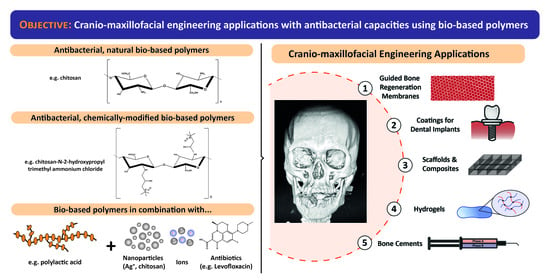
Antibacterial Bio-Based Polymers for Cranio-Maxillofacial Regeneration Applications
Abstract
:1. Introduction
1.1. Bio-Based Polymers
1.2. Cranio-Maxillary Tissue Engineering
- Membranes for Guided Tissue/Bone Regeneration. During bone-regenerative treatments, soft tissues of the oral cavity tend to proliferate too and can impede the correct restoration of the area. For this reason, it is necessary to employ a physical barrier (a membrane) to exclude the gingival epithelium and connective tissue ingrowth, while enhancing the formation of bone and/or periodontal tissues [28,29,30,31].
- Scaffolds and Implanting Medical Devices for Soft and Hard Maxillofacial Tissues:
- Hybrid scaffolds act as extracellular matrix substitutes, improving cell viability, attachment, proliferation and differentiation, as well as vascularization, host integration, and load bearing. They are mainly used for cranial and maxillary regenerative therapies like preventing oroantral fistulae or for treating maxillary peri-implantitis [32,33].
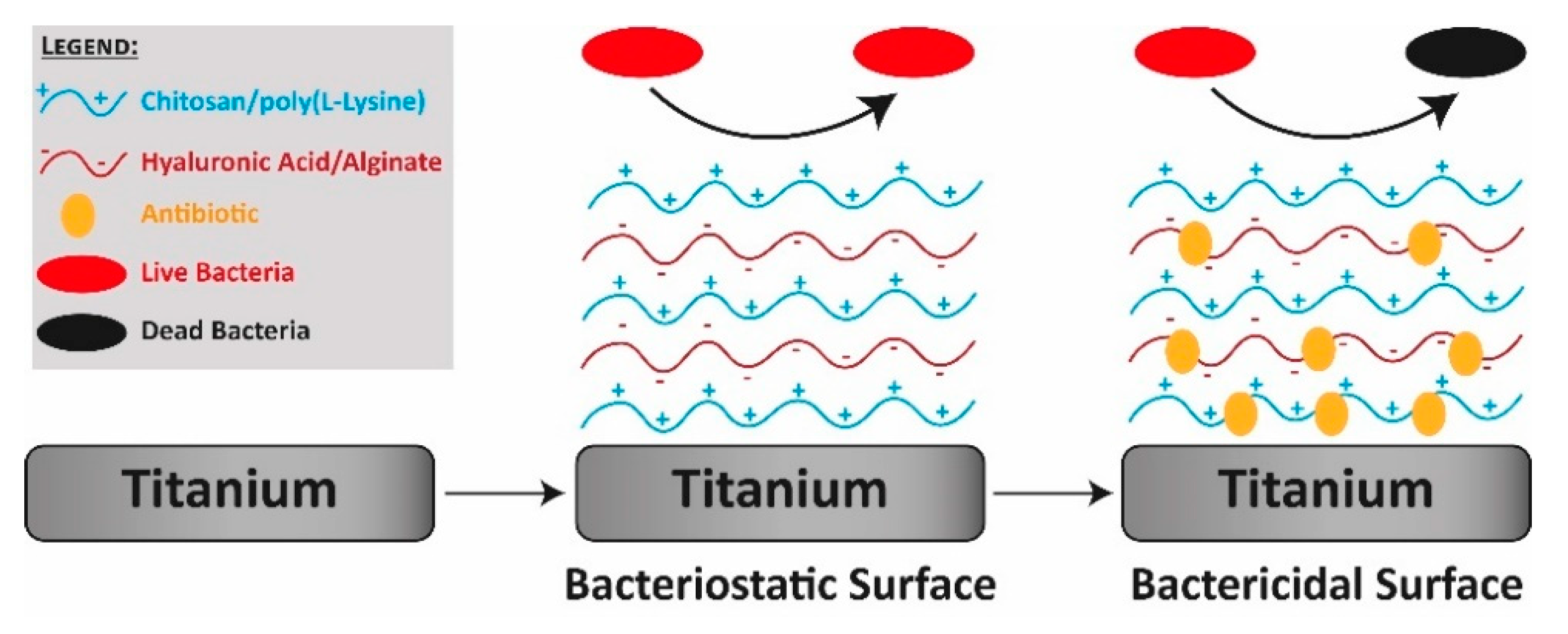
- Dental implants are a common choice for substituting missing teeth due to the excellent mechanical and chemical properties of the employed alloys. However, the application of bacterial biofilms on their surfaces may affect their outcome. For this reason, it is necessary to use materials and composites as coatings to provide implants with antibacterial properties [34,35,36].
