The Laboratory-Based HySpex Features of Chlorite as the Exploration Tool for High-Grade Iron Ore in Anshan-Benxi Area, Liaoning Province, Northeast China
Abstract
:1. Introduction
2. The Application Principle of Hyperspectral Imaging in Geoscience
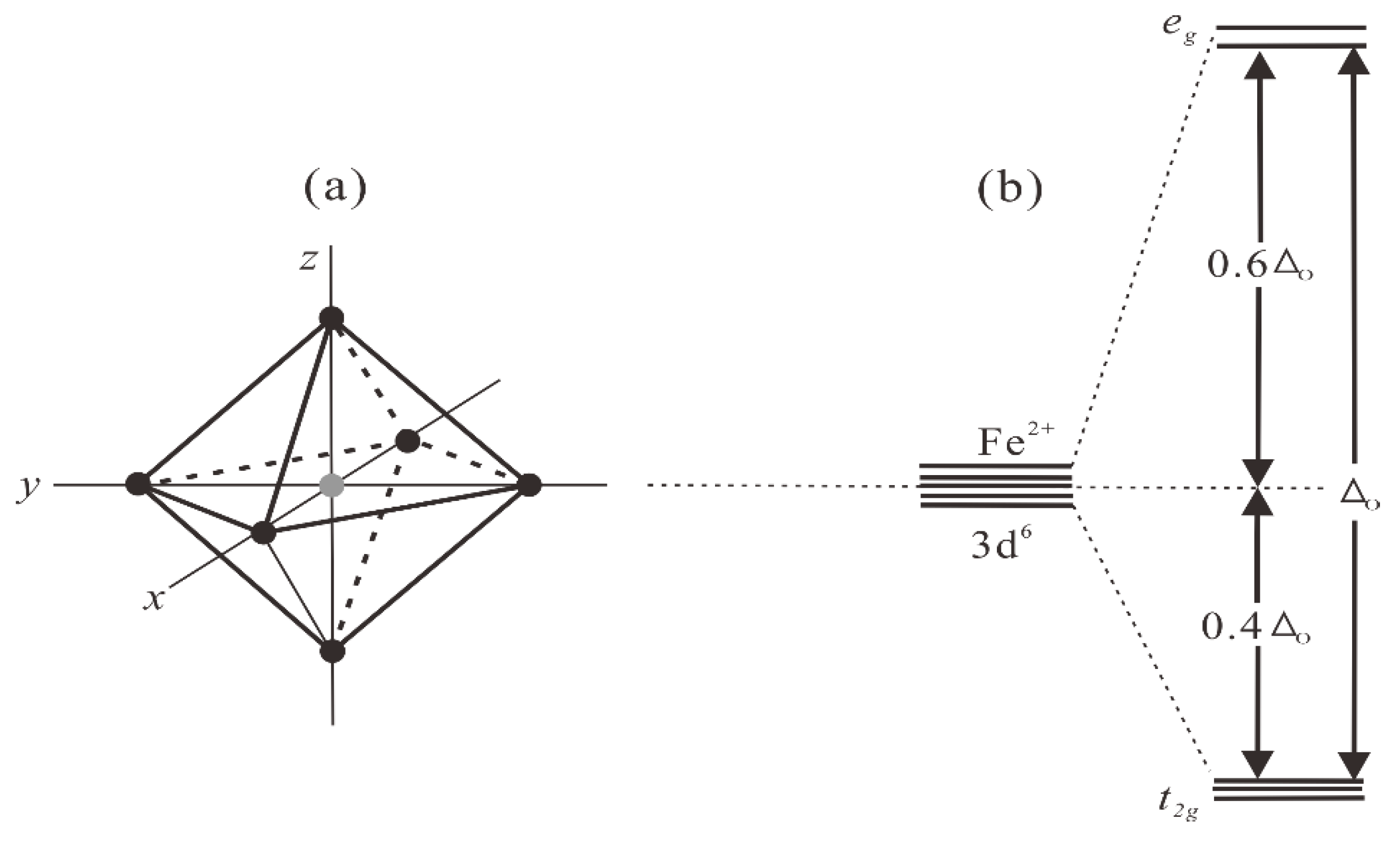
2.1. The Causes of Absorption Features of Minerals
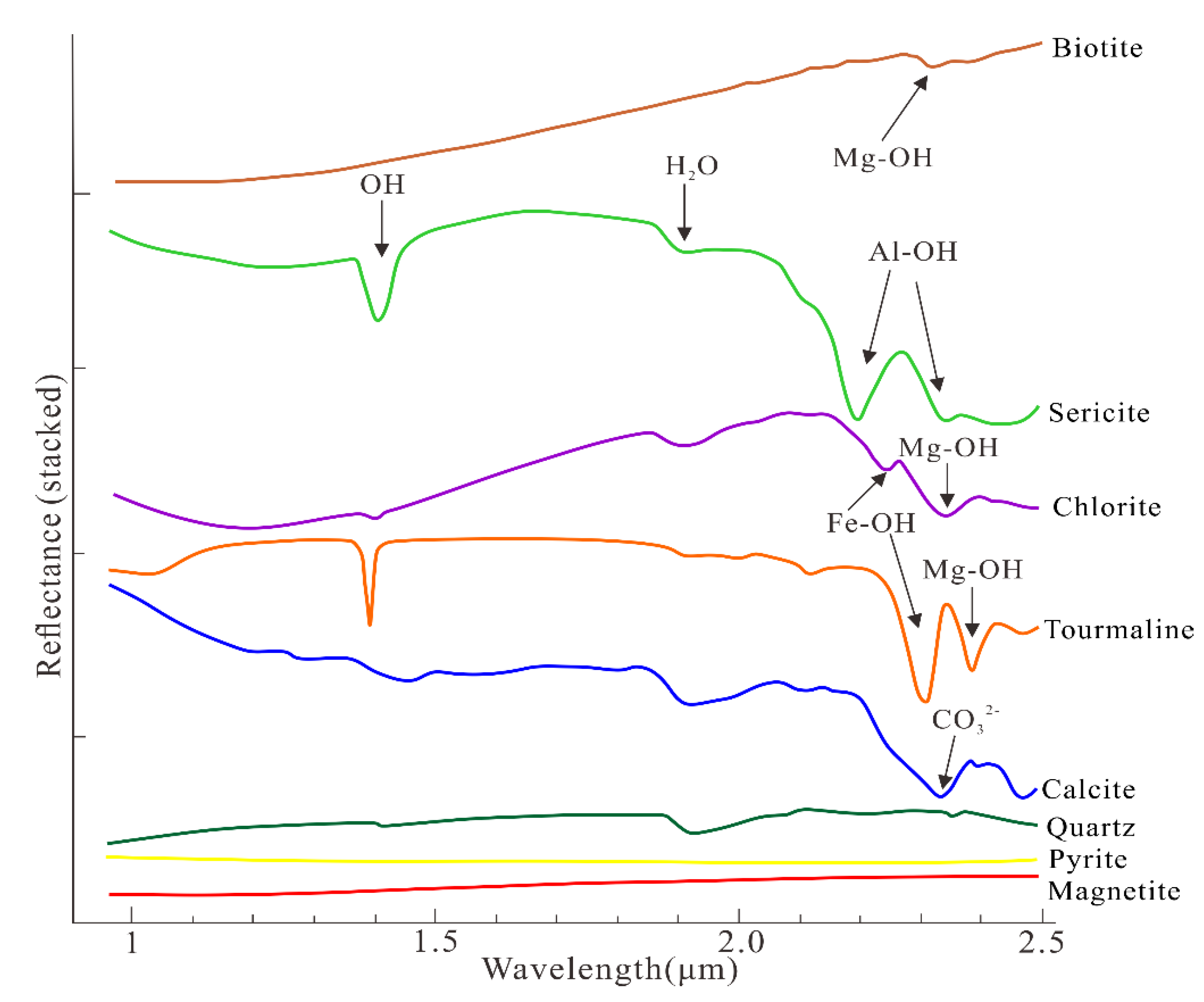
2.2. The Spectral Absorption Features of Chlorite
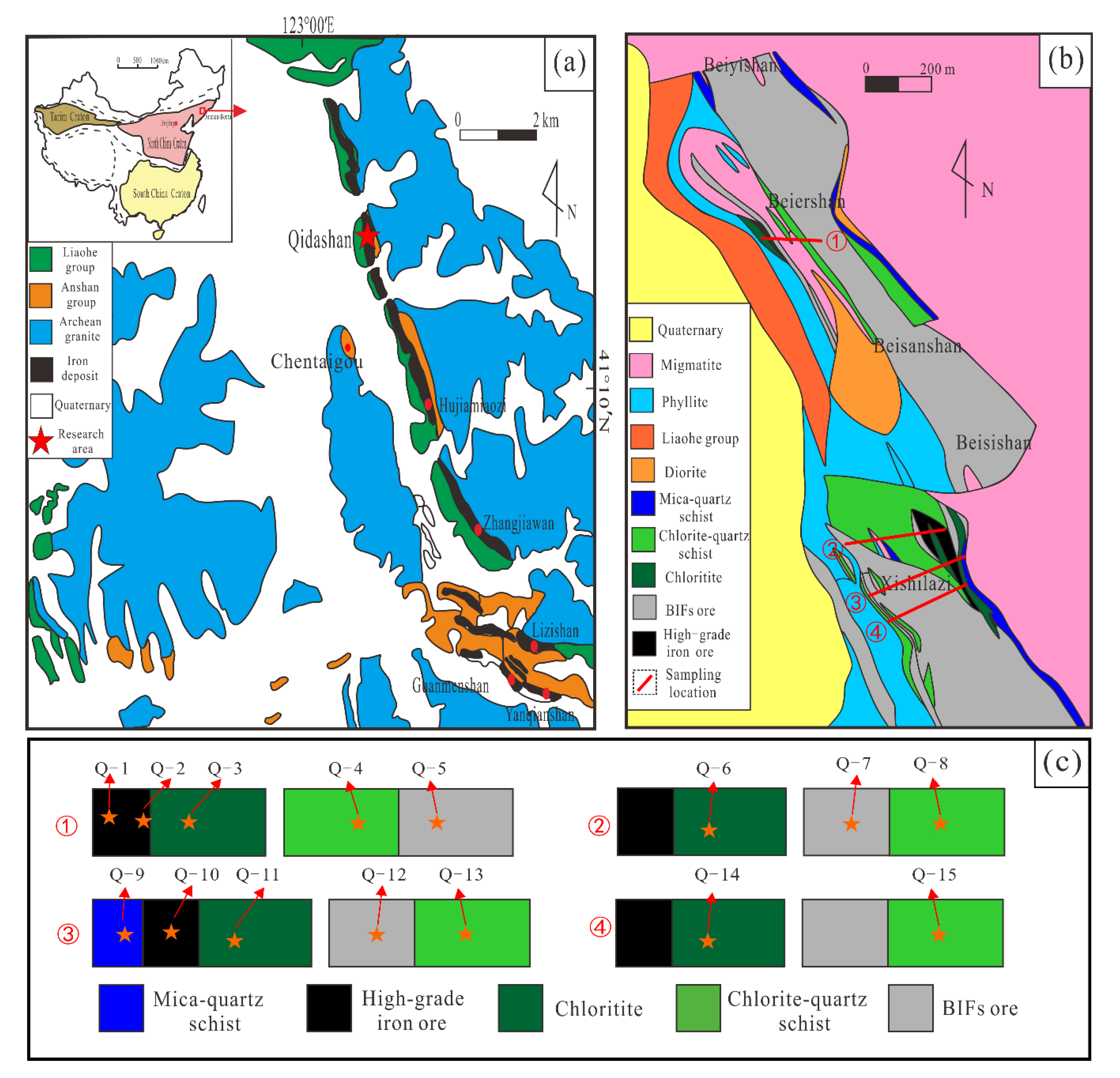
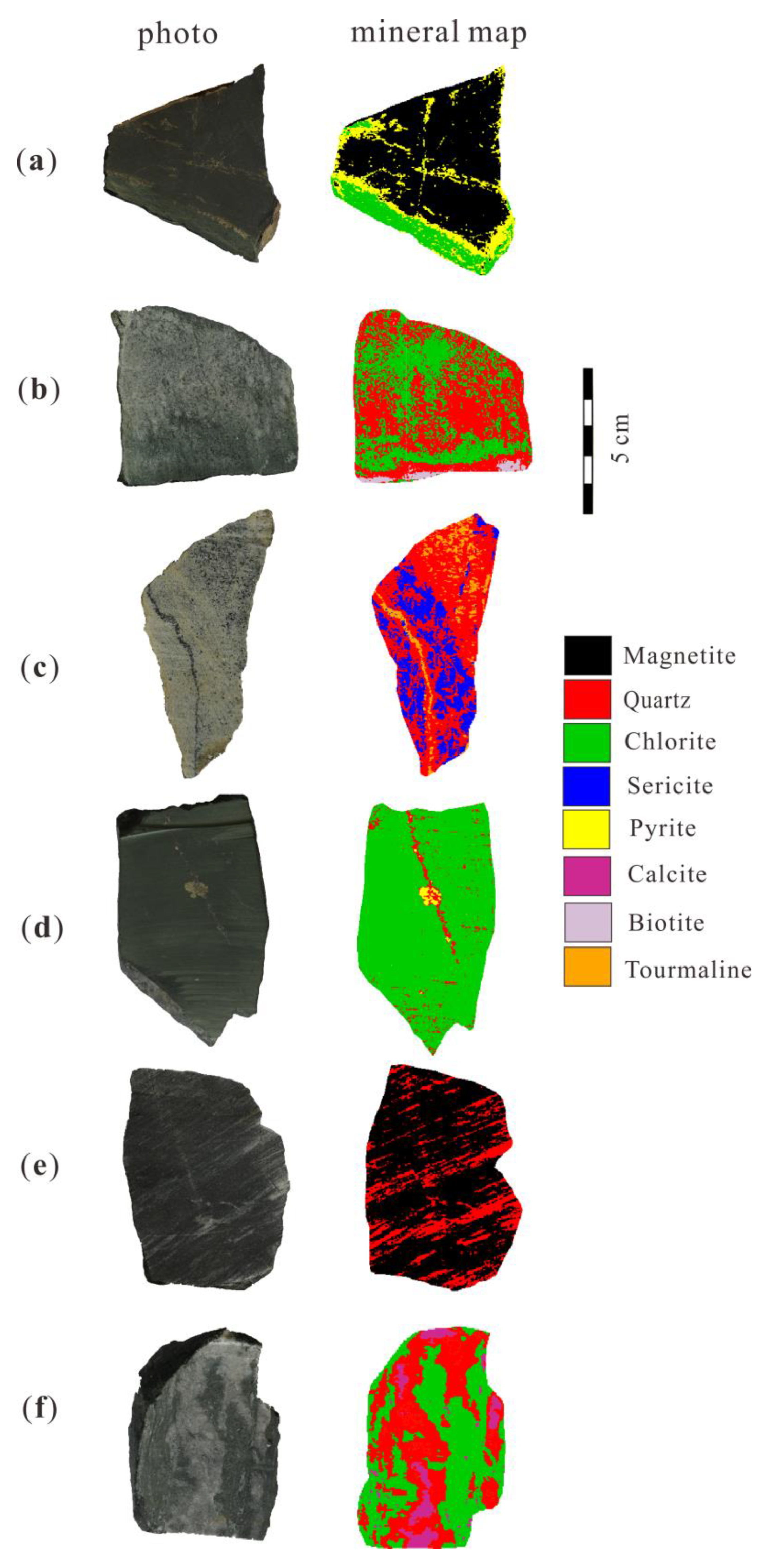
