A Math. Phys. Sci. 1950, 201, 392–405. [Google Scholar]
- Marshall, T.J. A relationship between permeability and size distribution of pores. J. Soil Sci. 1958, 9. [Google Scholar]
- Nielsen, D.R.; Kirkham, D.; Perrier, E.R. Soil capillary conductivity: Comparison of measured and calculated values1. Soil Sci. Soc. Am. J. 1960, 24, 157. [Google Scholar] [CrossRef]
- Jackson, R.D.; Reginato, R.J.; Van Bavel, C.H.M. Comparison of measured and calculated hydraulic conductivities of unsaturated soils. Water Resour. Res. 1965, 1, 375–380. [Google Scholar] [CrossRef]
- Chaney, K.; Swift, R.S. The influence of organic matter on aggregate stability in some British soils. J. Soil Sci. 1984, 35, 223–230. [Google Scholar] [CrossRef]
- Doornbos, R.F.; Van Loon, L.C.; Bakker, P.A.H. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Ogdahl, M.; Chorover, J.; Chadwick, O.A.; Oleksyn, J.; Zytkowiak, R.; Reich, P.B. Tree species effects on soil organic matter dynamics: The role of soil cation composition. Ecosystems 2007, 10, 999–1018. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Jiménez, C.C.; Tejedor, M.; Morillas, G.; Neris, J. Infiltration rate in Andisols: Effect of changes in vegetation cover (Tenerife, Spain). J. Soil Water Conserv. 2006, 61, 153–158. [Google Scholar]
- Sun, T.; Hobbie, S.E.; Berg, B.; Zhang, H.; Wang, Q.; Wang, Z. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. USA 2018, 115, 10392–10397. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.R.; Ellsworth, T.R.; Meek, B.D. Effect of root systems on preferential flow in swelling soil. Commun. Soil Sci. Plant Anal. 1995, 26, 2655–2666. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The influence of plant root systems on subsurface flow: Implications for slope stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Ngao, J.; Longdoz, B.; Granier, A.; Epron, D. Estimation of autotrophic and heterotrophic components of soil respiration by trenching is sensitive to corrections for root decomposition and changes in soil water content. Plant Soil 2007, 301, 99–110. [Google Scholar] [CrossRef]
- Lesturgez, G.; Poss, R.; Hartmann, C.; Bourdon, E.; Noble, A.; Ratana-Anupap, S. Roots of stylosanthes hamata create macropores in the compact layer of a sandy soil. Plant Soil 2004, 260, 101–109. [Google Scholar] [CrossRef]
- Lange, B.; Lüescher, P.; Germann, P.F. Significance of tree roots for preferential infiltration in stagnic soils. Hydrol. Earth Syst. Sci. 2009, 13, 1809–1821. [Google Scholar] [CrossRef] [Green Version]
- Beven, K.; Germann, P. Macropores and water flow in soils. Water Resour. Res. 1982, 18, 1311–1325. [Google Scholar] [CrossRef] [Green Version]
- Beven, K.; Germann, P. Macropores and water flow in soils revisited. Water Resour. Res. 2013, 49, 3071–3092. [Google Scholar] [CrossRef] [Green Version]
- Shortle, W.C.; Dudzik, K.R. Wood Decay in Living and Dead Trees: A Pictorial Overview; United States Department of Agriculture: Washington, DC, USA, 2012. [Google Scholar]
- Hamblin, A.P.; Davies, D.B. Influence of organic matter on the physical properties of some east anglian soils of high silt content. J. Soil Sci. 1973, 28, 11–22. [Google Scholar] [CrossRef]
- Su, X.X.; Liu, R.; Kang, X.Y. The cross-compatibility of euphrates poplar (populus euphratica) with pollcns radiated gamma-ray and the selection and breeding of hybrids. Hereditas 1995, 17, 24–26. [Google Scholar]
- Dickmann, D.I.; Isebrands, J.G.; Eckenwalder, J.E.; Richardson, E. Poplar Culture in North America; NRC Research Press: Ottawa, ON, Canada, 2001; pp. 77–118. [Google Scholar]
- Block, R.M.A.; Van Rees, K.C.J.; Knight, J.D. A review of fine root dynamics in populus plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Moret-Fernández, D.; González-Cebollada, C.; Latorre, B. Microflowmeter-tension disc infiltrometer—Part I: Measurement of the transient infiltration rate. J. Hydrol. 2012, 466–467, 151–158. [Google Scholar]
- Kambale, J.B.; Singh, D.K.; Sarangi, A. Impact of climate change on groundwater recharge in a semi-arid region of northern India. Appl. Ecol. Environ. Res. 2017, 15, 335–362. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Wen, X.; Chang, A.C. Integration of HYDRUS-1D and MODFLOW for evaluating the dynamics of salts and nitrogen in groundwater under long-term reclaimed water irrigation. Irrig. Sci. 2019, 37, 35–47. [Google Scholar] [CrossRef]





| pH | TDS | TOC | Cl− | SO42− | HCO3− | CO32− | Ca2+ | K+ | Mg2+ | Na+ |
|---|---|---|---|---|---|---|---|---|---|---|
| (-) | (μS cm−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) |
| 8.02 | 284 | 2.40 | 9.94 | 24.90 | 114 | <5 | 39.0 | 1.98 | 7.77 | 8.16 |
| Test Design | Without Roots | Living Roots | Dead Roots |
|---|---|---|---|
| Penetration time (min) | 198 | 178 | 171 |
| Steady-state water flux at the bottom of the container (cm d−1) | 54.75 ± 0.80 | 61.31 ± 0.61 | 55.97 ± 0.59 |
| Water storage capacity of soil (cm3 cm−3) | 0.279 | 0.317 | 0.322 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Dai, Y.; Wang, L.; Chen, L. Influence of Living and Dead Roots of Gansu Poplar on Water Infiltration and Distribution in Soil. Appl. Sci. 2020, 10, 3593. https://doi.org/10.3390/app10103593
Zhang D, Dai Y, Wang L, Chen L. Influence of Living and Dead Roots of Gansu Poplar on Water Infiltration and Distribution in Soil. Applied Sciences. 2020; 10(10):3593. https://doi.org/10.3390/app10103593
Chicago/Turabian StyleZhang, Dashuai, Yao Dai, Lingli Wang, and Liang Chen. 2020. "Influence of Living and Dead Roots of Gansu Poplar on Water Infiltration and Distribution in Soil" Applied Sciences 10, no. 10: 3593. https://doi.org/10.3390/app10103593