Real-Time Multidepth Multiphoton Microscopy Using Pixel-to-Pixel Focus-Switching
Abstract
:1. Introduction
- Real-time multidepth microscopy using non-imaging detection enables localization of biological objects in three dimensions within scattering specimens.
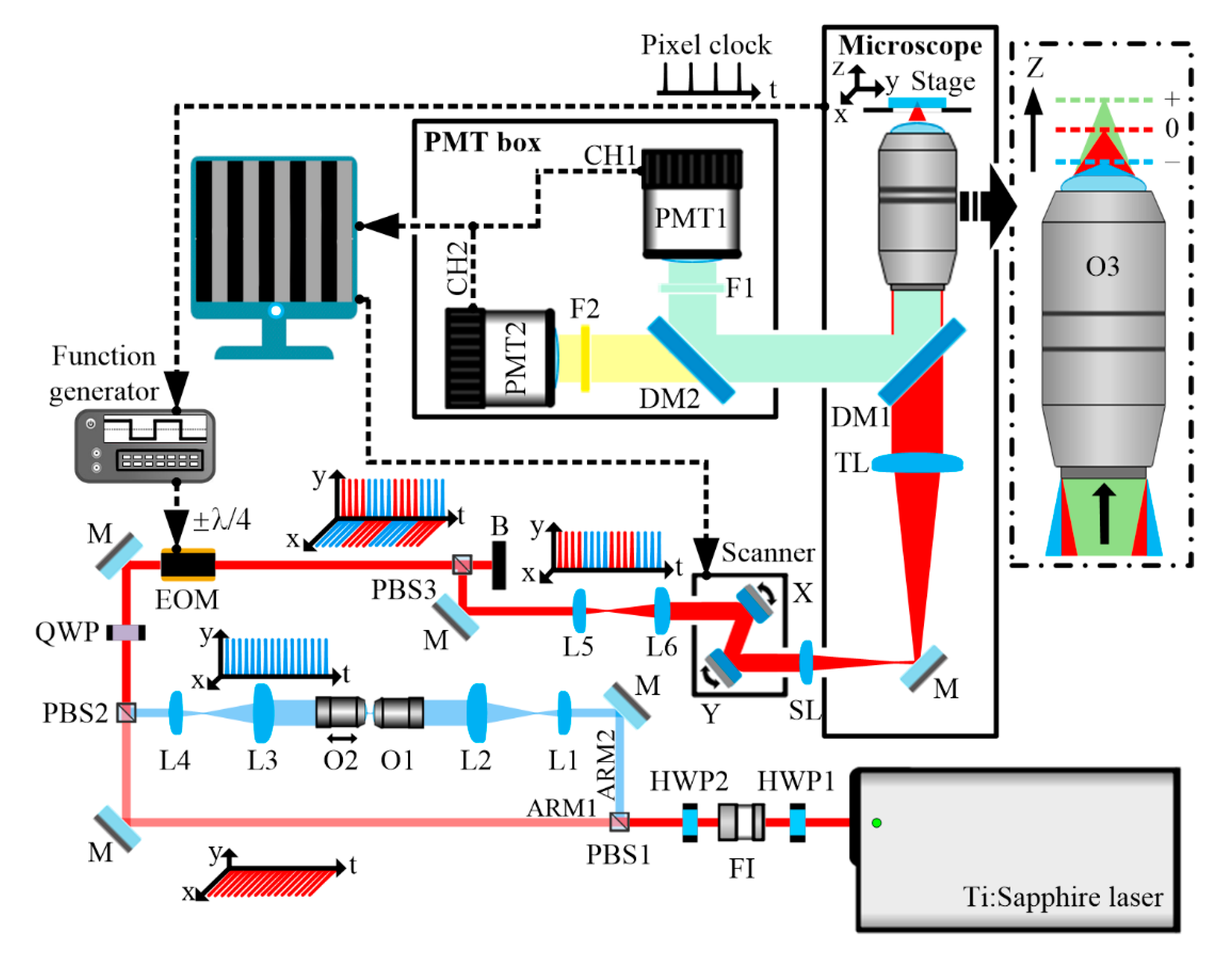
- As the key technique of the imaging system, the electro-optic modulator (EOM)-based fast-switching light paths are cost-effective and can be easily added to any commercial multiphoton microscope without internal modification.