- Hydrogels. Due to their unique properties, hydrogels are a perfect choice for filling irregular cavities during many clinical interventions such as maxillary sinus lift, replacement of bone structure caused by oroantral fistulae, maxillary peri-implantitis, sinusitis of zygomatic and bone defects of dental implant origin, among others [37,38].
- Polymeric and Bioactive Glass-based composites are used to induce and conduct the mineralization of tissues in cranial and periodontal defects repair. In this regard, bioactive glasses (BGs) are mainly employed as bone substitutes in orthopaedics for maxillofacial reconstruction and as bioactive coatings in dental implants [39,40,41,42].
- Cements are applied during the rehabilitation and placement of dental crowns, bridges, inlays, onlays or veneers, which are treatments where bacteria may be still present due to a partial removal of a caries or due to certain microleakage after cementing [43].
2. Membranes for Guided Tissue/Bone Regeneration
3. Scaffolds and Implanting Medical Devices for Soft and Hard Maxillofacial Tissues
3.1. Hybrid Scaffolds
3.2. Dental Implants
3.3. Hydrogels
3.4. Polymeric and Bioactive Glass-Based Composites
3.5. Other Approaches
4. Cements
4.1. Polymethyl Methacrylate-Based Cements
4.2. Glass Ionomer Cements
4.3. Calcium Phosphate Composites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BG | Bioactive glass |
| CPC | Calcium phosphate cement |
| CS | Chitosan |
| GIC | Glass ionomer cement |
| GTR/GBR | Guided tissue/bone regeneration |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| Nano-HA | Nano-sized hydroxyapatite |
| PAA | Poly(acrylic acid) |
| PCL | Polycaprolactone |
| pDA | Polydopamine |
| PEEK | Polyetheretherketone |
| PGA | Polygalacturonic acid |
| PHAs | Polyhydroxyalkanoates |
| PHB | Polyhydroxybutyrate |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PMMA | Polymethyl methacrylate |
| PP | PLGA/PCL blend |
| PTFE | Polytetrafluorethylene |
| PVA | Poly(vinyl alcohol) |
| Ti | Titanium |
Appendix A
| Clinical System | Clinical Applications | Bio-Based Polymer Employed | References | |
|---|---|---|---|---|
| Membranes for Guided Tissue/Bone Regeneration | 1. Augmentation of alveolar bone, reconstruction of oral tissues during the placement of dental implants, or during the regeneration of periodontal and endodontic tissues. 2. Enhancing the formation of bone and/or periodontal tissues | PTFE | [29,45,47,48] | |
| PLA | [29,49] | |||
| PGA | [29,45] | |||
| Collagen | [51,52] | |||
| Collagen (with chitosan, chlorhexidine or minocycline) | [29,44,46,56,57,65,101] | |||
| Chitosan (with chlorhexidine, minocycline or collagen) | [44,58,59,60,101,111] | |||
| Chitosan (with silver particles) | [31,67] | |||
| PLGA (with nanoapatite and lauric acid) | [53,68] | |||
| Gelatin (with PCL) | [62] | |||
| Scaffolds and Implanting Medical Devices for Soft and Hard Maxillofacial Tissues | Hybrid Scaffolds | 1. Sponge grafting for preventing oroantral fistulae, maxillary peri-implantitis, sinusitis of zygomatic and bone defects of dental implant. 2. Scaffolds for oral soft tissue (such as palatal, gingival and periodontal tissues). 3. Augmentation to support wound healing. | PHB/PCL | [54,70,73,74,75,76] |
| PLGA (with PCL, silver-modified/collagen) | [54] | |||
| Collagen | [23,33,54,78,79] | |||
| Silver-modified/collagen | [80,81] | |||
| Chitosan | [80,81,82,83,84] | |||
| Chitosan/PCL | [76] | |||
| Scaffolds and Implanting Medical Devices for Soft and Hard Maxillofacial Tissues | Dental Implants | Device in dental restoration for substituting missing teeth | Chitosan | [35,36,86,87,88] |
| Chitosan and Hyaluronic Acid | [35,89] | |||
| Silver-conjugated chitosan nanoparticles | [36] | |||