3. Geological Settings and Sampling
4. Methods
4.1. Data Acquisition
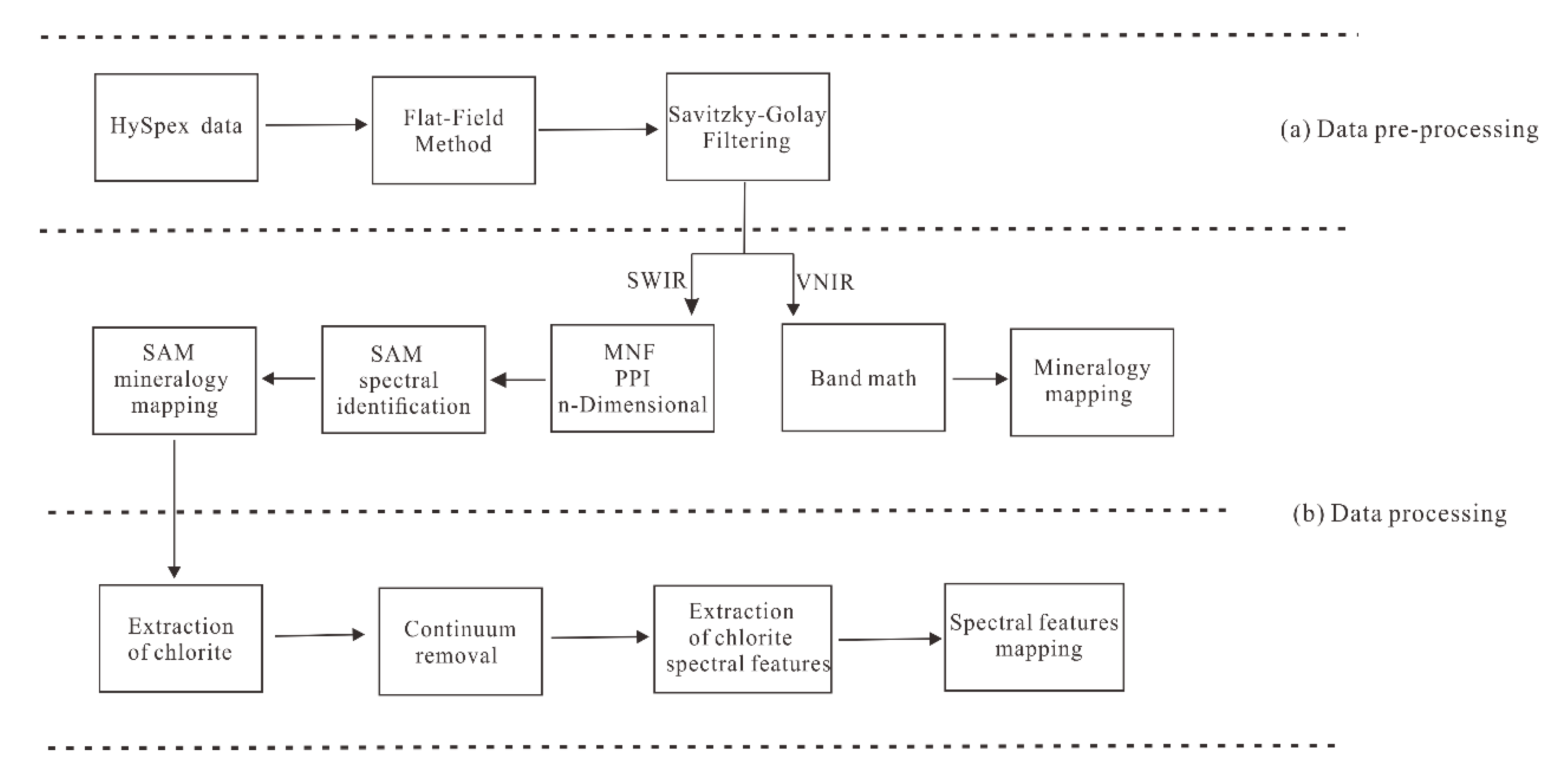
4.2. The HySpex Data Preprocessing
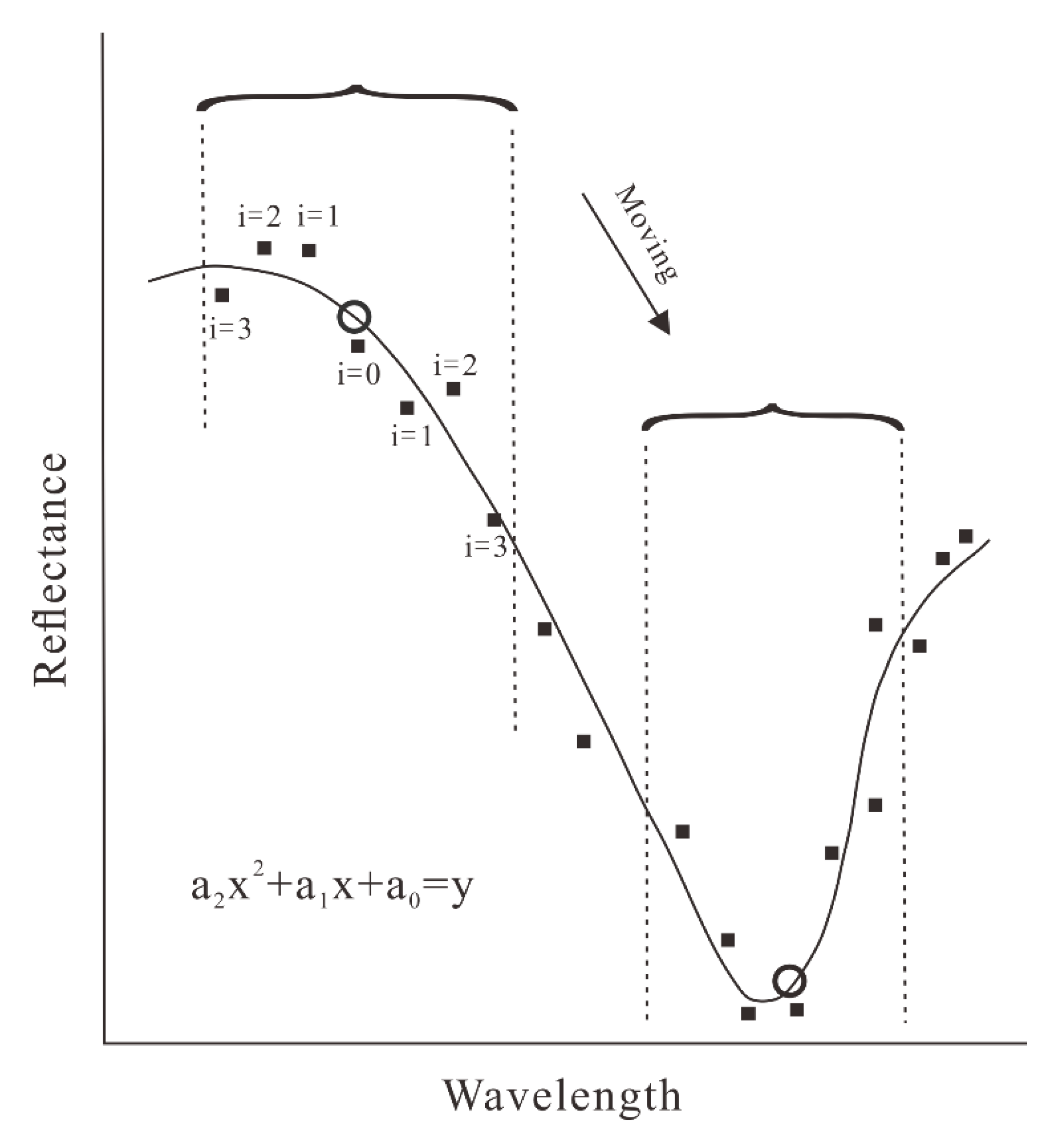
4.3. The HySpex Data Processing
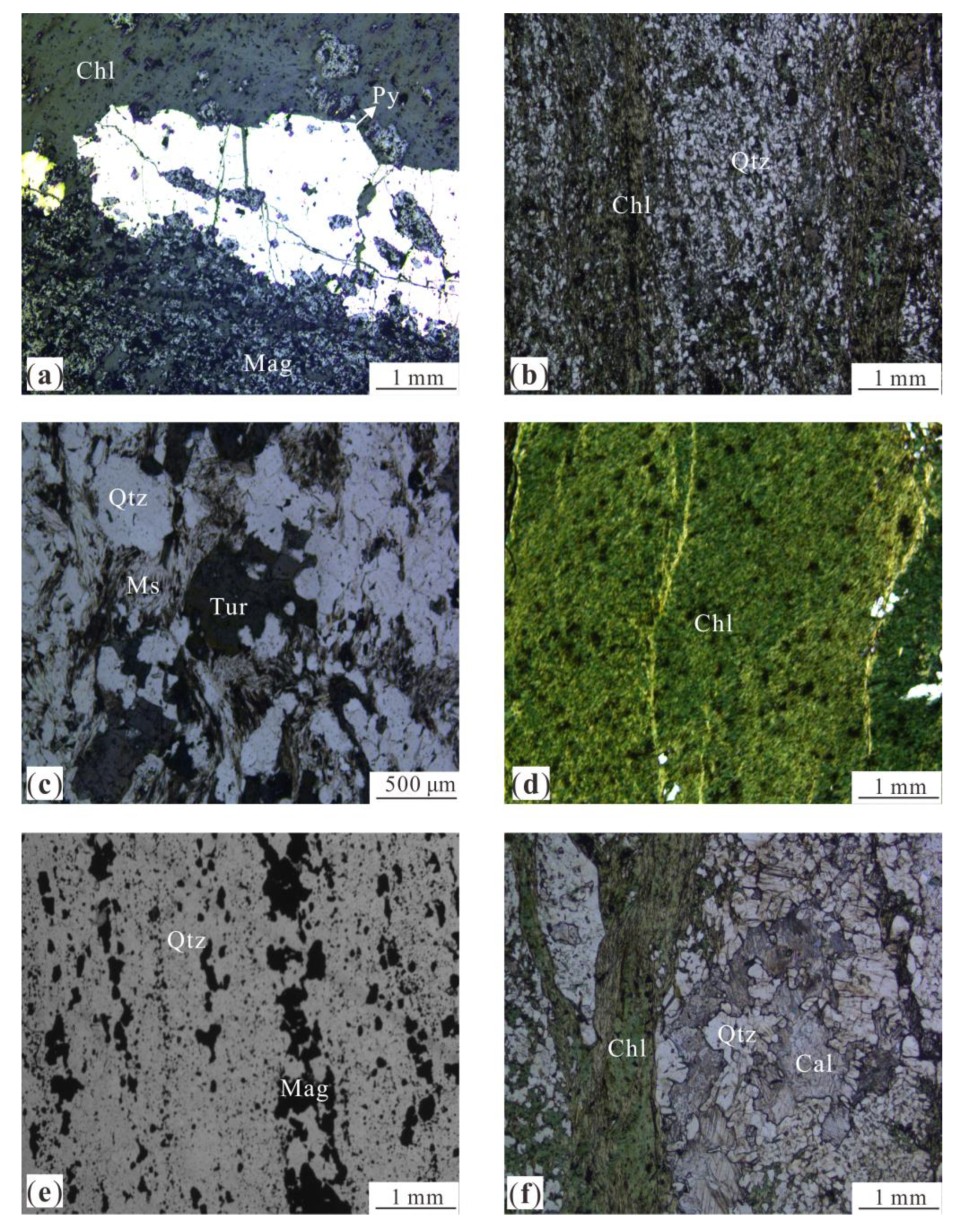
5. Results
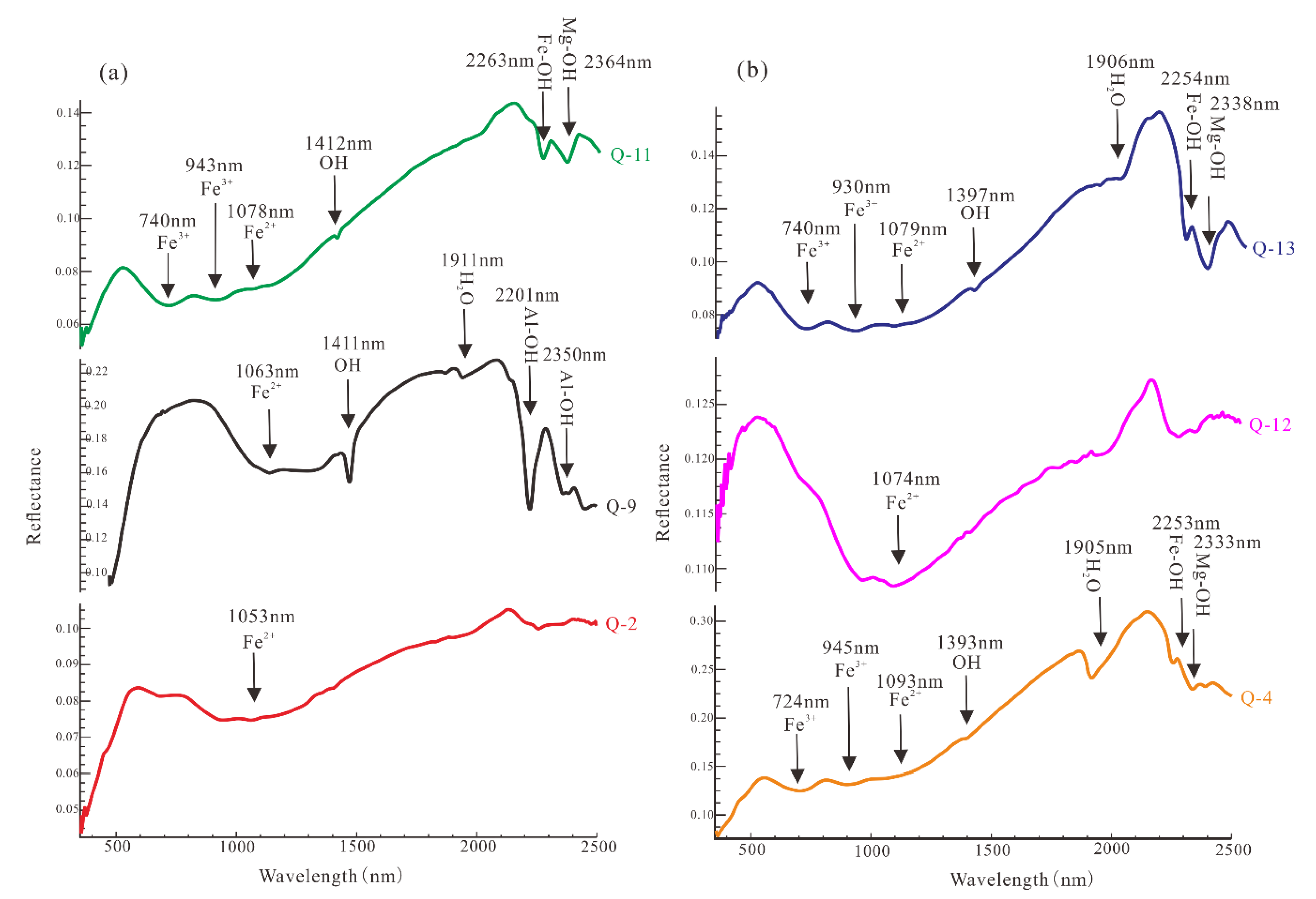
5.1. The Spectra of Point Measurement
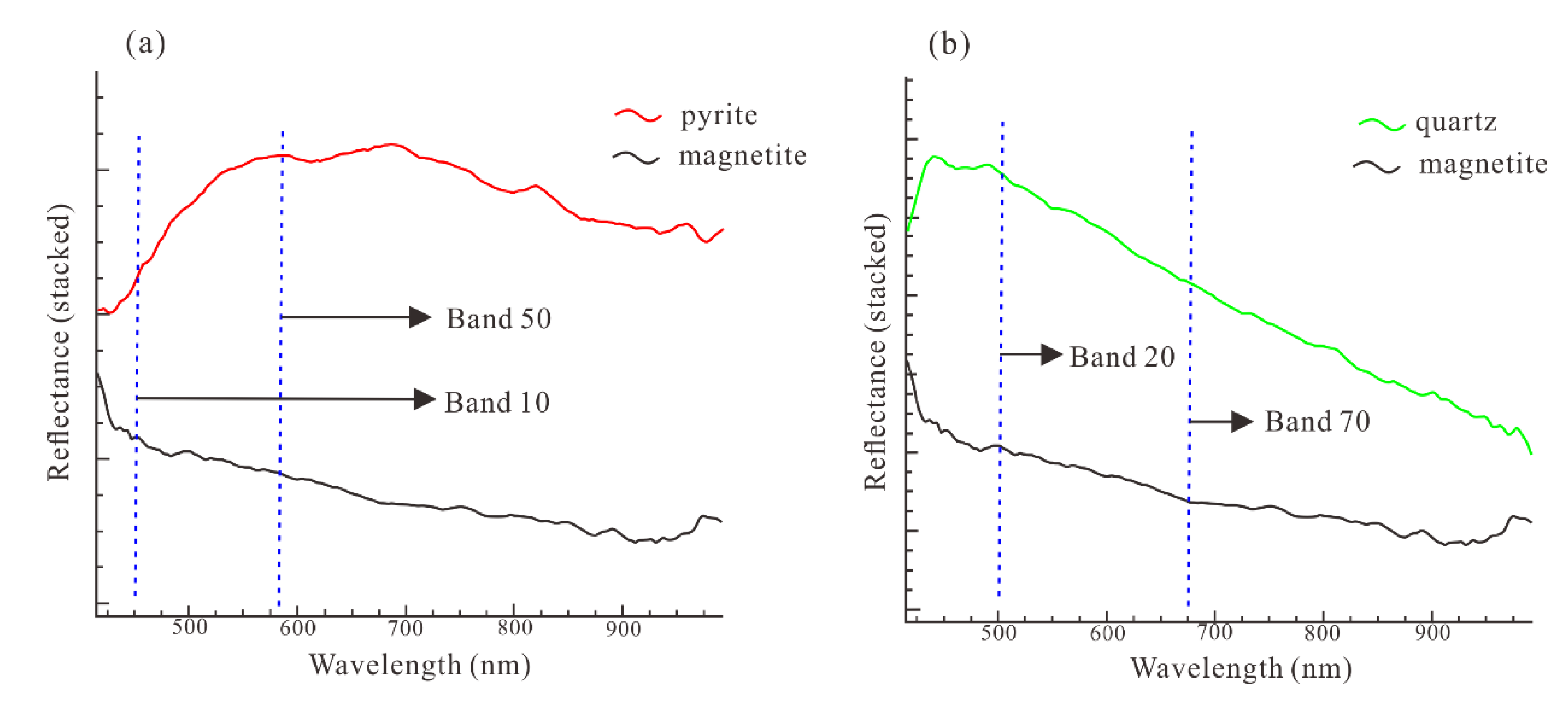
5.2. Minerals Identification from the HySpex Data
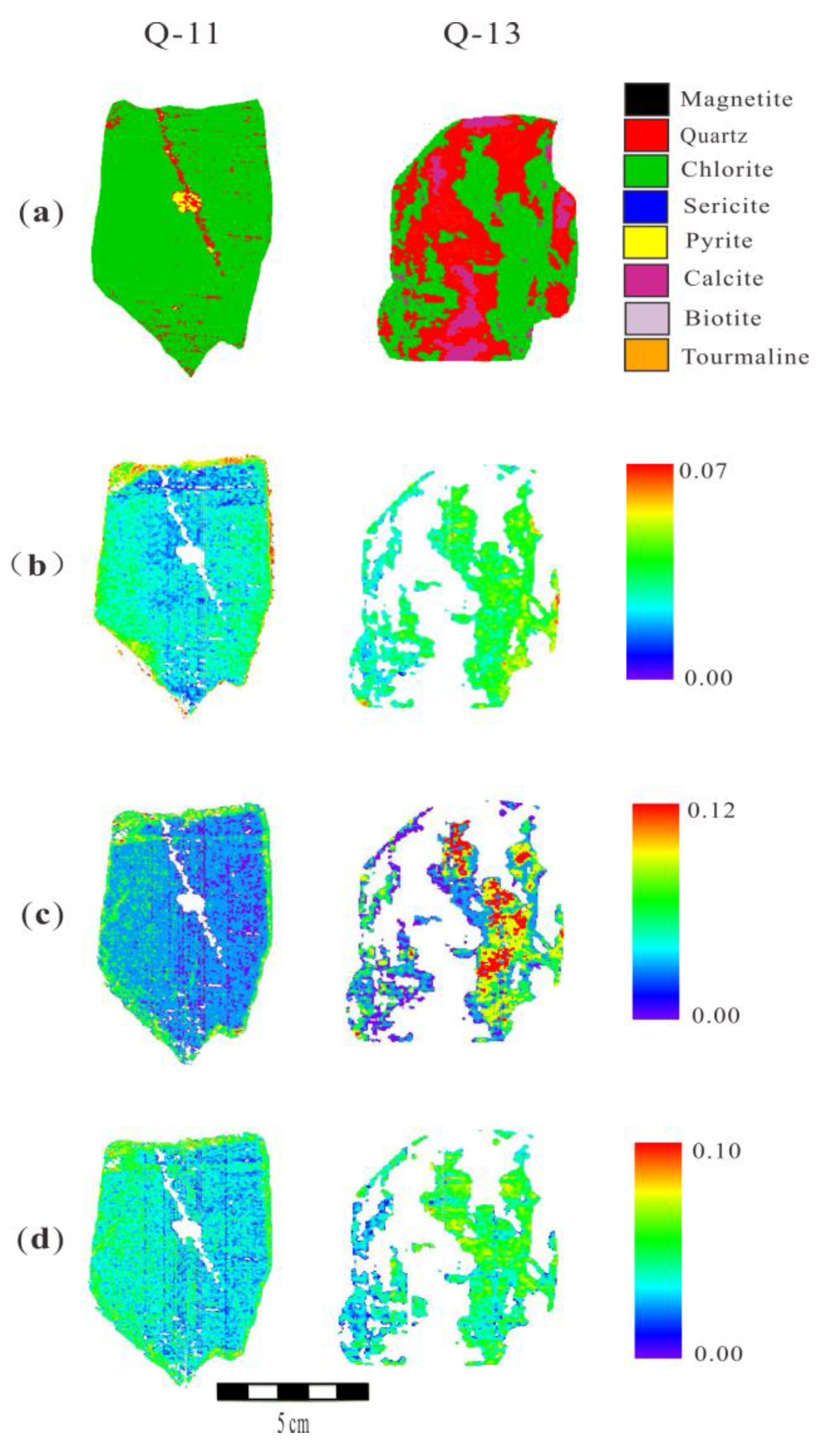
