A Novel Microchip Technique for Quickly Identifying Nanogranules in an Aqueous Solution by Transmission Electron Microscopy: Imaging of Platelet Granules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Collection and Platelet Preparation
2.2. Platelet Granules Isolation
2.3. Conventional TEM
2.4. Negative Staining of Isolated Platelet Granules with the K-kit Microchip
2.5. Immunoelectron Microscopy Using the K-kit Microchip
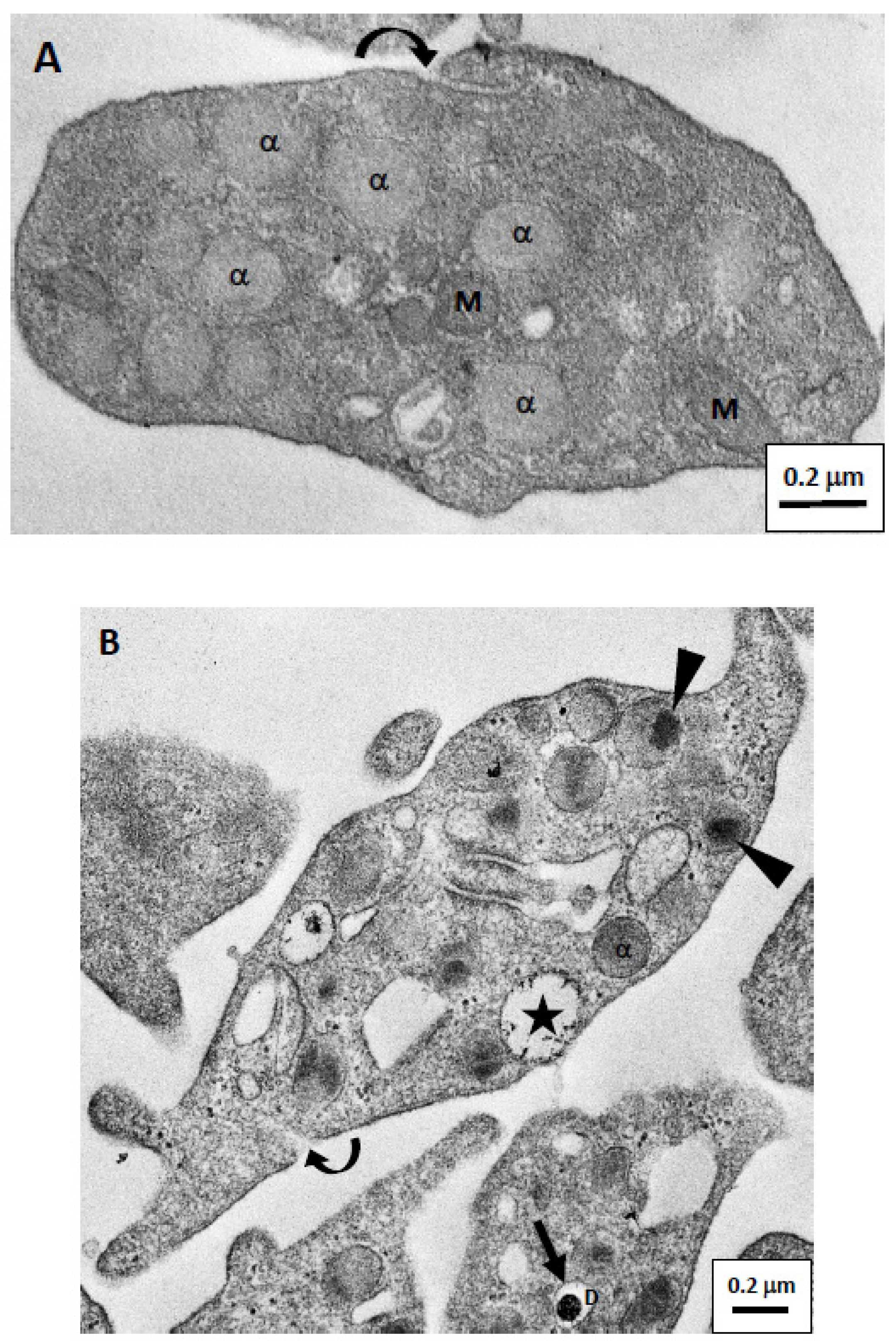
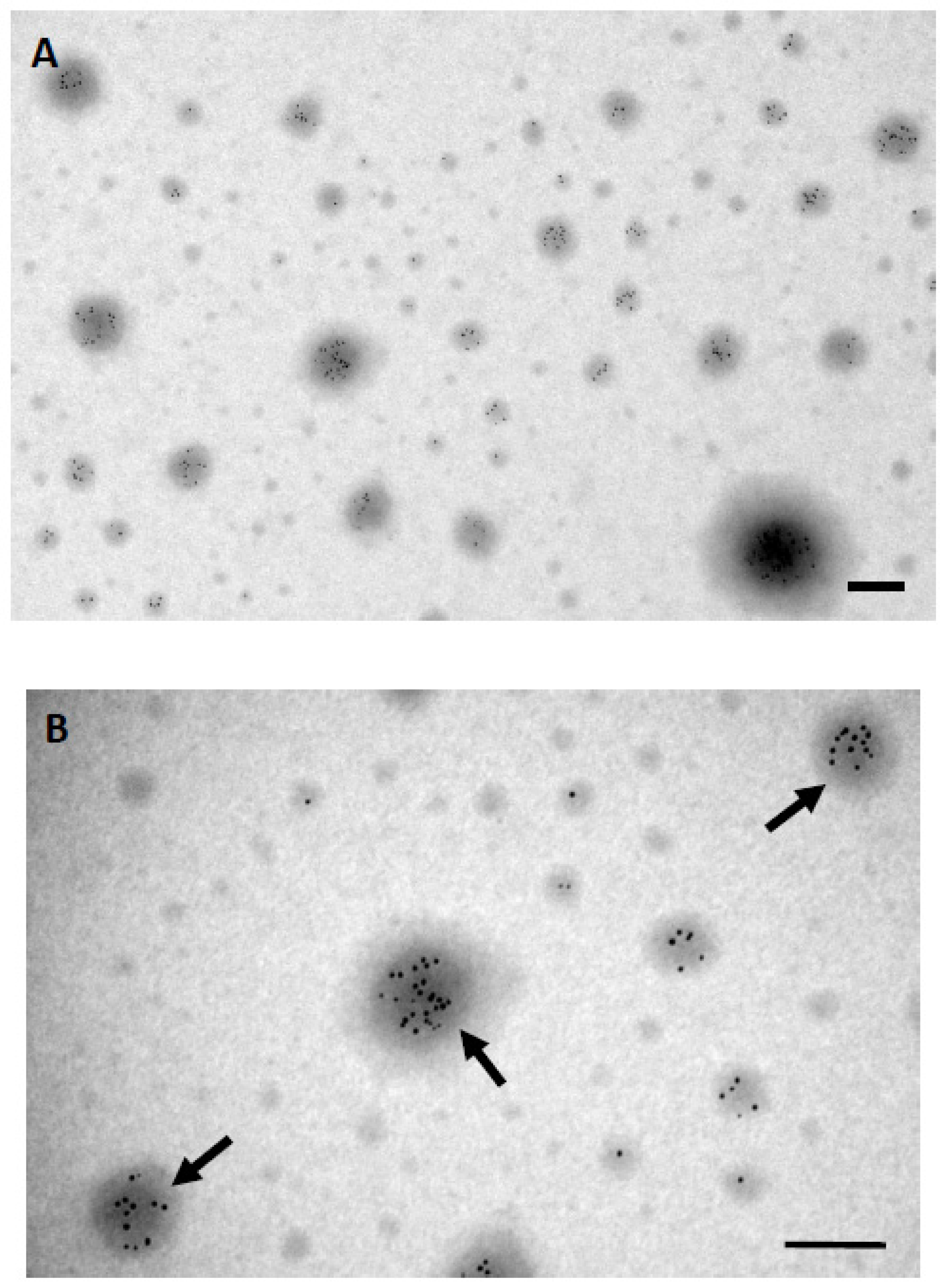
3. Results
3.1. The Structure and Instructions of the K-Kit Microchip
3.2. Images of Platelets and Granules by Traditional TEM
3.3. Negative Staining Images of Platelet Granules with the K-kit Microchip
3.4. Identification of α-Granules by Immunogold Labeling Using the K-Kit Microchip
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bozzola, J.J.; Russell, L.D. Specimen Preparation for Transmission Electron Microscopy. In Electron Microscopy: Principles and Techniques for Biologists; Jones and Bartlett Publishers: Boston, NV, USA, 1992; pp. 14–37. [Google Scholar]
- Sabatini, D.D.; Bensch, K.; Barrnett, R.J. Cytochemistry and electron microscopy: The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 1963, 17, 19–58. [Google Scholar] [CrossRef]
- Liu, K.L.; Wu, C.C.; Huang, Y.J.; Peng, H.L.; Chang, H.Y.; Chang, P.; Hsu, L.; Yew, T.R. Novel microchip for in situ TEM imaging of living organisms and bio-reactions in aqueous conditions. Lab Chip 2008, 8, 1915–1921. [Google Scholar] [CrossRef]
- Lu, P.J.; Cheng, W.L.; Huang, S.C.; Chen, Y.P.; Chou, H.K.; Cheng, H.F. Characterizing titanium dioxide and zinc oxide nanoparticles in sunscreen spray. Int. J. Cosmet. Sci. 2015, 37, 620–626. [Google Scholar] [CrossRef]
- Lai, S.E.; Hong, Y.J.; Chen, Y.T.; Kang, Y.T.; Chang, P.; Yew, T.R. Direct-writing of Cu nano-patterns with an electron beam. Microsc. Microanal. 2015, 21, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.A.; Kang, Y.T.; Chen, Y.C.; Wang, Y.C.; Wang, Y.J.; Wu, Y.T.; Liu, K.L.; Wang, C.Y.; Ko, Y.F.; Chen, C.Y.; et al. Quantitative characterization of nanoparticles in blood by transmission electron microscopy with a window-type microchip nanopipet. Anal. Chem. 2012, 84, 6312–6316. [Google Scholar] [CrossRef]
