Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions
Abstract
:1. Introduction
2. Reaction Pathways
2.1. Mechanism of ORR
2.2. Mechanism of HER
3. Approaches for Screening the ORR and HER Performances
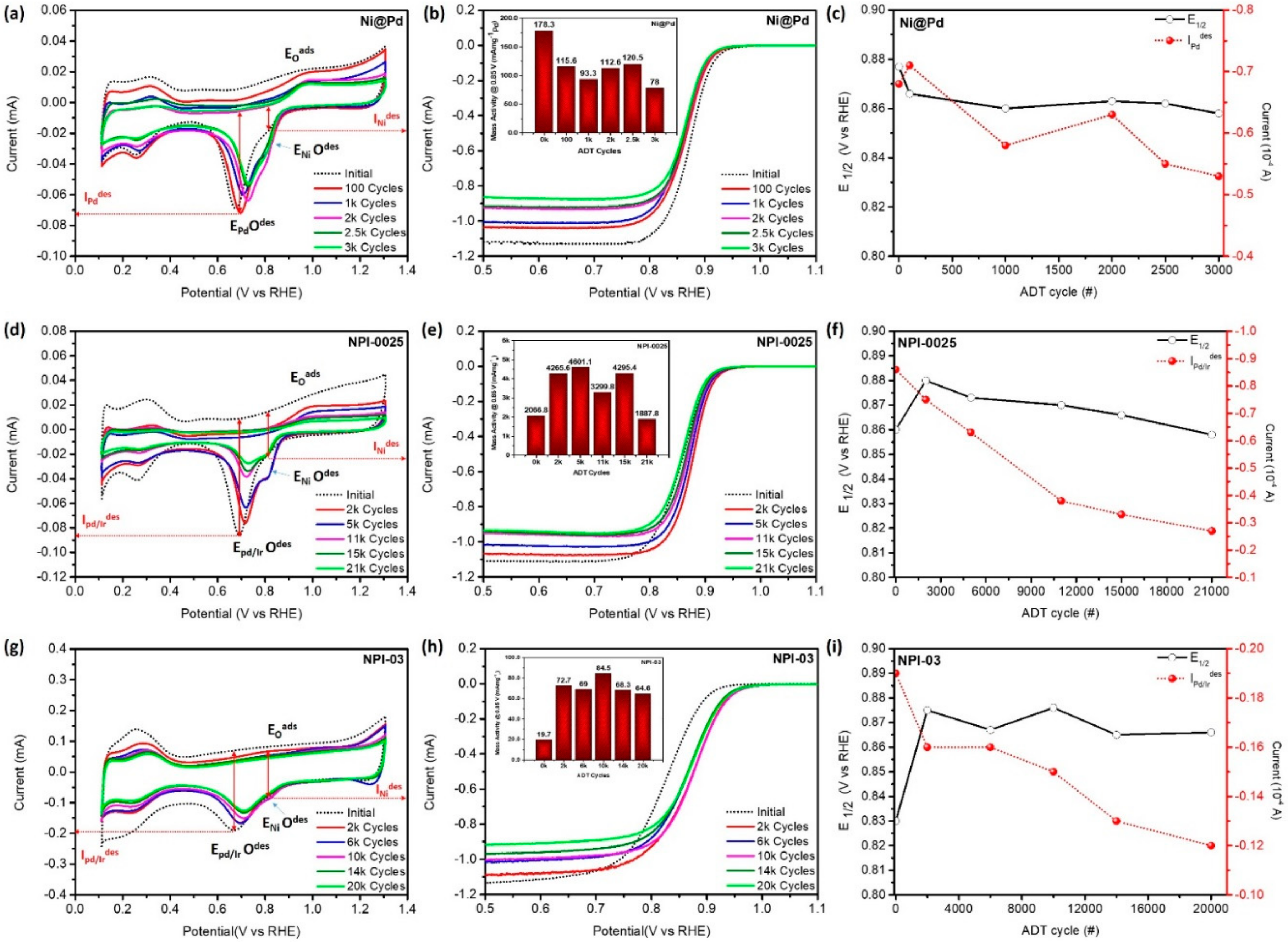
3.1. Evolution of ORR Performance
- (i)
- Mass activity: The mass activity (MA) is the most important parameter to evaluate the ORR performance of any catalyst, which is the measure of performance per unit loading of metal. Generally, the MA is calculated by the following equation:
- (ii)
- Specific activity: The specific activity (SA) indicates the number of active sites presents on the surface of the catalyst. The electrochemically active surface area (ECSA) plays an important role in evaluating the SA. The ECSA of the experimental catalysts can be calculated by acquiring the columbic charge for reduction of the monolayer Pt or Pd oxide after integration and double-layer correction using the following equation:
- (iii)
- Onset and half-wave potential: The onset potential (Eoc) and half-wave potential (E1/2) are the techniques for the quick screen of ORR performance. Both of these parameters can be obtained from the linear sweep voltammetry (LSV) curve (Figure 2).