| PEEK | [90] | |||
| PLGA (with norfloxacin) | [91] | |||
| Hydrogels | 1. Filling irregular cavities (bone and cartilage tissue engineering). 2. Elevation treatments of the maxillary sinus. 3. Ligament and bone regeneration in maxillofacial floor regeneration. | Chitosan | [34,92] | |
| Polymeric and Bioactive Glass-based composites | 1. To induce and conduct the mineralization of tissues in maxillofacial reconstruction, cranial and periodontal defects repair, alveolar ridge augmentation. 2. Bone substitute in orthopedics. 3. Bioactive coatings in dental implants. 4. Incorporation into various restorative dental materials. | Chitosan (with BG) | [98] | |
| PEEK (with BG) | [98] | |||
| Self-gelling pectin (with BG) | [99] | |||
| PHBV | [100] | |||
| Gelatin and Collagen | [101] | |||
| Other Approaches | Novel bioactive molecules with potential therapeutic effects for soft and hard tissue regeneration processes and periodontal applications | Quercitrin | [103,104] | |
| Eugenol derivative (with hydroxyapatite, ZnO2-containing Bis-GMA/TEGDMA) | [105,106] | |||
| Cements | PMMA-based cements | Standard vehicles for loading antibiotics used in maxillary orthopedics. | Chitosan | [112,113,114] |
| Chitosan hydrogels (with silver ions) | [110,111] | |||
| Quaternized chitosan (HACC) | [107] | |||
| Chitosan and HACC nanoparticles | [108,109] | |||
| Glass Ionomer Cements | 1. Applied during the rehabilitation and placement of dental crowns, bridges, inlays, onlays or veneers. 2. Standard vehicle for loading antibiotics in maxillary orthopedics treatment. | Chitosan | [116,117,118,119,121] | |
| Chitosan (with TiO2 nano-powder) | [120] | |||
| Calcium Phosphate Cements | 1. Bone fillers for fixating orthopedics and dental implants. 2. To avoid movements between the prosthesis and bone, being applied as an interface between both. 3. Performing osteoconductive actions in fractures and bone defects. | Chitosan | [122,123] | |
References
- Buchanan, E.P.; Xue, A.S.; Hollier, L.H. Craniofacial syndromes. Plast. Reconstr. Surg. 2014, 134, 128e–153e. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for cleft lip and palate regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef] [Green Version]
- Heggie, A.A. Craniofacial disorders. Aust. Dent. J. 2018, 63, S58–S68. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.; Caccamese, J.; Costello, B.J.; Woerner, J. Cleft and Craniofacial Surgery. J. Oral Maxillofac. Surg. 2017, 75, e126–e150. [Google Scholar] [CrossRef]
- Reddy, R.R.; Reddy, S.G.; Banala, B.; Bronkhorst, E.M.; Kummer, A.W.; Kuijpers-Jagtman, A.M.; Bergé, S.J. Placement of an antibiotic oral pack on the hard palate after primary cleft palatoplasty: A randomized controlled trial into the effect on fistula rates. Clin. Oral Investig. 2018, 22, 1953–1958. [Google Scholar] [CrossRef] [Green Version]
- Straccia, M.C.; D’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for The Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Quirino, R.L.; Garrison, T.F.; Kessler, M.R. Matrices from vegetable oils, cashew nut shell liquid, and other relevant systems for biocomposite applications. Green Chem. 2014, 16, 1700–1715. [Google Scholar] [CrossRef] [Green Version]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials: (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Capellán-Pérez, I.; Mediavilla, M.; de Castro, C.; Carpintero, Ó.; Miguel, L.J. Fossil fuel depletion and socio-economic scenarios: An integrated approach. Energy 2014, 77, 641–666. [Google Scholar] [CrossRef]
- Research and Markets Global Bio-Based Polymer Market (2019–2025). Available online: https://www.researchandmarkets.com/reports/4837061/global-bio-based-polymer-market-2019-2025 (accessed on 15 November 2020).