- Simultaneous label-free TPF and SHG imaging can gain functional and structural information together from unstained samples and eliminate possible artifacts from sample motion or laser power fluctuation.
2. Experimental Setup
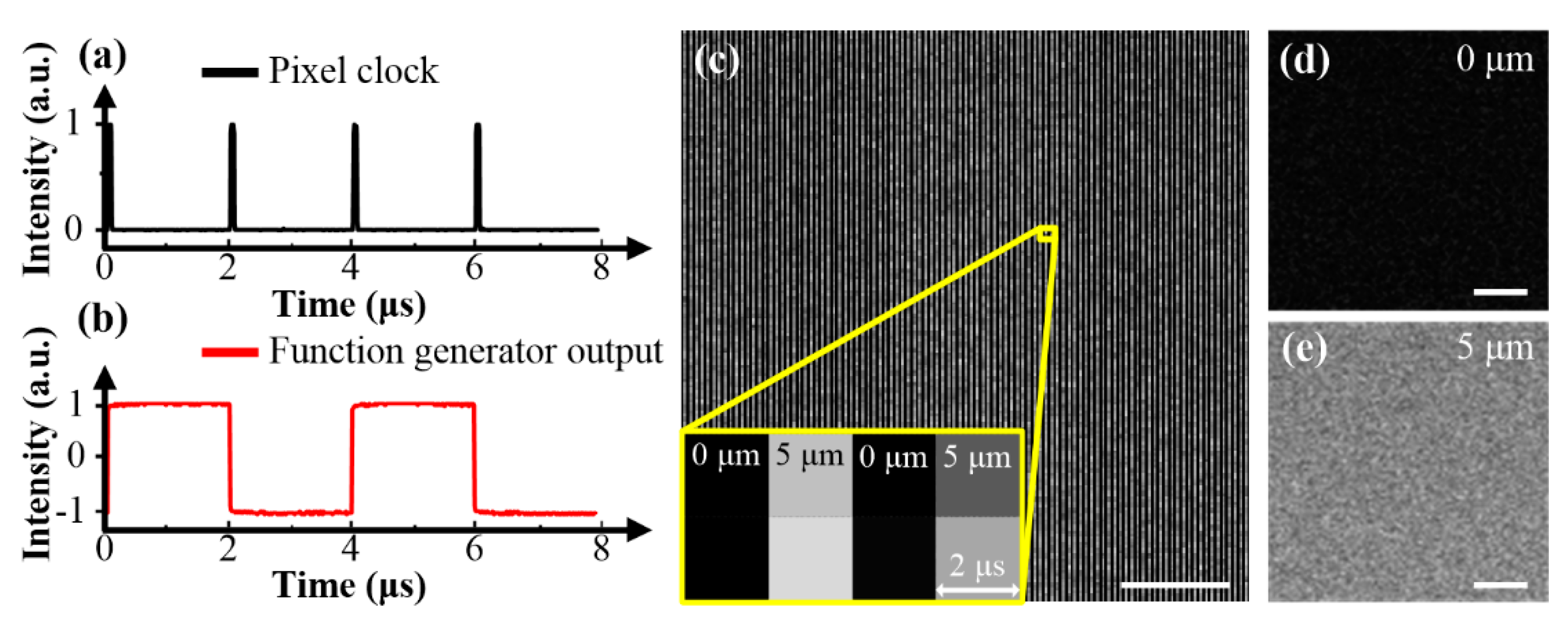
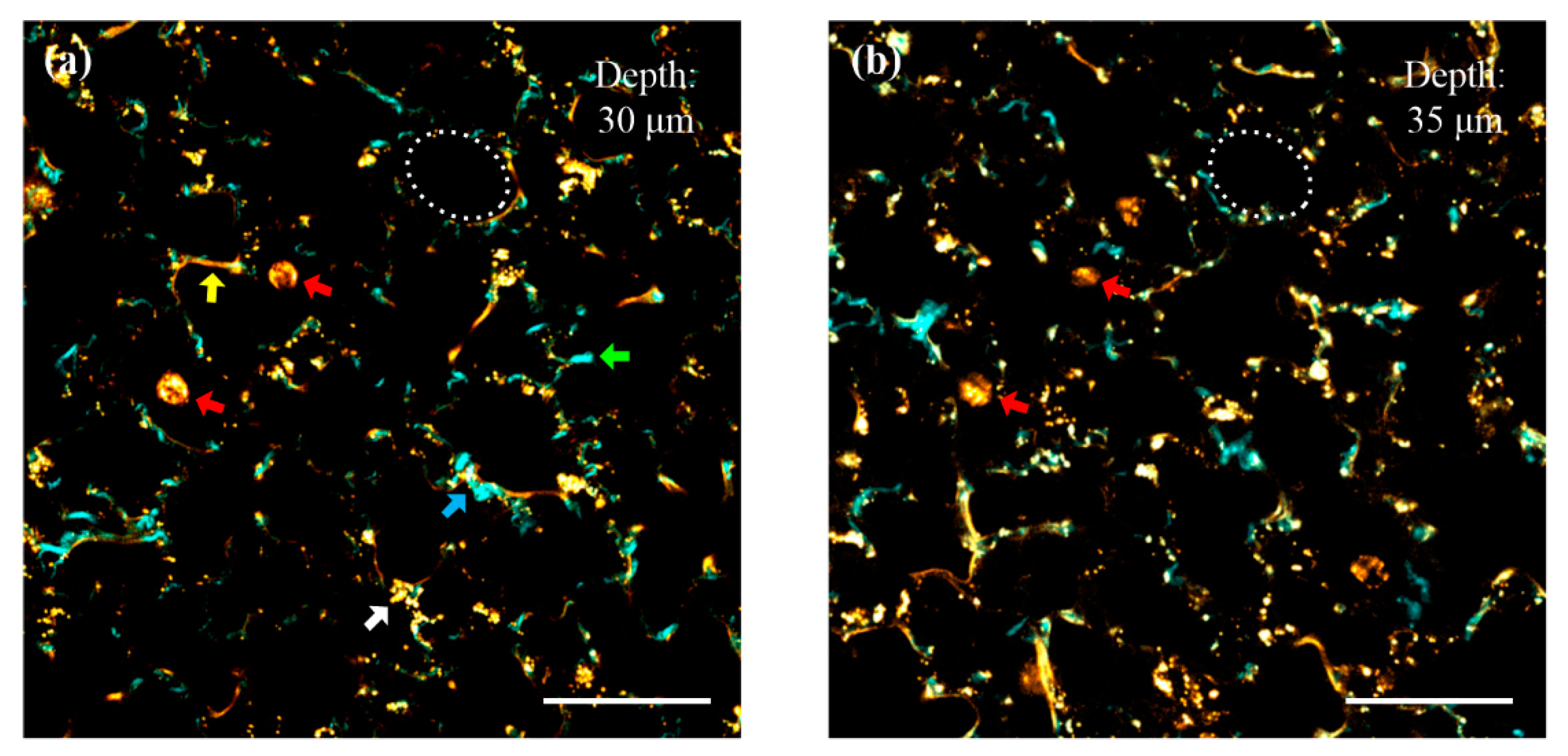
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Richter, V.; Piper, M.; Wagner, M.; Schneckenburger, H. Increasing resolution in live cell microscopy by structured illumination (SIM). Appl. Sci. 2019, 9, 1188. [Google Scholar] [CrossRef] [Green Version]
- Ustione, A.; Piston, D.W. A simple introduction to multiphoton microscopy. J. Microsc. 2011, 243, 221–226. [Google Scholar] [CrossRef]
- Xia, F.; Wu, C.; Sinefeld, D.; Li, B.; Qin, Y.; Xu, C. In vivo label-free confocal imaging of the deep mouse brain with long-wavelength illumination. Biomed. Opt. Express 2018, 9, 6545–6555. [Google Scholar] [CrossRef]
- Sato, R.; Shimizu, Y.; Chen, C.; Matsukuma, H.; Gao, W. Investigation and improvement of thermal stability of a chromatic confocal probe with a mode-locked femtosecond laser source. Appl. Sci. 2019, 9, 4084. [Google Scholar] [CrossRef] [Green Version]
- Richter, V.; Bruns, S.; Bruns, T.; Weber, P.; Wagner, M.; Cremer, C.M.; Schneckenburger, H. Axial tomography in live cell laser microscopy. J. Biomed. Opt. 2017, 22, 091505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Shimizu, Y.; Saito, T.; Matsukuma, H.; Gao, W. Measurement uncertainty analysis of a stitching linear-scan method for the evaluation of roundness of small cylinders. Appl. Sci. 2020, 10, 4750. [Google Scholar] [CrossRef]