3.2. Evolution of HER Performance
- (i)
- Overpotential: The overpotential is the difference between the applied (i.e., experimentally observed potential) and thermodynamically determined potential for any electrochemical reaction. The overpotential is the most important parameter for evaluating the HER performance, and a desired HER catalyst should be capable of lowering the overpotential.
- (ii)
- Electrochemically active surface area (ECSA): ECSA is the active area (i.e., number of active sites) present on the NC surface accessible to the electrolyte for charge transfer. The ECSA can be determined by using electrochemical techniques such as cyclic voltammetry (CV) and CO-stripping analysis.
- (iii)
- Tafel slope: Tafel slope, which is actually the slope of the linear region of a Tafel plot (i.e., the plot between overpotential vs. log |current density|) is used to achieve insight into the reaction pathways (mechanisms) of HER on a NC surface. The HER pathways on NC surfaces can be determined by comparing the Tafel slope value with that of theoretical ones. The theoretical Tafel slope values for the Volmer (i.e., the discharge step), the Heyrovsky (i.e., electrochemical desorption step), and the Tafel (i.e., the chemical desorption/combination) steps are 120 mVdec−1, 40 mVdec−1, and 30 mVdec−1, respectively. For instance, previous studies reported that the Tafel slope is 30 mVdec−1 for a commercial Pt catalyst in an acidic medium (i.e., 0.5 M H2SO4), which indicates that HER proceeds through the Volmer–Tafel process on the Pt surface.
- (iv)
- Exchange current density: The exchange current density is the current density at the equilibrium potential (i.e., both the cathodic and anodic currents are equal) and reflects the inherent nature of the NC. The exchange current density can be determined via the intersection of the extrapolated linear part of the Tafel plots to the X-axis.
- (v)
- Faradic efficiency: Faradic efficiency is the ratio of the amount of experimentally produced H2 to the theoretically determined H2 amount.
- (vi)
- Turnover efficiency: Turnover efficiency refers to the number of desired product molecules that a NC can produce per catalytic site per unit of time.
- (vii)
- Stability: Long term durability/stability is another important aspect for HER catalysts, which describes the ability of a catalyst to maintain its activity when operated for a particular time. The stability can be easily determined by comparing the overpotential and Tafel slopes of the as-prepared condition and after degradation test cycles.