- Lewyllie, A.; De Llano-Pérula, M.C.; Verdonck, A.; Willems, G. Three-dimensional imaging of soft and hard facial tissues in patients with craniofacial syndromes: A systematic review of methodological quality. Dentomaxillofac. Radiol. 2018, 47. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Caccamese, J.F.; Coletti, D.P.; Sauk, J.J.; Fisher, J.P. Tissue Engineering Solutions for Cleft Palates. J. Oral Maxillofac. Surg. 2007, 65, 2503–2511. [Google Scholar] [CrossRef]
- Massenburg, B.B.; Riesel, J.N.; Hughes, C.D.; Meara, J.G. Global Cleft Lip and Palate Care: A Brief Review. Cleft Lip Palate Treat. 2018, 15–23. [Google Scholar] [CrossRef]
- Jones, K.L.; Jones, M.C.; del Campo, M. Facial Defects as Major Feature. In Smith’s Recognizable Patterns of Human Malformation, 7th ed.; Saunders: Yonkers, NY, USA, 2014; p. 1016. ISBN 9781455738113. [Google Scholar]
- Hamze, H.; Mengiste, A.; Carter, J. The impact and cost-effectiveness of the amref health Africa-smile train cleft lip and palate surgical repair programme in eastern and central Africa. Pan Afr. Med. J. 2017, 28, 1–12. [Google Scholar] [CrossRef]
- Seifeldin, S.A. Is alveolar cleft reconstruction still controversial? (Review of literature). Saudi Dent. J. 2015, 28, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Hatayama, T.; Nakada, A.; Nakamura, H.; Mariko, W.; Tsujimoto, G.; Nakamura, T. Regeneration of gingival tissue using in situ tissue engineering with collagen scaffold. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Peacock, Z.S. Controversies in Oral and Maxillofacial Pathology. Oral Maxillofac. Surg. Clin. N. Am. 2017, 29, 475–486. [Google Scholar] [CrossRef]
- Roode, G.J.; Bütow, K.W.; Naidoo, S. Preoperative evaluation of micro-organisms in non-operated cleft in soft palate: Impact on use of antibiotics. Br. J. Oral Maxillofac. Surg. 2017, 55, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Cocco, J.F.; Antonetti, J.W.; Burns, J.L.; Heggers, J.P.; Blackwell, S.J. Characterization of the nasal, sublingual, and oropharyngeal mucosa microbiota in cleft lip and palate individuals before and after surgical repair. Cleft Palate-Craniofac. J. 2010, 47, 151–155. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Sawada, K.; Miron, R.J.; Bosshardt, D.D.; Buser, D.; Gruber, R. Collagen barrier membranes adsorb growth factors liberated from autogenous bone chips. Clin. Oral Implants Res. 2017, 28, 236–241. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Peñarrocha, M.; Hernández-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/ chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Jensen, O.T.; Adams, M.; Cottam, J.R.; Ringeman, J. Occult Peri-implant Oroantral Fistulae: Posterior Maxillary Peri-implantitis/Sinusitis of Zygomatic or Dental Implant Origin. Treatment and Prevention with Bone Morphogenetic Protein-2/Absorbable Collagen Sponge Sinus Grafting. Int. J. Oral Maxillofac. Implants 2013, 28, e512–e520. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Cai, X.; Chen, R.; Zhang, H.; Jiang, T.; Wang, Y. A thermosensitive chitosan-based hydrogel for sealing and lubricating purposes in dental implant system. Clin. Implant Dent. Relat. Res. 2019, 21, 324–335. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF 165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Deepa, V.; Ahamed, S.; Sathish, E.; Meyappan, R.; Satheesh Kumar, K.; Narayana, S. Remineralization efficiency of bioactive glass on artificially induced carious lesion an in-vitro study. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 19. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate Bioglass® against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Mokhtari, H.; Ghasemi, Z.; Kharaziha, M.; Karimzadeh, F.; Alihosseini, F. Chitosan-58S bioactive glass nanocomposite coatings on TiO 2 nanotube: Structural and biological properties. Appl. Surf. Sci. 2018, 441, 138–149. [Google Scholar] [CrossRef]
- Silva-Herzog Rivera, D.; Pozos-Guillen, A.; Aragón-Piña, A.; Cerda-Cristerna, B.I.; Masuoka-Ito, D.; Sánchez-Vargas, L.O. Glass coatings to enhance the interfacial bond strength between veneering ceramic and zirconia. Odontology 2020, 108, 415–423. [Google Scholar] [CrossRef]