- Weiss, H.J. Platelet physiology and abnormalities of platelet function. N. Engl. J. Med. 1975, 293, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Bennett, J.S. Platelets and their membranes in hemostasis: Physiology and pathophysiology. Ann. Intern. Med. 1981, 94, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.G. Interaction of vascular endothelial cells with leukocytes, platelets and cancer cells in inflammation, thrombosis and cancer growth and metastasis. Acta Pharmacol. Sin. 2003, 24, 1297–1300. [Google Scholar]
- Mezouar, S.; Mege, D.; Darbousset, R.; Farge, D.; Debourdeau, P.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin. Oncol. 2014, 41, 346–358. [Google Scholar] [CrossRef]
- Varon, D.; Shai, E. Platelets and their microparticles as key players in pathophysiological responses. J. Thromb. Haemost. 2015, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.G.; Michelson, A.D.; Flaumenhaft, R. Granule exocytosis is required for platelet spreading: Differential sorting of α-granules expressing VAMP-7. Blood 2012, 120, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Mirlashari, M.R.; Ryningen, A.; Mikkelsen, H.M.; Fukami, M.H. Differential secretion of blood platelet storage granules. Platelets 1996, 7, 313–320. [Google Scholar] [CrossRef]
- King, S.M.; Reed, G.L. Development of platelet secretory granules. Semin. Cell Dev. Biol. 2002, 13, 293–302. [Google Scholar]
- Stenberg, P.E.; McEver, R.P.; Schuman, M.A.; Jacques, Y.V.; Bainton, D.F. A platelet alpha-granule membrane protein (GMP140) is expressed on the plasma membrane after activation. J. Cell Biol. 1985, 101, 880–886. [Google Scholar] [CrossRef]
- Harrison, P.; Cramer, E.M. Platelet alpha-granules. Blood Rev. 1993, 7, 52–62. [Google Scholar] [CrossRef]
- Reverter, J.C.; Escolar, G.; Sanz, C.; Cases, A.; Villamor, N.; Nieuwenhuis, H.K.; López, J.; Ordinas, A. Platelet activation during hemodialysis measured through exposure of p-selectin: Analysis by flow cytometric and ultrastructural techniques. J. Lab. Clin. Med. 1994, 124, 79–85. [Google Scholar]