4. Noble Metal-Based Electrocatalysts for ORR and HER
4.1. Pt-Based Catalysts in ORR and HER
4.2. Pd-Based Catalysts in ORR and HER
4.3. Ir-Based Catalysts in ORR and HER
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef] [Green Version]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nat. Cell Biol. 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Markovic, N.; Gasteiger, H.; Ross, P.N. Kinetics of Oxygen Reduction on Pt(hkl) Electrodes: Implications for the Crystallite Size Effect with Supported Pt Electrocatalysts. J. Electrochem. Soc. 1997, 144, 1591–1597. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nat. Cell Biol. 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Peter, S.C. An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Sci. 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nat. Cell Biol. 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Bhalothia, D.; Huang, T.-H.; Chou, P.-H.; Chen, P.-C.; Wang, K.-W.; Chen, T.-Y. CO-Reductive and O2-Oxidative Annealing Assisted Surface Restructure and Corresponding Formic Acid Oxidation Performance of PdPt and PdRuPt Nanocatalysts. Sci. Rep. 2020, 10, 8457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Mei, D.; Liang, X. The OH−-driven synthesis of Pt–Ni nanocatalysts with atomic segregation for alkaline hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 5475–5481. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.-H.; Lin, M.; Bhalothia, D.; Yang, S.-S.; Fan, G.-J.; Wang, J.-L.; Chan, T.-S.; Wang, Y.-L.; Tu, X.; et al. Local synergetic collaboration between Pd and local tetrahedral symmetric Ni oxide enables ultra-high-performance CO2 thermal methanation. J. Mater. Chem. A 2020, 8, 12744–12756. [Google Scholar] [CrossRef]
- Kuttiyiel, K.A.; Sasaki, K.; Chen, W.-F.; Su, D.; Adzic, R.R. Core–shell, hollow-structured iridium–nickel nitride nanoparticles for the hydrogen evolution reaction. J. Mater. Chem. A 2014, 2, 591–594. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 4174–4192. [Google Scholar] [CrossRef]