5.3. Mapping Absorption Features of Chlorite from the HySpex Data
6. Discussion
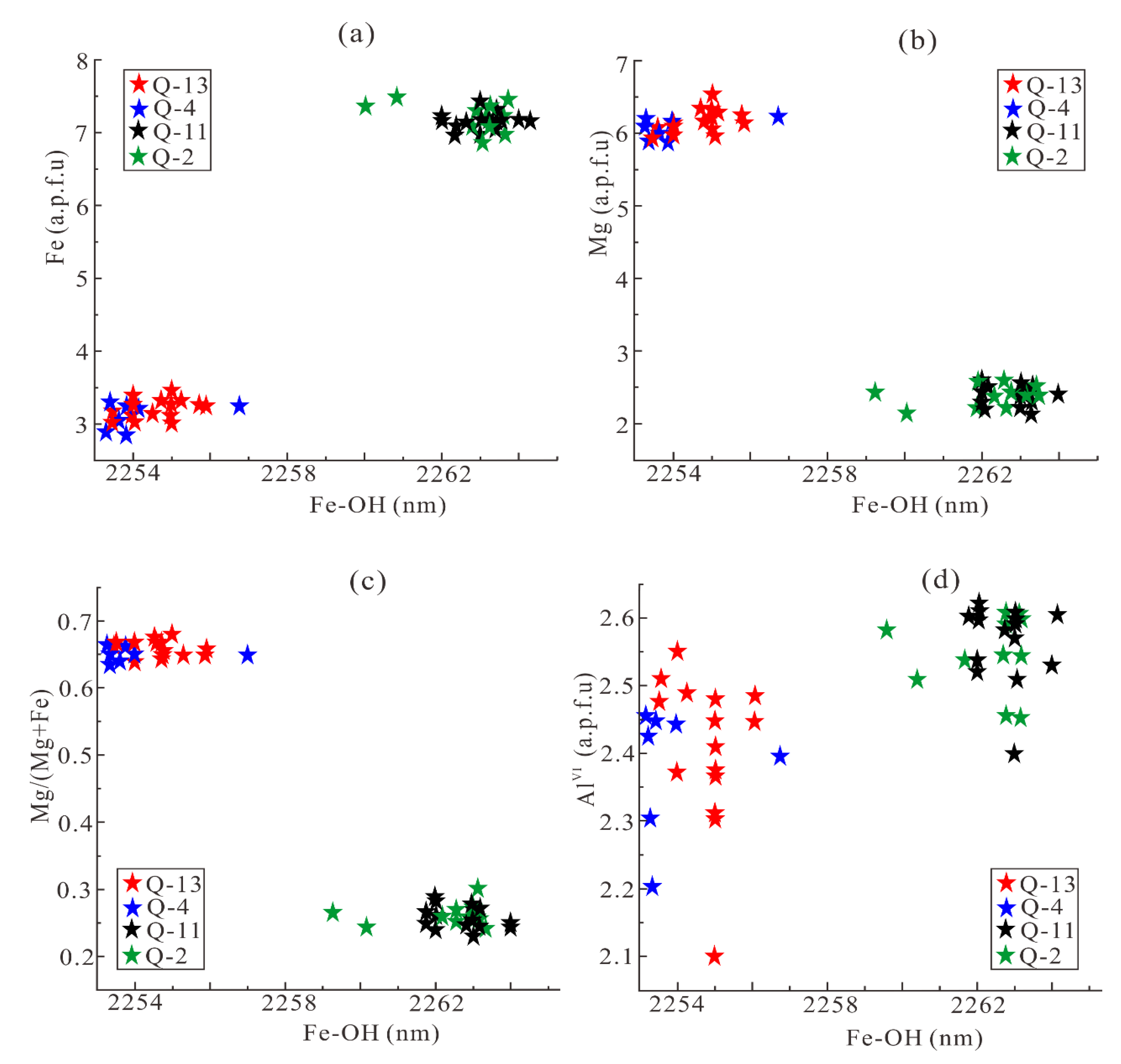
6.1. The Cause of Spectral Variation of Different Chlorites in Qidashan Iron Deposit
6.2. Geological Significance and Application to Mineral Exploration
6.3. Advantages and Disadvantages of Laboratory-Based Hyperspectral Imaging
7. Conclusions
- (1)
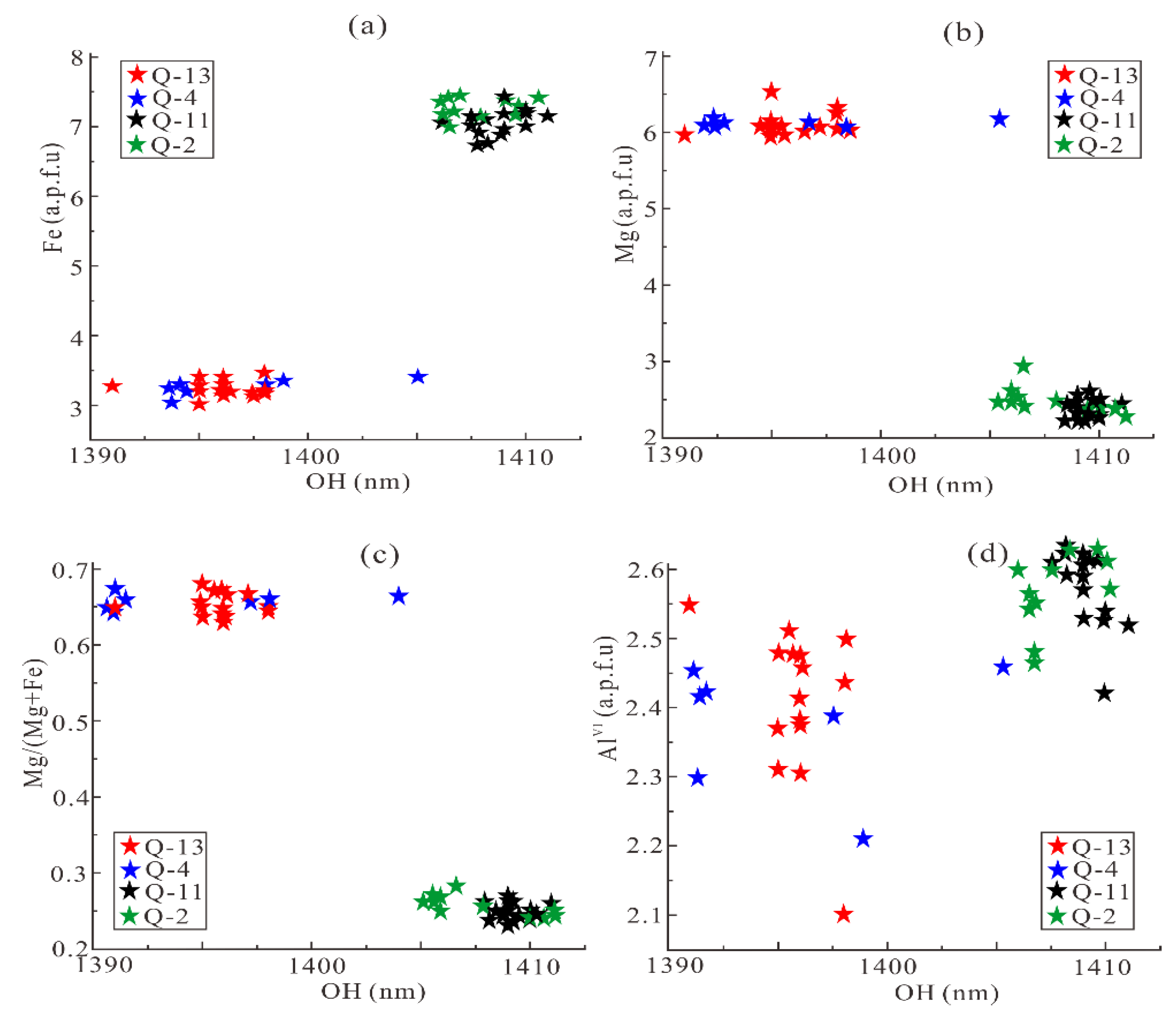
- The results of mineral mapping and chlorite spectral features extraction in Qidashan Iron Deposit show that chlorite both occurs in the wall rocks around BIFs and high-grade iron ore. The wavelength position of OH, Fe-OH, and Mg-OH absorptions of chlorite around high-grade iron ore are between 1400 and 1410, 2260 and 2265, and 2360 and 2370 nm, respectively, whereas the corresponding wavelengths are between 1390 and 1400, 2245 and 2255, and 2330 and 2340 nm around BIFs.
- (2)
- The higher spatial resolution laboratory-based hyperspectral imaging can reflect the specific information of mineral species, mineral content, structure, and even the polytypes of the rock sample.
- (3)
- The relationship between cations in the chlorite octahedral layer and the absorption wavelengths of OH, Fe-OH, and Mg-OH indicates that Fe is positively related to the wavelength, whereas Mg and Mg/(Mg + Fe) are inversely related. The wavelength appears to be weakly influenced by AlVI. Nevertheless, the relative absorption depths of the OH, Fe-OH, and Mg-OH are a poor guide to chemical composition of chlorite.