- Daugela, P.; Oziunas, R.; Zekonis, G. Antibacterial potential of contemporary dental luting cements. Stomatol. Balt. Dent. Maxillofac. J. 2008, 10, 16–21. [Google Scholar]
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Kumar, A.; Khan, M.A.; Lal, N. Comparative study of nonabsorbable and absorbable barrier membranes in periodontal osseous defects by guided tissue regeneration. J. Oral Biol. Craniofac. Res. 2016, 6, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Won, J.Y.; Park, C.Y.; Bae, J.H.; Ahn, G.; Kim, C.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Shim, J.H.; Huh, J.B. Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11, 055013. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Fu, Y.; Giegengack, M.; Tseng, S.C.G. Oral Mucosal Graft with Amniotic Membrane Transplantation for Total Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2011, 152, 739–747. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Bohm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine dermis-derived collagen membranes induce implantation bed vascularization via multinucleated giant cells: A physiological reaction? J. Oral Implantol. 2015, 41, e238–e251. [Google Scholar] [CrossRef]
- Wessing, B.; Urban, I.; Montero, E.; Zechner, W.; Hof, M.; Alández Chamorro, J.; Alández Martin, N.; Polizzi, G.; Meloni, S.; Sanz, M. A multicenter randomized controlled clinical trial using a new resorbable non-cross-linked collagen membrane for guided bone regeneration at dehisced single implant sites: Interim results of a bone augmentation procedure. Clin. Oral Implants Res. 2017, 28, e218–e226. [Google Scholar] [CrossRef]
- Hoornaert, A.; D’Arros, C.; Heymann, M.F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 45012. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Wu, G.; Deng, H.; Jiang, T.; Tu, H.; Chen, J.; Zhan, Y.; Wang, Y.; Ma, X. Regulating the gaps between folds on the surface of silk fibroin membranes via LBL deposition for improving their biomedical properties. Colloids Surf. B Biointerfaces 2017, 154, 228–238. [Google Scholar] [CrossRef]
- Lee, S.B.; Kwon, J.S.; Lee, Y.K.; Kim, K.M.; Kim, K.N. Bioactivity and mechanical properties of collagen composite membranes reinforced by chitosan and β-tricalcium phosphate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1935–1942. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological Evaluation (In Vitro and In Vivo) of Bilayered Collagenous Coated (Nano Electrospun and Solid Wall) Chitosan Membrane for Periodontal Guided Bone Regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tai, H.Y.; Fu, E.; Don, T.M. Guided bone regeneration activity of different calcium phosphate/chitosan hybrid membranes. Int. J. Biol. Macromol. 2019, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration: A review of ceramics and polymers. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.; Trocolli, R.; Wellen, R.M.R.; Rojo, L.; Lopez-Manchado, M.A.; Fook, M.V.L.; Silva, S.M.L. HDPE/chitosan composites modified with PE-g-MA. thermal, morphological and antibacterial analysis. Polymers 2019, 11, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/β-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Zhao, Y. Effect of Molecular Weight, Acid, and Plasticizer on the Physicochemical and Antibacterial Properties of β-Chitosan Based Films. J. Food Sci. 2012, 77, 127–136. [Google Scholar] [CrossRef]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Aşan Özüsağlam, M.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric collagen/chitosan membrane containing minocycline-loaded chitosan nanoparticles for guided bone regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarani, N.N.; Jamuna-Thevi, K.; Shahab, N.; Hermawan, H.; Saidin, S. Antibacterial efficacy of triple-layered poly(Lactic-co-glycolic acid)/nanoapatite/lauric acid guided bone regeneration membrane on periodontal bacteria. Dent. Mater. J. 2017, 36, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Martín-del-Campo, M.; Sampedro, J.G.; Flores-Cedillo, M.L.; Rosales-Ibañez, R.; Rojo, L. Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules 2019, 24, 1660. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Li, W.; Correia, A.; Yang, Y.; Zheng, K.; Liu, D.; Schubert, D.W.; Boccaccini, A.R.; Santos, H.A.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ϵ-caprolactone)/Sol-Gel-Derived Silica Hybrid Scaffolds with Drug Releasing Function for Bone Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 14540–14548. [Google Scholar] [CrossRef] [Green Version]