- Yu, L.; Song, S.; McElhenny, B.; Ding, F.; Luo, D.; Yu, Y.; Chen, S.; Ren, Z. A universal synthesis strategy to make metal nitride electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 19728–19732. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.-Y.; Sun, Y.-K. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2016, 4, 10379–10393. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2019, 30. [Google Scholar] [CrossRef]
- Murata, T.; Kotsuki, K.; Murayama, H.; Tsuji, R.; Morita, Y. Metal-free electrocatalysts for oxygen reduction reaction based on trioxotriangulene. Commun. Chem. 2019, 2, 46. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
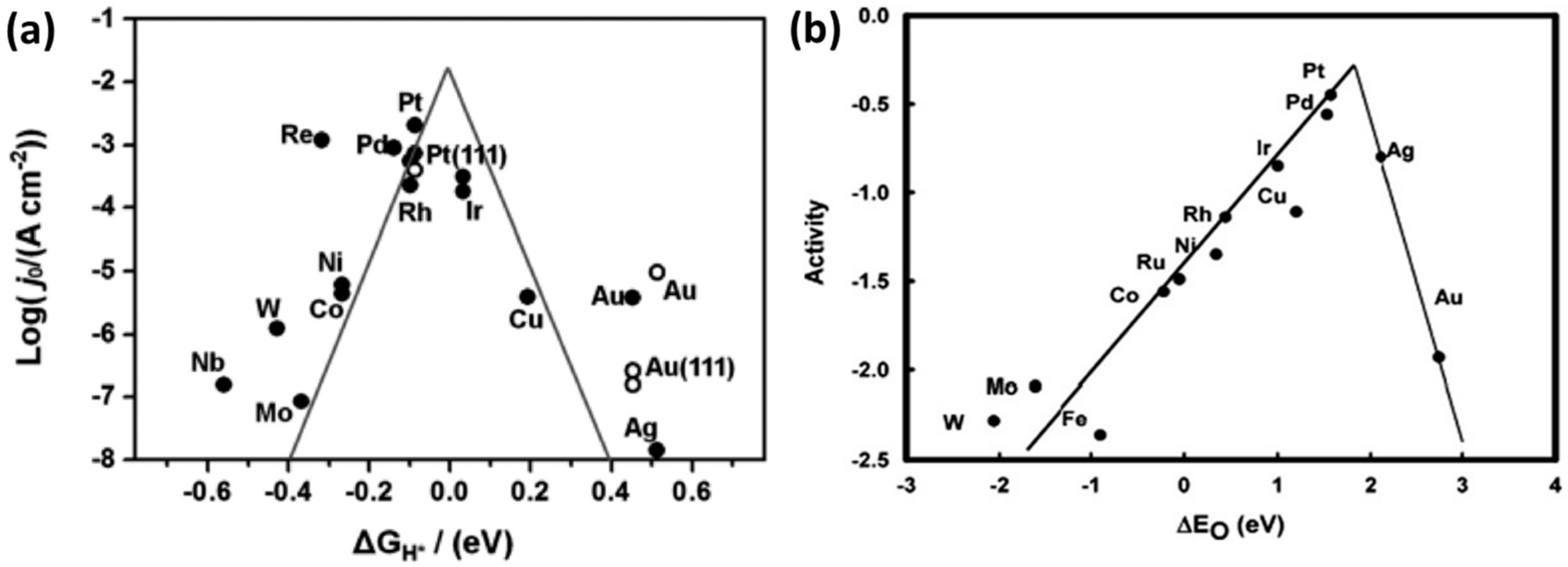
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Yano, H.; Watanabe, M.; Iiyama, A.; Uchida, H. Particle-size effect of Pt cathode catalysts on durability in fuel cells. Nano Energy 2016, 29, 323–333. [Google Scholar] [CrossRef]
- Oh, H.-S.; Nong, H.N.; Reier, T.; Bergmann, A.; Gliech, M.; De Araújo, J.F.; Willinger, E.; Schlögl, R.; Teschner, D.; Strasser, P. Electrochemical Catalyst–Support Effects and Their Stabilizing Role for IrOxNanoparticle Catalysts during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2016, 138, 12552–12563. [Google Scholar] [CrossRef]
- Chalgin, A.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Wu, J. Effect of supporting materials on the electrocatalytic activity, stability and selectivity of noble metal-based catalysts for oxygen reduction and hydrogen evolution reactions. Prog. Nat. Sci. 2020, 30, 289–297. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum Alloy Catalysts for Oxygen Reduction Reaction: Advances, Challenges and Perspectives. ChemNanoMat 2019, 6, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Oezaslan, M.; Hasché, F.; Strasser, P. Oxygen Electroreduction on PtCo3, PtCo and Pt3Co Alloy Nanoparticles for Alkaline and Acidic PEM Fuel Cells. J. Electrochem. Soc. 2012, 159, B394–B405. [Google Scholar] [CrossRef]
- Oezaslan, M.; Hasché, F.; Strasser, P. PtCu3, PtCu and Pt3Cu Alloy Nanoparticle Electrocatalysts for Oxygen Reduction Reaction in Alkaline and Acidic Media. J. Electrochem. Soc. 2012, 159, B444–B454. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Wan, M.; Dong, L.; Zhang, M.; Du, M. Highly efficient and durable PtCo alloy nanoparticles encapsulated in carbon nanofibers for electrochemical hydrogen generation. Chem. Commun. 2016, 52, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hwang, S.Y.; Trout, A.; Peng, Z. Solid-State Chemistry-Enabled Scalable Production of Octahedral Pt–Ni Alloy Electrocatalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2014, 136, 7805–7808. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; Li, J.; Liu, J. Facile fabrication of Pt–Ni alloy nanoparticles supported on reduced graphene oxide as excellent electrocatalysts for hydrogen evolution reaction in alkaline environment. J. Nanoparticle Res. 2019, 21, 13. [Google Scholar] [CrossRef]
- Stamenković, V.; Schmidt, T.J.; Ross, A.P.N.; Marković, N.M. Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Chou, J.-P.; Liu, P.-Y.; Chen, T.-Y.; Kai, J.-J.; Hu, A.; Chen, H.-Y.T. Pt3 clusters-decorated Co@Pd and Ni@Pd model core–shell catalyst design for the oxygen reduction reaction: A DFT study. J. Mater. Chem. A 2018, 6, 23326–23335. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.C.; Yan, C.; Yeh, W.; Tsai, D.-L.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y. Heterogeneous assembly of Pt-clusters on hierarchically structured CoOx@SnPd2@SnO2 quaternary nanocatalysts manifesting oxygen reduction reaction performance. New J. Chem. 2020, 44, 9712–9724. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, Y.; Li, L.; Liao, M.; Fu, Y.; Sun, C.; Shao, Z.; Shen, P.K. Electrochemically synthesized Cu/Pt core-shell catalysts on a porous carbon electrode for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 180, 84–91. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Wang, F. Synthesis and characterization of Cu@Pt/C core-shell structured catalysts for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2011, 36, 9151–9154. [Google Scholar] [CrossRef]
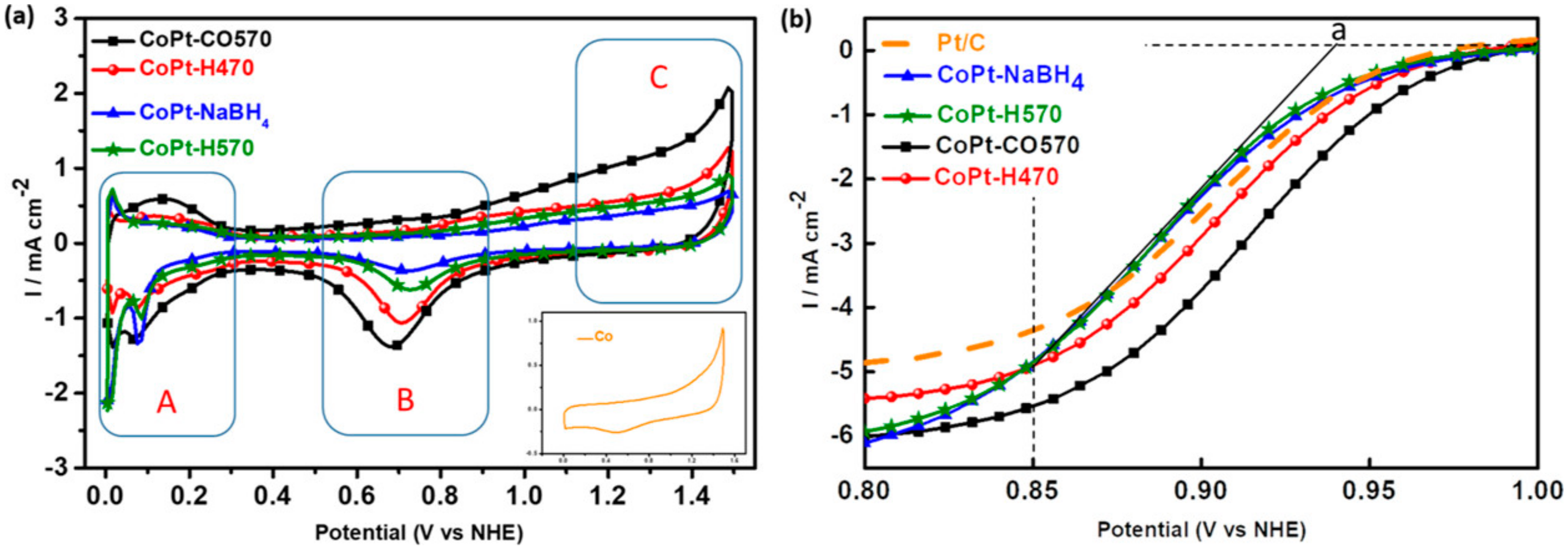
- Bhalothia, D.; Fan, Y.-J.; Huang, T.-H.; Lin, Z.-J.; Yang, Y.-T.; Wang, K.-W.; Chen, T.-Y. Local Structural Disorder Enhances the Oxygen Reduction Reaction Activity of Carbon-Supported Low Pt Loading CoPt Nanocatalysts. J. Phys. Chem. C 2019, 123, 19013–19021. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed Pt-Cu Core-Shell Nanoparticle Electrocatalysts for Use in PEM Fuel Cell Cathodes. J. Phys. Chem. C 2008, 112, 2770–2778. [Google Scholar] [CrossRef]
- Sarkar, A.; Manthiram, A. Synthesis of Pt@Cu Core-Shell Nanoparticles by Galvanic Displacement of Cu by Pt4+Ions and Their Application as Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. J. Phys. Chem. C 2010, 114, 4725–4732. [Google Scholar] [CrossRef]