- Chen, C.; Sato, R.; Shimizu, Y.; Nakamura, T.; Matsukuma, H.; Gao, W. A method for expansion of Z-directional measurement range in a mode-locked femtosecond laser chromatic confocal probe. Appl. Sci. 2019, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Mandras, N.; Alovisi, M.; Roana, J.; Crosasso, P.; Luganini, A.; Pasqualini, D.; Genta, E.; Arpicco, S.; Banche, G.; Cuffini, A.; et al. Evaluation of the bactericidal activity of a hyaluronic acid-vehicled clarithromycin antibiotic mixture by confocal laser scanning microscopy. Appl. Sci. 2020, 10, 761. [Google Scholar] [CrossRef]
- Xia, F.; Wu, C.; Sinefeld, D.; Li, B.; Qin, Y.; Xu, C. In vivo label-free confocal imaging of adult mouse brain up to 1.3-mm depth with NIR-II illumination. In Proceedings of the SPIE Photonics West BiOS 2019, San Francisco, CA, USA, 2–7 February 2019. [Google Scholar]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef]
- Carriles, R.; Schafer, D.N.; Sheetz, K.E.; Field, J.J.; Cisek, R.; Barzda, V.; Sylvester, A.W.; Squier, J.A. Invited Review Article: Imaging techniques for harmonic and multiphoton absorption fluorescence microscopy. Rev. Sci. Instrum. 2009, 80, 081101. [Google Scholar] [CrossRef] [Green Version]
- Alovisi, M.; Pasqualini, D.; Carpegna, G.; Comba, A.; Moccia, E.; Multari, S.; Dioguardi, M.; Scotti, N.; Berutti, E. The influence of brushing movement on geometrical shaping outcomes: A micro‐CT study. Appl. Sci. 2020, 10, 4805. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, B.; Xia, F.; Xia, Y.; Xu, C. Time-lens based multi-color background-free coherent anti-Stokes Raman scattering microscopy. In Proceedings of the SPIE Photonics West BiOS 2019, San Francisco, CA, USA, 2–7 February 2019. [Google Scholar]
- Ouzounov, D.G.; Wang, T.; Wang, M.; Feng, D.D.; Horton, N.G.; Cruz-Hernández, J.C.; Cheng, Y.-T.; Reimer, J.; Tolias, A.S.; Nishimura, N.; et al. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 2017, 14, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, C.; Wang, M.; Charan, K.; Xu, C. An adaptive excitation source for high-speed multiphoton microscopy. Nat. Methods 2020, 17, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, A.; Olarte, O.E.; Villamaina, V.; Loza-Alvarez, P.; Resan, B. Multiphoton imaging with blue-diode-pumped SESAM-modelocked Ti:sapphire oscillator generating 5 nJ 82 fs pulses. Opt. Express 2017, 25, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, B.; Xia, F.; Xia, Y.; Xu, C. Multi-color background-free coherent anti-Stokes Raman scattering microscopy using a time-lens source. Opt. Express 2018, 26, 34474–34483. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Edwards, R.A.; Pate, K.T.; Waterman, M.L.; Donovan, P.J.; Gratton, E. Metabolic trajectory of cellular differentiation in small intestine by phasor fluorescence lifetime microscopy of NADH. Sci. Rep. 2012, 2, 568. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Rico-Jimenez, J.J.; Zhang, C.; Alex, A.; Chaney, E.J.; Barkalifa, R.; Spillman, D.R., Jr.; Marjanovic, M.; Arp, Z.; Hood, S.R.; et al. Simultaneous label-free autofluorescence and multi-harmonic imaging reveals in vivo structural and metabolic changes in murine skin. Biomed. Opt. Express 2019, 10, 5431–5444. [Google Scholar] [CrossRef]
- You, S.; Tu, H.; Chaney, E.J.; Sun, Y.; Zhao, Y.; Bower, A.J.; Liu, Y.-Z.; Marjanovic, M.; Sinha, S.; Pu, Y.; et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat. Commun. 2018, 9, 2125. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Handley, M.; Carles, G.; Harveya, A.R. Advances in 3D single particle localization microscopy. APL Photon. 2019, 4, 060901. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, R.; Chao, J.; Ram, S.; Ward, E.S.; Ober, R.J. Intensity-based axial localization approaches for multifocal plane microscopy. Opt. Express 2017, 25, 3394–3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahmasbi, A.; Ram, S.; Chao, J.; Abraham, A.V.; Tang, F.W.; Ward, E.S.; Ober, R.J. Designing the focal plane spacing for multifocal plane microscopy. Opt. Express 2014, 22, 16706–16721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajj, B.; Oudjedi, L.; Fiche, J.