- Pokrovskaya, I.D.; Yadav, S.; Rao, A.; McBride, E.; Kamykowski, J.A.; Zhang, G.; Aronova, M.A.; Leapman, R.D.; Storrie, B. 3D ultrastructural analysis of α-granule, dense granule, mitochondria, and canalicular system arrangement in resting human platelets. Res. Pract. Thromb. Haemost. 2019, 4, 72–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamykowski, J.; Carlton, P.; Sehgal, S.; Storrie, B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet α-granules. Blood 2011, 118, 1370–1373. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Storrie, B. The cellular basis of platelet secretion: Emerging structure/function relationships. Platelets 2017, 28, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Lo, R.W.; Urban, D.; Pluthero, F.G.; Kahr, W.H. α-Granule biogenesis: From disease to discovery. Platelets 2017, 28, 147–154. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, L.H.; Cui, X.; Lei, X.X.; Tang, J.B.; Cheng, B. Investigating the effect of age on platelet ultrastructure using transmission electron microscopy. Int. Wound J. 2019, 16, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Fukami, M.H.; Salganicoff, L. Human platelet storage organelles. A review. Thromb. Haemost. 1977, 38, 963–970. [Google Scholar] [CrossRef] [PubMed]
- McNicol, A.; Israels, S.J. Platelet dense granules: Structure, function and implications for haemostasis. Thromb. Res. 1999, 95, 1–18. [Google Scholar] [CrossRef]
- Israels, S.J.; McMillan, E.M.; Robertson, C.; Singhory, S.; McNicol, A. The lysosomal granule membrane protein, LAMP-2, is also present in platelet dense granule membranes. Thromb. Haemost. 1996, 75, 623–629. [Google Scholar] [CrossRef]
- Jedlitschky, G.; Tirschmann, K.; Lubenow, L.E.; Nieuwenhuis, H.K.; Akkerman, J.W.; Greinacher, A.; Kroemer, H.K. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 2004, 104, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Niessen, J.; Jedlitschky, G.; Greinacher, A.; Kroemer, H.K. Isolation of platelet granules. Curr. Protoc. Cell Biol. 2010, 46, 3.35.1–3.35.14. [Google Scholar]
- Hsiao, G.; Lee, J.J.; Lin, K.H.; Shen, C.H.; Fong, T.H.; Chou, D.S.; Sheu, J.R. Characterization of a novel and potent collagen antagonist, caffeic acid phenethyl ester, in human platelets: In vitro and in vivo studies. Cardiovasc. Res. 2007, 75, 782–792. [Google Scholar] [CrossRef]