- (4)
- The difference in the chemical composition of chlorite caused by the different formation conditions of BIFs and high-grade iron ore in Qidashan Iron Deposit is well recorded in the wavelength position of chlorite. This difference of the wavelength, in turn, can be used as a favorable tool for the exploration of high-grade iron ore in the Anshan-Benxi area. This chlorite hyperspectral imaging tool may also provide a basis for the aerial or satellite hyperspectral remote sensing mineral exploration in Anshan-Benxi area.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, R.N. Water frost and ice: The near-infrared spectral reflectance 0.65–2.5 μm. J. Geophys. Res. Space Phys. 1981, 86, 3087–3096. [Google Scholar] [CrossRef]
- Hapke, B. Bidirectional reflectance spectroscopy: 1. Theory. J. Geophys. Res. Space Phys. 1981, 86, 3039–3054. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Space Phys. 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Bedini, E. Mineral mapping in the Kap Simpson complex, central East Greenland, using HyMap and ASTER remote sensing data. Adv. Space Res. 2011, 47, 60–73. [Google Scholar] [CrossRef]
- Dalm, M.; Buxton, M.W.; Van Ruitenbeek, F.J.; Voncken, J.H. Application of near-infrared spectroscopy to sensor based sorting of a porphyry copper ore. Miner. Eng. 2014, 58, 7–16. [Google Scholar] [CrossRef]
- Dalm, M.; Buxton, M.; Van Ruitenbeek, F. Discriminating ore and waste in a porphyry copper deposit using short-wavelength infrared (SWIR) hyperspectral imagery. Miner. Eng. 2017, 105, 10–18. [Google Scholar] [CrossRef]
- Neal, L.C.; Wilkinson, J.J.; Mason, P.J.; Chang, Z. Spectral characteristics of propylitic alteration minerals as a vectoring tool for porphyry copper deposits. J. Geochem. Explor. 2018, 184, 179–198. [Google Scholar] [CrossRef] [Green Version]
- De Linaje, V.A.; Khan, S.D.; Nicolás, V.A.D.L.D. Mapping of diagenetic processes in sandstones using imaging spectroscopy: A case study of the Utrillas Formation, Burgos, Spain. Sediment. Geol. 2017, 353, 114–124. [Google Scholar] [CrossRef]
- Greenberger, R.N.; Ehlmann, B.L.; Jewell, P.W.; Birgenheier, L.P.; Green, R.O. Detection of organic-rich oil shales of the green river formation, Utah, with ground-based imaging spectroscopy. In Proceedings of the 2016 8th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing, Los Angeles, CA, USA, 23 August 2016. [Google Scholar]
- Zaini, N.; Van Der Meer, F.; Van Der Werff, H. Determination of Carbonate Rock Chemistry Using Laboratory-Based Hyperspectral Imagery. Remote Sens. 2014, 6, 4149–4172. [Google Scholar] [CrossRef] [Green Version]
- Baissa, R.; Labbassi, K.; Launeau, P.; Gaudin, A.; Ouajhain, B. Using HySpex SWIR-320m hyperspectral data for the identification and mapping of minerals in hand specimens of carbonate rocks from the Ankloute Formation (Agadir Basin, Western Morocco). J. Afr. Earth Sci. 2011, 61, 1–9. [Google Scholar] [CrossRef]
- Lampinen, H.M.; Laukamp, C.; Occhipinti, S.A.; Hardy, L. Mineral footprints of the Paleoproterozoic sediment-hosted Abra Pb-Zn-Cu-Au deposit Capricorn Orogen, Western Australia. Ore Geol. Rev. 2018, 104, 436–461. [Google Scholar] [CrossRef]
- Wang, E.-D.; Xia, J.-M.; Fu, J.-F.; Jia, S.-S.; Men, Y.-K. Formation mechanism of Gongchangling high-grade magnetite deposit hosted in Archean BIF, Anshan-Benxi area, Northeastern China. Ore Geol. Rev. 2014, 57, 308–321. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.-Q.; Li, L.-X.; Zhang, Z.-C.; Liu, M.-J.; Yao, T.; Chen, J. Desilicification and iron activation–reprecipitation in the high-grade magnetite ores in BIFs of the Anshan-Benxi area, China: Evidence from geology, geochemistry and stable isotopic characteristics. J. Asian Earth Sci. 2015, 113, 998–1016. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of Rocks and Minerals, and Principles of Spectroscopy. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing, 3rd ed.; Rencz, A.N., Ed.; John Wiley and Sons: New York, NY, USA, 1999; Volume 3, pp. 3–58. [Google Scholar]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. Space Phys. 1990, 95, 12653–12680. [Google Scholar] [CrossRef] [Green Version]
- King, T.V.V.; Clark, R.N. Spectral characteristics of chlorites and Mg-serpentines using high-resolution reflectance spectroscopy. J. Geophys. Res. Space Phys. 1989, 94, 13997–14008. [Google Scholar] [CrossRef]
- Hunt, G.R. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Hunt, G.R. Near-infrared (1.3–2.4) μm spectra of alteration minerals—Potential for use in remote sensing. Geophysics 1979, 44, 1974–1986. [Google Scholar] [CrossRef]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; Brown, A.J. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Min. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Bishop, J.L. Infrared Spectroscopic Analyses on the Nature of Water in Montmorillonite. Clays Clay Min. 1994, 42, 702–716. [Google Scholar] [CrossRef]
- Yang, M.; Ye, M.; Han, H.; Ren, G.; Han, L.; Zhang, Z. Near-Infrared Spectroscopic Study of Chlorite Minerals. J. Spectrosc. 2018, 2018, 6958260. [Google Scholar] [CrossRef]
- Burns, R.G. Mineralogical Applications of Crystal Field Theory; Cambridge University Press: London, UK, 1993; pp. 149–152. [Google Scholar]
- Yang, K.; Lian, C.; Huntington, J.F.; Peng, Q.; Wang, Q. Infrared spectral reflectance characterization of the hydrothermal alteration at the Tuwu Cu–Au deposit, Xinjiang, China. Miner. Depos. 2005, 40, 324–336. [Google Scholar] [CrossRef]
- James, H.L. Sedimentary facies of iron-formation. Econ. Geol. 1954, 49, 235–293. [Google Scholar] [CrossRef]
- Zhai, M.; Santosh, M. Metallogeny of the North China Craton: Link with secular changes in the evolving Earth. Gondwana Res. 2013, 24, 275–297. [Google Scholar] [CrossRef]
- Vaiphasa, C. Consideration of smoothing techniques for hyperspectral remote sensing. ISPRS J. Photogramm. Remote Sens. 2006, 60, 91–99. [Google Scholar] [CrossRef]
- Fraser, S.J.; Whitbourn, L.; Yang, K.; Ramanaidou, E.; Connor, P.; Poropat, G.; Soole, P.; Mason, P.; Coward, D.; Phillips, R. Mineralogical Face-Mapping Using Hyperspectral Scanning for Mine Mapping and Control. In Proceedings of the 6th International Mining Geology Conference, Darwin, NT, Australia, 21 August 2006; pp. 227–232. [Google Scholar]
- Ruffin, C.; King, R.L.; Younan, N.H. A Combined Derivative Spectroscopy and Savitzky-Golay Filtering Method for the Analysis of Hyperspectral Data. GISci. Remote Sens. 2008, 45, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky–Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Ostertagov, E.; Ostertag, O. Methodology and application of Savitzky-Golay moving average polynomial smoother. Glob. J. Pure Appl. Math. 2016, 12, 3201–3210. [Google Scholar]
- Tong, D.; Jia, Z.; Qin, X.; Cao, C.; Chang, C. An Optimal Savitzky-golay Filtering Based Vertical Handoff Algorithm in Heterogeneous Wireless Networks. J. Comput. 2014, 9, 2685–2690. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Wang, D.; Liu, S.; Song, L.; Wang, Y.; Zhao, Z. Research and Verification of a Remote Sensing BIF Model Based on Spectral Reflectance Characteristics. J. Indian Soc. Remote Sens. 2019, 47, 1051–1061. [Google Scholar] [CrossRef]