-B.; Dahan, M.; Nollmann, M. Highly efficient multicolor multifocus microscopy by optimal design of diffraction binary gratings. Sci. Rep. 2017, 7, 5284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, Q.; Xia, Y.; Liu, B.; Zhang, S. Construction and application of femtosecond laser two-photon fluorescence microscopy system. J. Harbin Inst. Technol. 2015, 47, 1–5. [Google Scholar]
- Wang, S.; Qin, Y.; Guo, M.; Zhang, S.; Xia, Y. High speed 3D two-photon fluorescence microscopy by femtosecond laser pulses. In Proceedings of the LIDAR Imaging Detection and Target Recognition 2017, Changchun, China, 23–25 July 2017. [Google Scholar]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Abraham, T.; Hirota, J.A.; Wadsworth, S.; Knight, D.A. Minimally invasive multiphoton and harmonic generation imaging of extracellular matrix structures in lung airway and related diseases. Pulm. Pharmacol. Ther. 2011, 24, 487–496. [Google Scholar] [CrossRef]
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef] [Green Version]
- Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 091403. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Chen, D.; Xia, Y. Real-Time Multidepth Multiphoton Microscopy Using Pixel-to-Pixel Focus-Switching. Appl. Sci. 2020, 10, 7173. https://doi.org/10.3390/app10207173
Qin Y, Chen D, Xia Y. Real-Time Multidepth Multiphoton Microscopy Using Pixel-to-Pixel Focus-Switching. Applied Sciences. 2020; 10(20):7173. https://doi.org/10.3390/app10207173
Chicago/Turabian StyleQin, Yifan, Deying Chen, and Yuanqin Xia. 2020. "Real-Time Multidepth Multiphoton Microscopy Using Pixel-to-Pixel Focus-Switching" Applied Sciences 10, no. 20: 7173. https://doi.org/10.3390/app10207173