- Jena, B.P.; Stemmer, P.M.; Wang, S.; Mao, G.; Lewis, K.T.; Walz, D.A. Human platelet vesicles exhibit distinct size and proteome. J. Proteome Res. 2017, 16, 2333–2338. [Google Scholar] [CrossRef]
- Bozzola, J.J.; Russell, L.D. Specimen Preparation for Scanning Electron Microscopy. In Electron Microscopy: Principles and Techniques for Biologists; Jones and Bartlett Publishers: Boston, NV, USA, 1992; pp. 40–62. [Google Scholar]
- Nogales, E.; Scheres, S.H. Cryo-EM: A unique tool for the visualization of macromolecular complexity. Mol. Cell 2015, 58, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Nogales, E. Cryo-EM. Curr. Biol. 2018, 28, R1127–R1128. [Google Scholar] [CrossRef] [Green Version]
- Picot, J.; Guerin, C.L.; Kim, C.L.V.; Boulanger, C.M. Flow cytometry: Retrospective, fundamentals and recent instrumentation. Cytotechnology 2012, 64, 109–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasalic, L.; Pennings, G.J.; Connor, D.; Campbell, H.; Kritharides, L.; Chen, V.M. Flow cytometry protocols for assessment of platelet function in whole blood. Methods Mol. Biol. 2017, 1646, 369–389. [Google Scholar] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trang, N.T.T.; Chang, J.; Chen, W.-A.; Chen, C.-C.; Chen, H.-M.; Chang, C.-C.; Fong, T.-H. A Novel Microchip Technique for Quickly Identifying Nanogranules in an Aqueous Solution by Transmission Electron Microscopy: Imaging of Platelet Granules. Appl. Sci. 2020, 10, 4946. https://doi.org/10.3390/app10144946
Trang NTT, Chang J, Chen W-A, Chen C-C, Chen H-M, Chang C-C, Fong T-H. A Novel Microchip Technique for Quickly Identifying Nanogranules in an Aqueous Solution by Transmission Electron Microscopy: Imaging of Platelet Granules. Applied Sciences. 2020; 10(14):4946. https://doi.org/10.3390/app10144946
Chicago/Turabian StyleTrang, Nguyen Thi Thu, Jungshan Chang, Wei-An Chen, Chih-Chun Chen, Hui-Min Chen, Chao-Chien Chang, and Tsorng-Harn Fong. 2020. "A Novel Microchip Technique for Quickly Identifying Nanogranules in an Aqueous Solution by Transmission Electron Microscopy: Imaging of Platelet Granules" Applied Sciences 10, no. 14: 4946. https://doi.org/10.3390/app10144946