- Hsu, I.J.; Hansgen, D.A.; McCandless, B.E.; Willis, B.G.; Chen, J.G. Atomic Layer Deposition of Pt on Tungsten Monocarbide (WC) for the Oxygen Reduction Reaction. J. Phys. Chem. C 2011, 115, 3709–3715. [Google Scholar] [CrossRef]
- Shao, F.-Q.; Lin, X.-X.; Feng, J.-J.; Yuan, J.; Chen, J.-R.; Wang, A.-J. Simple fabrication of core-shell AuPt@Pt nanocrystals supported on reduced graphene oxide for ethylene glycol oxidation and hydrogen evolution reactions. Electrochim. Acta 2016, 219, 321–329. [Google Scholar] [CrossRef]
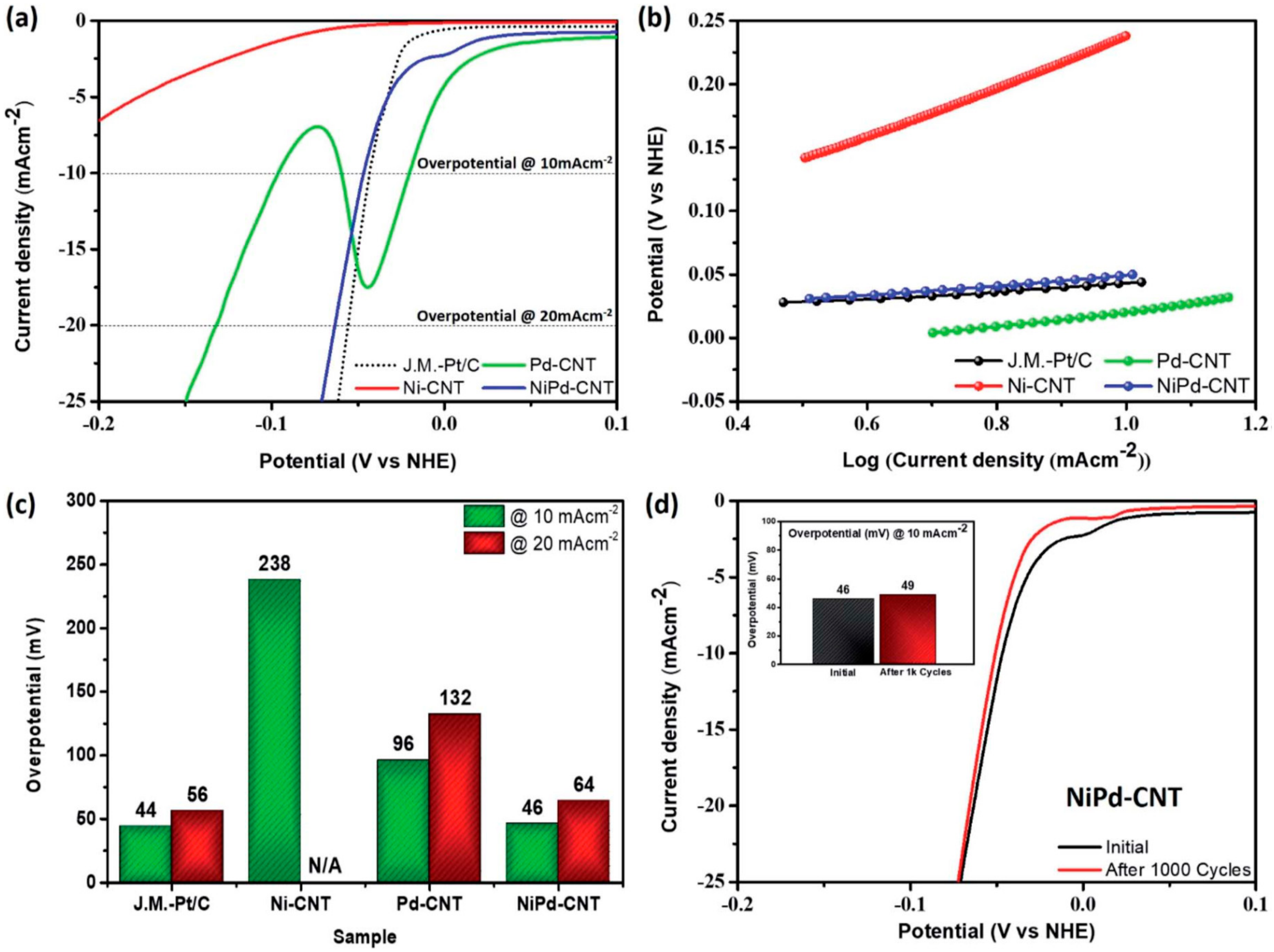
- Bhalothia, D.; Chou, J.-P.; Yan, C.; Hu, A.; Yang, Y.-T.; Chen, T.-Y. Programming ORR Activity of Ni/NiOx@Pd Electrocatalysts via Controlling Depth of Surface-Decorated Atomic Pt Clusters. ACS Omega 2018, 3, 8733–8744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalothia, D.; Dai, S.; Wang, S.-P.; Yan, C.; Huang, T.-H.; Chen, P.C.; Hiraoka, N.; Wang, K.-W.; Chen, T.-Y. Sub-nanometer Pt cluster decoration enhances the oxygen reduction reaction performances of NiOx supported Pd nano-islands. Sustain. Energy Fuels 2020, 4, 809–823. [Google Scholar] [CrossRef]
- Bhalothia, D.; Wang, S.-P.; Lin, S.; Yan, C.; Wang, K.-W.; Chen, P.-C. Atomic Pt-Clusters Decoration Triggers a High-Rate Performance on Ni@Pd Bimetallic Nanocatalyst for Hydrogen Evolution Reaction in Both Alkaline and Acidic Medium. Appl. Sci. 2020, 10, 5155. [Google Scholar] [CrossRef]
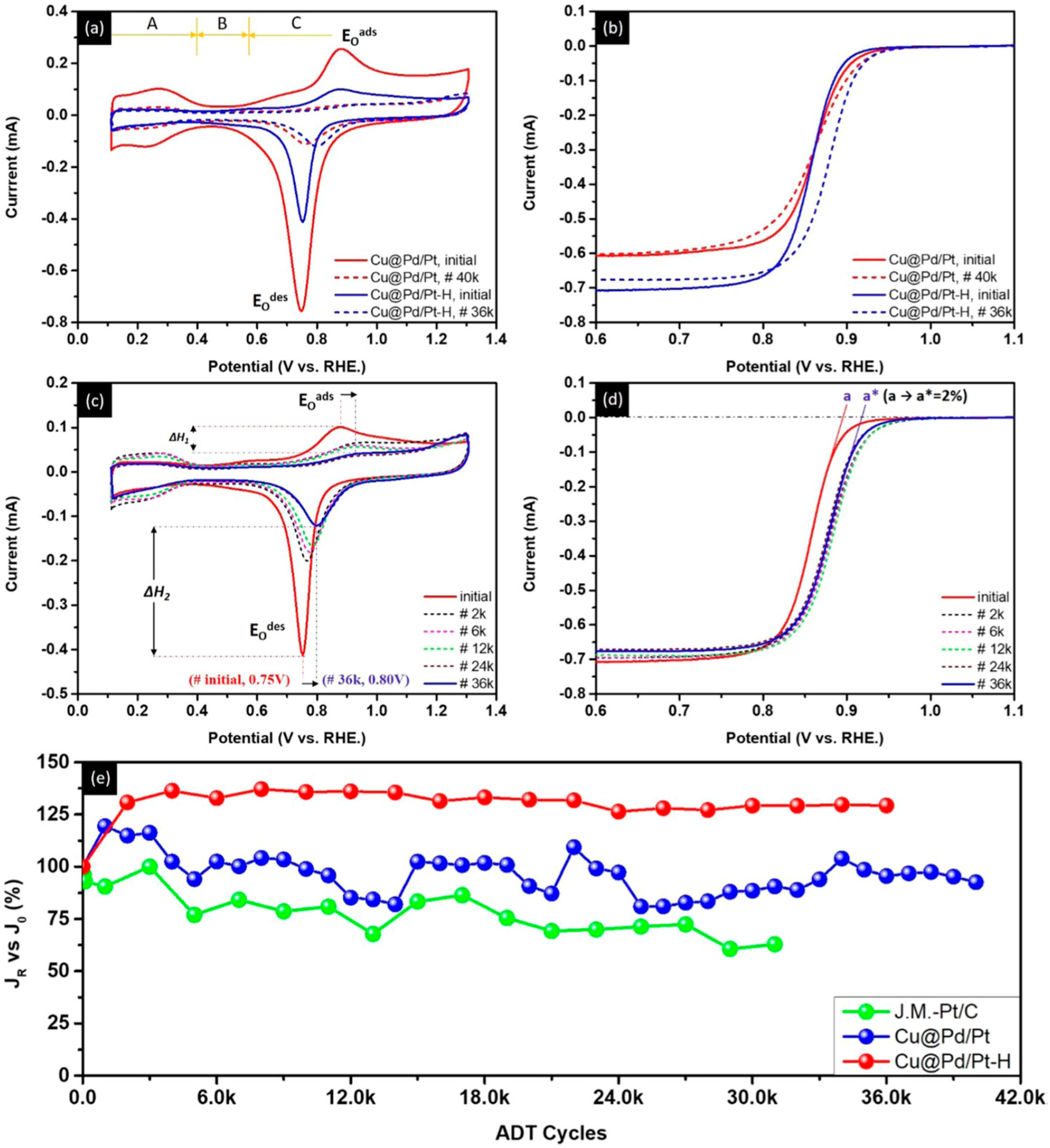
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. Effects of Pt metal loading on the atomic restructure and oxygen reduction reaction performance of Pt-cluster decorated Cu@Pd electrocatalysts. Sustain. Energy Fuels 2019, 3, 1668–1681. [Google Scholar] [CrossRef]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. H2 Reduction Annealing Induced Phase Transition and Improvements on Redox Durability of Pt Cluster-Decorated Cu@Pd Electrocatalysts in Oxygen Reduction Reaction. ACS Omega 2019, 4, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Bhalothia, D.; Fan, Y.J.; Lai, Y.C.; Yang, Y.T.; Yang, Y.W.; Lee, C.H.; Chen, T.Y. Conformational Effects of Pt-Shells on Nanostructures and Corresponding Oxygen Reduction Reaction Activity of Au-Cluster-Decorated NiO x @Pt Nanocatalysts. Nanomaterials 2019, 9, 1003. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Chou, J.-P.; Wang, K.-W.; Hsu, Y.-Y.; Hu, A.; Pan, X.; Chen, T.-Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Kędra, T.; Czerwiński, A. Hydrogen electrosorption into Pd–Pt–Au ternary alloys. Electrochim. Acta 2010, 55, 1150–1159. [Google Scholar] [CrossRef]