- Kruse, F.A.; Bedell, R.L.; Taranik, J.V.; Peppin, W.A.; Weatherbee, O.; Calvin, W.M. Mapping alteration minerals at prospect, outcrop and drill core scales using imaging spectrometry. Int. J. Remote Sens. 2011, 33, 1780–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boardman, J.W. Analysis, understanding and visualization of hyperspectral data last convex sets in n-space. In Proceedings of the SPIE Defense and Security-The International Scoiety for Optical Engineering, Orlando, FL, USA, 17 April 1995. [Google Scholar]
- Kruse, F.; Lefkoff, A.; Boardman, J.; Heidebrecht, K.; Shapiro, A.; Barloon, P.; Goetz, A. The spectral image processing system (SIPS)—Interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Duke, E.F. Near infrared spectra of muscovite, Tschermak substitution, and metamorphic reaction progress: Implications for remote sensing. Geology 1994, 22, 621–624. [Google Scholar] [CrossRef]
- Van Der Meer, F. Spectral reflectance of carbonate mineral mixtures and bidirectional reflectance theory: Quantitative analysis techniques for application in remote sensing. Remote Sens. Rev. 1995, 13, 67–94. [Google Scholar] [CrossRef]
- Kurz, T.H.; Dewit, J.; Buckley, S.J.; Thurmond, J.B.; Hunt, D.W.; Swennen, R. Hyperspectral image analysis of different carbonate lithologies (limestone, karst and hydrothermal dolomites): The Pozalagua Quarry case study (Cantabria, North-west Spain). Sedimentology 2011, 59, 623–645. [Google Scholar] [CrossRef]
- Zaini, N.; Van Der Meer, F.D.; Van Der Werff, H. Effect of Grain Size and Mineral Mixing on Carbonate Absorption Features in the SWIR and TIR Wavelength Regions. Remote Sens. 2012, 4, 987–1003. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Huntington, J.F.; Browne, P.R.; Ma, C. An infrared spectral reflectance study of hydrothermal alteration minerals from the Te Mihi sector of the Wairakei geothermal system, New Zealand. Geothermics 2000, 29, 377–392. [Google Scholar] [CrossRef]
- McLeod, R.L.; Gabell, A.R.; Gardavsky, V. Chlorite infrared spectral data as proximity indicators of volcanogenic massive sulfide mineralisation. In Proceedings of the Pacific Rim Congress 87, Gold Coast, Australia, 1 January 1987; pp. 321–324. [Google Scholar]
- Li, H.; Zhang, Z.; Li, L.; Zhang, Z.; Chen, J.; Yao, T. Types and general characteristics of the BIF-related iron deposits in China. Ore Geol. Rev. 2014, 57, 264–287. [Google Scholar] [CrossRef]
- Liu, M.J.; Zeng, Q.D.; Li, H.M.; Li, L.X.; Wen, Y.; Yao, L.D.; Gao, Y.S. Metallogenic epoch of high-grade iron ore deposits of iron activation and enrichment genesis in Anshan-Benxi area of Liaoning: Re-Os isotopic dating evidence of molybdenite from Qidashan iron deposit. Miner. Depos. 2017, 36, 237–249. [Google Scholar]
- Li, Y.H.; Zhang, Z.J.; Hou, K.J.; Duan, C.; Wang, D.F.; Hu, G.Y. The genesis of Gongchangling high-grade iron ores, Anshan-Benxi area, Liaoning province, NE China: Evidence from Fe-Si-O-S isotopes. Acta Geol. Sin. 2014, 88, 2351–2372. [Google Scholar]
- Chen, G.Y.; Sun, D.S.; Sun, C.M.; Li, M.H.; Wang, X.F.; Wang, Z.F. Genesis of Gongchanglin iron deposit. J. Mineral. Petrol. 1984, 4, 1–226. [Google Scholar]
- Thompson, A.J.B.; Hauff, P.L.; Robitaille, A.J. Alteration mapping in exploration: Application of short-wave infrared (SWIR) spectroscopy. Soc. Econ. Geol. Newsl. 1999, 39, 15–27. [Google Scholar]








| Comment | K2O | Na2O | SiO2 | TiO2 | Al2O3 | FeO | MgO | MnO | CaO | Cl | P2O5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q-2-01 | 0.01 | 0.01 | 22.39 | 0.05 | 19.16 | 35.87 | 6.60 | 0.19 | 0 | 0.01 | 0.02 | 84.31 |
| Q-2-02 | 0.00 | 0.01 | 22.65 | 0.04 | 19.20 | 36.63 | 6.46 | 0.15 | 0.04 | 0.03 | 0.00 | 85.21 |
| Q-2-03 | 0.00 | 0.00 | 22.87 | 0.04 | 19.15 | 35.80 | 7.13 | 0.17 | 0.02 | 0.02 | 0.00 | 85.20 |
| Q-2-04 | 0.01 | 0.02 | 23.40 | 0.07 | 18.71 | 36.48 | 7.13 | 0.13 | 0.03 | 0.01 | 0.01 | 86.00 |
| Q-2-05 | 0.03 | 0.02 | 22.76 | 0.03 | 19.51 | 36.35 | 6.93 | 0.19 | 0.01 | 0.01 | 0.03 | 85.87 |
| Q-2-06 | 0.00 | 0.03 | 22.84 | 0.03 | 18.56 | 36.76 | 6.97 | 0.17 | 0.20 | 0.01 | 0.01 | 85.58 |
| Q-2-07 | 0.00 | 0.00 | 22.82 | 0.06 | 19.66 | 37.28 | 6.88 | 0.20 | 0.00 | 0.01 | 0.00 | 86.91 |
| Q-2-08 | 0.01 | 0.01 | 23.21 | 0.03 | 18.72 | 37.51 | 6.18 | 0.15 | 0.00 | 0.01 | 0.01 | 85.84 |
| Q-2-09 | 0.00 | 0.00 | 23.74 | 0.06 | 18.78 | 36.62 | 7.12 | 0.13 | 0.00 | 0.01 | 0.00 | 86.46 |
| Q-2-10 | 0.00 | 0.00 | 23.28 | 0.05 | 19.83 | 37.13 | 7.00 | 0.15 | 0.00 | 0.00 | 0.00 | 87.44 |
| Q-2-11 | 0.01 | 0.01 | 23.73 | 0.07 | 18.64 | 35.53 | 8.02 | 0.17 | 0.00 | 0.01 | 0.00 | 86.19 |
| Q-11-01 | 0.01 | 0.03 | 22.81 | 0.03 | 19.77 | 37.11 | 7.05 | 0.15 | 0.00 | 0.01 | 0.00 | 86.97 |
| Q-11-02 | 0.00 | 0.00 | 22.89 | 0.05 | 19.64 | 36.96 | 7.17 | 0.19 | 0.00 | 0.01 | 0.00 | 89.91 |
| Q-11-03 | 0.01 | 0.00 | 23.18 | 0.04 | 19.53 | 37.56 | 6.77 | 0.16 | 0.00 | 0.00 | 0.00 | 87.25 |
| Q-11-04 | 0.01 | 0.01 | 23.00 | 0.02 | 20.11 | 36.38 | 7.62 | 0.15 | 0.00 | 0.02 | 0.01 | 87.33 |
| Q-11-05 | 0.01 | 0.01 | 22.68 | 0.06 | 18.23 | 37.22 | 6.33 | 0.13 | 0.23 | 0.00 | 0.01 | 84.93 |
| Q-11-06 | 0.00 | 0.01 | 22.94 | 0.03 | 19.42 | 37.14 | 7.08 | 0.21 | 0.00 | 0.01 | 0.00 | 86.84 |
| Q-11-07 | 0.02 | 0.04 | 23.31 | 0.03 | 19.54 | 36.58 | 7.08 | 0.16 | 0.01 | 0.02 | 0.00 | 86.79 |
| Q-11-08 | 0.00 | 0.00 | 22.99 | 0.02 | 19.98 | 36.25 | 7.35 | 0.20 | 0.00 | 0.02 | 0.00 | 86.81 |
| Q-11-09 | 0.00 | 0.00 | 23.39 | 0.01 | 19.86 | 36.47 | 7.20 | 0.19 | 0.00 | 0.00 | 0.00 | 87.12 |
| Q-11-10 | 0.00 | 0.00 | 17.84 | 0.03 | 13.74 | 46.61 | 6.02 | 0.18 | 0.06 | 0.01 | 0.01 | 84.5 |
| Q-11-11 | 0.00 | 0.00 | 22.96 | 0.05 | 19.94 | 36.52 | 7.05 | 0.12 | 0.05 | 0.01 | 0.01 | 86.71 |
| Q-11-12 | 0.00 | 0.01 | 22.61 | 0.05 | 19.98 | 36.43 | 7.11 | 0.16 | 0.00 | 0.01 | 0.00 | 86.36 |
| Q-11-13 | 0.00 | 0.00 | 23.02 | 0.03 | 19.49 | 37.19 | 6.46 | 0.16 | 0.00 | 0.01 | 0.00 | 86.36 |
| Q-11-14 | 0.00 | 0.03 | 22.75 | 0.05 | 19.89 | 37.19 | 7.00 | 0.18 | 0.00 | 0.03 | 0.00 | 87.12 |
| Q-11-15 | 0.00 | 0.00 | 22.75 | 0.04 | 20.06 | 37.17 | 6.91 | 0.19 | 0.00 | 0.00 | 0.02 | 87.14 |
| Q-13-01 | 0.00 | 0.00 | 26.62 | 0.02 | 19.87 | 18.60 | 19.05 | 0.40 | 0.00 | 0.00 | 0.00 | 84.56 |
| Q-13-02 | 0.03 | 0.02 | 26.51 | 0.04 | 18.61 | 18.95 | 19.12 | 0.39 | 0.02 | 0.00 | 0.00 | 83.69 |
| Q-13-03 | 0.03 | 0.02 | 27.01 | 0.05 | 19.70 | 18.84 | 19.49 | 0.39 | 0.00 | 0.00 | 0.02 | 85.55 |
| Q-13-04 | 0.01 | 0.00 | 28.36 | 0.01 | 16.89 | 19.75 | 20.28 | 0.38 | 0.04 | 0.01 | 0.01 | 85.74 |
| Q-13-05 | 0.03 | 0.03 | 27.87 | 0.04 | 18.61 | 17.38 | 21.20 | 0.40 | 0.00 | 0.01 | 0.00 | 85.57 |
| Q-13-06 | 0.00 | 0.00 | 25.65 | 0.05 | 20.26 | 18.84 | 19.91 | 0.39 | 0.03 | 0.00 | 0.00 | 85.13 |
| Q-13-07 | 0.01 | 0.03 | 26.41 | 0.03 | 19.55 | 19.07 | 19.77 | 0.38 | 0.02 | 0.00 | 0.00 | 85.27 |
| Q-13-08 | 0.01 | 0.01 | 27.1 | 0.02 | 18.91 | 18.78 | 19.87 | 0.38 | 0.00 | 0.01 | 0.01 | 85.1 |
| Q-13-09 | 0.00 | 0.01 | 26.29 | 0.04 | 20.13 | 18.99 | 19.61 | 0.41 | 0.00 | 0.00 | 0.00 | 85.48 |
| Q-13-10 | 0.01 | 0.01 | 26.9 | 0.05 | 19.7 | 18.89 | 19.80 | 0.36 | 0.00 | 0.01 | 0.01 | 85.74 |
| Q-13-11 | 0.00 | 0.00 | 27.17 | 0.04 | 19.32 | 18.84 | 19.31 | 0.40 | 0.00 | 0.01 | 0.02 | 85.11 |