- Zuo, Y.; Rao, D.; Li, S.; Li, T.; Zhu, G.; Chen, S.; Song, L.; Chai, Y.; Han, K. Atomic Vacancies Control of Pd-Based Catalysts for Enhanced Electrochemical Performance. Adv. Mater. 2017, 30, 1704171. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Merisalu, M.; Rähn, M.; Sammelselg, V.; Tammeveski, K. Electrochemical reduction of oxygen in alkaline solution on Pd/C catalysts prepared by electrodeposition on various carbon nanomaterials. J. Electroanal. Chem. 2019, 834, 223–232. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Heterogeneous NiO2-to-Pd Epitaxial Structure Performs Outstanding Oxygen Reduction Reaction Activity. J. Phys. Chem. C 2019, 124, 2295–2306. [Google Scholar] [CrossRef]
- Bhalothia, D.; Shuan, L.; Wu, Y.-J.; Yan, C.; Wang, K.-W.; Chen, T.-Y.; Lin, S. A highly mismatched NiO2-to-Pd hetero-structure as an efficient nanocatalyst for the hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 2541–2550. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, K.; Zhang, J. The effect of heat treatment on nanoparticle size and ORR activity for carbon-supported Pd–Co alloy electrocatalysts. Electrochim. Acta 2007, 52, 3088–3094. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, K.; Zhang, J. Effect of synthetic reducing agents on morphology and ORR activity of carbon-supported nano-Pd–Co alloy electrocatalysts. Electrochim. Acta 2007, 52, 7964–7971. [Google Scholar] [CrossRef]
- Mech, K.; Żabiński, P.; Kowalik, R.; Tokarski, T.; Fitzner, K. Electrodeposition of Co–Pd alloys from ammonia solutions and their catalytic activity for hydrogen evolution reaction. J. Appl. Electrochem. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Bozzini, B.; Zangari, G.; Cavallotti, P. Electrolytic hydrogen evolution on Co-alloy thin films prepared by electrochemical and autocatalytic deposition techniques. Electrochim. Acta 1994, 39, 1787–1793. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Nabipour, S.; Asen, P. Palladium nanoparticles supported on graphene as an efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2015, 40, 16184–16191. [Google Scholar] [CrossRef]