| Q-13-12 | 0.02 | 0.00 | 26.82 | 0.02 | 19.68 | 18.36 | 19.44 | 0.36 | 0.00 | 0.01 | 0.00 | 84.71 |
| Q-13-13 | 0.02 | 0.01 | 27.05 | 0.03 | 19.50 | 18.56 | 19.39 | 0.42 | 0.00 | 0.01 | 0.00 | 84.99 |
| Q-13-14 | 0.08 | 0.01 | 27.01 | 0.03 | 19.04 | 18.66 | 20.55 | 0.36 | 0.00 | 0.00 | 0.01 | 85.75 |
| Q-13-15 | 0.02 | 0.00 | 26.67 | 0.03 | 19.74 | 18.92 | 19.68 | 0.40 | 0.00 | 0.00 | 0.00 | 85.46 |
| Q-4-01 | 0.00 | 0.06 | 26.10 | 0.02 | 19.49 | 18.84 | 19.33 | 0.39 | 0.01 | 0.10 | 0.01 | 84.35 |
| Q-4-02 | 0.06 | 0.01 | 30.32 | 0.11 | 16.18 | 18.63 | 19.50 | 0.35 | 0.04 | 0.01 | 0.00 | 85.21 |
| Q-4-03 | 0.04 | 0.00 | 27.34 | 0.04 | 19.23 | 19.02 | 19.39 | 0.34 | 0.00 | 0.00 | 0.00 | 85.46 |
| Q-4-04 | 0.00 | 0.02 | 26.84 | 0.02 | 19.27 | 19.06 | 19.44 | 0.39 | 0.03 | 0.01 | 0.00 | 85.08 |
| Q-4-05 | 0.00 | 0.03 | 28.48 | 0.03 | 18.25 | 17.46 | 19.67 | 0.42 | 0.00 | 0.00 | 0.01 | 84.71 |
| Q-4-06 | 0.01 | 0.00 | 26.74 | 0.05 | 19.08 | 18.98 | 19.62 | 0.38 | 0.00 | 0.01 | 0.00 | 84.87 |
| Q-4-07 | 0.03 | 0.10 | 27.65 | 0.02 | 17.96 | 18.80 | 19.67 | 0.37 | 0.00 | 0.13 | 0.00 | 84.73 |
| Comment | Si | AlIV | AlVI | Ti | Fe3+ | Fe2+ | Mn | Mg | Ca | Na | K | Cl | Mg/(Fe + Mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q-2-01 | 5.27 | 2.73 | 2.60 | 0.01 | 0.00 | 7.11 | 0.04 | 2.31 | 0.00 | 0.00 | 0.00 | 0.01 | 0.25 |
| Q-2-02 | 5.28 | 2.72 | 2.58 | 0.01 | 0.00 | 7.20 | 0.03 | 2.25 | 0.01 | 0.01 | 0.00 | 0.02 | 0.24 |
| Q-2-03 | 5.31 | 2.69 | 2.56 | 0.01 | 0.00 | 7.00 | 0.03 | 2.47 | 0.00 | 0.00 | 0.00 | 0.02 | 0.26 |
| Q-2-04 | 5.39 | 2.61 | 2.48 | 0.01 | 0.00 | 7.07 | 0.03 | 2.45 | 0.01 | 0.02 | 0.01 | 0.01 | 0.26 |
| Q-2-05 | 5.25 | 2.75 | 2.57 | 0.01 | 0.00 | 7.09 | 0.04 | 2.38 | 0.01 | 0.02 | 0.01 | 0.01 | 0.25 |
| Q-2-06 | 5.31 | 2.69 | 2.42 | 0.01 | 0.00 | 7.26 | 0.03 | 2.42 | 0.00 | 0.02 | 0.00 | 0.00 | 0.25 |
| Q-2-07 | 5.21 | 2.79 | 2.53 | 0.01 | 0.00 | 7.22 | 0.04 | 2.34 | 0.00 | 0.02 | 0.00 | 0.01 | 0.24 |
| Q-2-08 | 5.40 | 2.60 | 2.53 | 0.01 | 0.00 | 7.32 | 0.03 | 2.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 |
| Q-2-09 | 5.44 | 2.56 | 2.52 | 0.01 | 0.00 | 7.03 | 0.02 | 2.43 | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 |
| Q-2-10 | 5.28 | 2.72 | 2.58 | 0.01 | 0.01 | 7.09 | 0.03 | 2.36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.25 |
| Q-2-11 | 5.42 | 2.58 | 2.45 | 0.01 | 0.00 | 6.83 | 0.03 | 2.73 | 0.00 | 0.00 | 0.01 | 0.01 | 0.29 |
| Q-11-01 | 5.20 | 2.80 | 2.53 | 0.00 | 0.00 | 7.18 | 0.03 | 2.40 | 0.00 | 0.02 | 0.00 | 0.01 | 0.25 |
| Q-11-02 | 5.22 | 2.78 | 2.52 | 0.01 | 0.00 | 7.15 | 0.04 | 2.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 |
| Q-11-03 | 5.28 | 2.72 | 2.54 | 0.01 | 0.00 | 7.23 | 0.03 | 2.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 |
| Q-11-04 | 5.19 | 2.81 | 2.57 | 0.00 | 0.00 | 6.97 | 0.03 | 2.56 | 0.00 | 0.01 | 0.01 | 0.02 | 0.27 |
| Q-11-05 | 5.33 | 2.67 | 2.40 | 0.01 | 0.00 | 7.43 | 0.03 | 2.22 | 0.06 | 0.03 | 0.01 | 0.00 | 0.23 |
| Q-11-06 | 5.24 | 2.76 | 2.49 | 0.00 | 0.00 | 7.21 | 0.04 | 2.41 | 0.00 | 0.01 | 0.00 | 0.01 | 0.25 |
| Q-11-07 | 5.31 | 2.69 | 2.58 | 0.00 | 0.00 | 7.02 | 0.03 | 2.41 | 0.00 | 0.03 | 0.01 | 0.01 | 0.26 |
| Q-11-08 | 5.23 | 2.77 | 2.60 | 0.00 | 0.00 | 6.96 | 0.04 | 2.49 | 0.00 | 0.00 | 0.00 | 0.01 | 0.26 |
| Q-11-09 | 5.30 | 2.70 | 2.62 | 0.00 | 0.00 | 6.95 | 0.04 | 2.43 | 0.02 | 0.00 | 0.00 | 0.00 | 0.26 |
| Q-11-10 | 4.42 | 3.58 | 2.61 | 0.00 | 0.00 | 6.87 | 0.04 | 2.22 | 0.01 | 0.00 | 0.00 | 0.00 | 0.24 |
| Q-11-11 | 5.24 | 2.76 | 2.61 | 0.01 | 0.00 | 7.02 | 0.02 | 2.40 | 0.00 | 0.00 | 0.00 | 0.01 | 0.25 |
| Q-11-12 | 5.18 | 2.82 | 2.59 | 0.01 | 0.00 | 7.07 | 0.03 | 2.43 | 0.00 | 0.01 | 0.00 | 0.01 | 0.26 |
| Q-11-13 | 5.30 | 2.70 | 2.60 | 0.01 | 0.00 | 7.20 | 0.03 | 2.22 | 0.00 | 0.00 | 0.00 | 0.01 | 0.24 |
| Q-11-14 | 5.18 | 2.82 | 2.54 | 0.01 | 0.00 | 7.19 | 0.03 | 2.37 | 0.00 | 0.03 | 0.00 | 0.02 | 0.25 |
| Q-11-15 | 5.18 | 2.82 | 2.58 | 0.01 | 0.00 | 7.17 | 0.04 | 2.34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 |
| Q-13-01 | 5.61 | 2.39 | 2.55 | 0.00 | 0.09 | 3.18 | 0.07 | 5.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.65 |
| Q-13-02 | 5.67 | 2.33 | 2.37 | 0.01 | 0.03 | 3.36 | 0.07 | 6.10 | 0.01 | 0.01 | 0.01 | 0.00 | 0.64 |
| Q-13-03 | 5.63 | 2.37 | 2.48 | 0.01 | 0.06 | 3.22 | 0.07 | 6.06 | 0.00 | 0.01 | 0.00 | 0.00 | 0.65 |
| Q-13-04 | 5.93 | 2.07 | 2.10 | 0.00 | 0.02 | 3.43 | 0.07 | 6.33 | 0.01 | 0.00 | 0.00 | 0.00 | 0.65 |
| Q-13-05 | 5.76 | 2.24 | 2.31 | 0.01 | 0.04 | 2.97 | 0.07 | 6.54 | 0.00 | 0.02 | 0.02 | 0.00 | 0.69 |
| Q-13-06 | 5.39 | 2.61 | 2.41 | 0.01 | 0.00 | 3.39 | 0.07 | 6.23 | 0.01 | 0.00 | 0.00 | 0.00 | 0.65 |
| Q-13-07 | 5.54 | 2.46 | 2.39 | 0.00 | 0.00 | 3.38 | 0.07 | 6.29 | 0.00 | 0.02 | 0.00 | 0.00 | 0.65 |
| Q-13-08 | 5.69 | 2.31 | 2.37 | 0.00 | 0.03 | 3.26 | 0.07 | 6.22 | 0.00 | 0.01 | 0.00 | 0.01 | 0.65 |
| Q-13-09 | 5.50 | 2.50 | 2.47 | 0.01 | 0.00 | 3.33 | 0.07 | 6.12 | 0.00 | 0.01 | 0.00 | 0.00 | 0.65 |
| Q-13-10 | 5.60 | 2.40 | 2.44 | 0.01 | 0.03 | 3.26 | 0.06 | 6.15 | 0.00 | 0.01 | 0.00 | 0.01 | 0.65 |
| Q-13-11 | 5.69 | 2.31 | 2.47 | 0.01 | 0.10 | 3.20 | 0.07 | 6.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.65 |
| Q-13-12 | 5.63 | 2.37 | 2.51 | 0.00 | 0.08 | 3.14 | 0.06 | 6.08 | 0.00 | 0.00 | 0.01 | 0.00 | 0.65 |
| Q-13-13 | 5.67 | 2.33 | 2.49 | 0.01 | 0.09 | 3.16 | 0.07 | 6.05 | 0.00 | 0.00 | 0.01 | 0.00 | 0.65 |
| Q-13-14 | 5.62 | 2.38 | 2.30 | 0.00 | 0.00 | 3.28 | 0.06 | 6.37 | 0.00 | 0.01 | 0.04 | 0.00 | 0.66 |
| Q-13-15 | 5.58 | 2.42 | 2.45 | 0.00 | 0.02 | 3.29 | 0.07 | 6.14 | 0.00 | 0.00 | 0.01 | 0.00 | 0.65 |
| Q-4-01 | 5.54 | 2.46 | 2.42 | 0.00 | 0.00 | 3.35 | 0.07 | 6.12 | 0.00 | 0.05 | 0.00 | 0.07 | 0.65 |
| Q-4-02 | 6.24 | 1.76 | 2.20 | 0.02 | 0.15 | 3.05 | 0.06 | 5.98 | 0.01 | 0.00 | 0.01 | 0.01 | 0.66 |
| Q-4-03 | 5.71 | 2.29 | 2.45 | 0.01 | 0.09 | 3.23 | 0.06 | 6.04 | 0.00 | 0.00 | 0.02 | 0.00 | 0.65 |
| Q-4-04 | 5.64 | 2.36 | 2.42 | 0.00 | 0.04 | 3.31 | 0.07 | 6.09 | 0.01 | 0.02 | 0.00 | 0.01 | 0.65 |
| Q-4-05 | 5.94 | 2.06 | 2.45 | 0.00 | 0.22 | 2.83 | 0.07 | 6.12 | 0.00 | 0.02 | 0.00 | 0.01 | 0.68 |
| Q-4-06 | 5.64 | 2.36 | 2.38 | 0.01 | 0.02 | 3.33 | 0.07 | 6.17 | 0.00 | 0.00 | 0.00 | 0.01 | 0.65 |
| Q-4-07 | 5.82 | 2.18 | 2.29 | 0.00 | 0.07 | 3.24 | 0.07 | 6.17 | 0.00 | 0.08 | 0.01 | 0.09 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, D.; Yao, Y.; Fu, J.; Michalski, J.R.; Song, K. The Laboratory-Based HySpex Features of Chlorite as the Exploration Tool for High-Grade Iron Ore in Anshan-Benxi Area, Liaoning Province, Northeast China. Appl. Sci. 2020, 10, 7444. https://doi.org/10.3390/app10217444
Hao D, Yao Y, Fu J, Michalski JR, Song K. The Laboratory-Based HySpex Features of Chlorite as the Exploration Tool for High-Grade Iron Ore in Anshan-Benxi Area, Liaoning Province, Northeast China. Applied Sciences. 2020; 10(21):7444. https://doi.org/10.3390/app10217444
Chicago/Turabian StyleHao, Dahai, Yuzeng Yao, Jianfei Fu, Joseph R. Michalski, and Kun Song. 2020. "The Laboratory-Based HySpex Features of Chlorite as the Exploration Tool for High-Grade Iron Ore in Anshan-Benxi Area, Liaoning Province, Northeast China" Applied Sciences 10, no. 21: 7444. https://doi.org/10.3390/app10217444