- Andonoglou, P.; Jannakoudakis, A. Palladium deposition on activated carbon fibre supports and electrocatalytic activity of the modified electrodes towards the hydrogen evolution reaction. Electrochim. Acta 1997, 42, 1905–1913. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, X.-W.; Sun, X.-F.; Sheng, G.-P.; Zhang, Y.; Yan, G.-M.; Wang, S.; Xu, A.-W.; Yu, H.-Q. A new cathodic electrode deposit with palladium nanoparticles for cost-effective hydrogen production in a microbial electrolysis cell. Int. J. Hydrog. Energy 2011, 36, 2773–2776. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, S.; Gu, S.; Xu, B.; Yan, Y. Size-Dependent Hydrogen Oxidation and Evolution Activities on Supported Palladium Nanoparticles in Acid and Base. J. Electrochem. Soc. 2016, 163, F499–F506. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Su, D.; Adzic, R.R. Platinum Monolayer on IrFe Core–Shell Nanoparticle Electrocatalysts for the Oxygen Reduction Reaction. Electrocatalysis 2011, 2, 134–140. [Google Scholar] [CrossRef]
- Strickler, A.L.; Jackson, A.; Jaramillo, T.F. Active and Stable Ir@Pt Core–Shell Catalysts for Electrochemical Oxygen Reduction. ACS Energy Lett. 2016, 2, 244–249. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsu, H.-C.; Wang, K.-C. Iridium-decorated Palladium–Platinum core–shell catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. J. Colloid Interface Sci. 2014, 427, 91–97. [Google Scholar] [CrossRef]
- Bhalothia, D.; Tsai, D.-L.; Wang, S.-P.; Yan, C.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y.; Chen, P.-C. Ir-oxide mediated surface restructure and corresponding impacts on durability of bimetallic NiOx@Pd nanocatalysts in oxygen reduction reaction. J. Alloy. Compd. 2020, 844, 156160. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Li, G.; Li, N.; Li, S.; Cano, Z.P.; Ma, L.; Cui, P.; Xu, P.; Jiang, G.; et al. A Single-Atom Iridium Heterogeneous Catalyst in Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 9640–9645. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. https://doi.org/10.3390/app10217708
Bhalothia D, Krishnia L, Yang S-S, Yan C, Hsiung W-H, Wang K-W, Chen T-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Applied Sciences. 2020; 10(21):7708. https://doi.org/10.3390/app10217708
Chicago/Turabian StyleBhalothia, Dinesh, Lucky Krishnia, Shou-Shiun Yang, Che Yan, Wei-Hao Hsiung, Kuan-Wen Wang, and Tsan-Yao Chen. 2020. "Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions" Applied Sciences 10, no. 21: 7708. https://doi.org/10.3390/app10217708