- Hamlekhan, A.; Moztarzadeh, F.; Mozafari, M.; Azami, M.; Nezafati, N. Preparation of laminated poly(ε-caprolactone)-gelatin-hydroxyapatite nanocomposite scaffold bioengineered via compound techniques for bone substitution. Biomatter 2011, 1, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Thai, T.H.; Nuntanaranont, T.; Kamolmatyakul, S.; Meesane, J. In vivo evaluation of modified silk fibroin scaffolds with a mimicked microenvironment of fibronectin/decellularized pulp tissue for maxillofacial surgery. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Saprykina, N.N.; Sergeev, V.S.; Afanasyev, B.V.; Vilesov, A.D.; Sukhorukov, G.B. Triple-responsive inorganic-organic hybrid microcapsules as a biocompatible smart platform for the delivery of small molecules. J. Mater. Chem. B 2016, 4, 7270–7282. [Google Scholar] [CrossRef] [Green Version]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef] [PubMed]
- Cornelsen, M.; Probst, F.A.; Schwarz, C.; Burian, E.; Tröltzsch, M.; Otto, S.; Saller, M.M.; Schieker, M.; Seitz, H. Mechanical and biological effects of infiltration with biopolymers on 3D printed tricalciumphosphate scaffolds. Dent. Mater. J. 2017, 36, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.R.; Brown, P.; Khajotia, S.S.; Dmytryk, J.J.; Madihally, S.V. Antibacterial activity of chitosan-based matrices on oral pathogens. J. Mater. Sci. Mater. Med. 2008, 19, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Thoma, D.S.; Jung, R.E.; Schneider, D.; Cochran, D.L.; Ender, A.; Jones, A.A.; Görlach, C.; Uebersax, L.; Graf-Hausner, U.; Hämmerle, C.H.F. Soft tissue volume augmentation by the use of collagen-based matrices: A volumetric analysis. J. Clin. Periodontol. 2010, 37, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Agis, H.; Collins, A.; Taut, A.D.; Jin, Q.; Kruger, L.; Görlach, C.; Giannobile, W.V. Cell population kinetics of collagen scaffolds in Ex Vivo oral wound repair. PLoS ONE 2014, 9, e112680. [Google Scholar] [CrossRef]
- Özmeriç, N.; Özcan, G.; Haytaç, C.M.; Alaaddinoǧlu, E.E.; Sargon, M.F.; Şenel, S. Chitosan film enriched with an antioxidant agent, taurine, in fenestration defects. J. Biomed. Mater. Res. 2000, 51, 500–503. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, H.J.; Koo, K.T.; Ben Amara, H.; Leesungbok, R.; Noh, K.; Lee, S.C.; Lee, S.W. Oral Soft Tissue Regeneration Using Nano Controlled System Inducing Sequential Release of Trichloroacetic Acid and Epidermal Growth Factor. Tissue Eng. Regen. Med. 2020, 17, 91–103. [Google Scholar] [CrossRef]
- Madi, M.; Kassem, A. Topical simvastatin gel as a novel therapeutic modality for palatal donor site wound healing following free gingival graft procedure. Acta Odontol. Scand. 2018, 76, 212–219. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, S.H.; Moon, I.S.; Cho, K.S.; Chai, J.K.; Kim, C.K. Eight-week histological analysis on the effect of chitosan on surgically created one-wall intrabony defects in beagle dogs. J. Clin. Periodontol. 2003, 30, 443–453. [Google Scholar] [CrossRef]
- Li, N.; Jiang, L.; Jin, H.; Wu, Y.; Liu, Y.; Huang, W.; Wei, L.; Zhou, Q.; Chen, F.; Gao, Y.; et al. An enzyme-responsive membrane for antibiotic drug release and local periodontal treatment. Colloids Surf. B Biointerfaces 2019, 183, 110454. [Google Scholar] [CrossRef] [PubMed]
- Asensio; Vázquez-Lasa; Rojo Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Chua, P.H.; Neoh, K.G.; Shi, Z.; Kang, E.T. Structural stability and bioapplicability assessment of hyaluronic acid-chitosan polyelectrolyte multilayers on titanium substrates. J. Biomed. Mater. Res. Part A 2008, 87, 1061–1074. [Google Scholar] [CrossRef]
- Pezzotti, G.; Marin, E.; Adachi, T.; Lerussi, F.; Rondinella, A.; Boschetto, F.; Zhu, W.; Kitajima, T.; Inada, K.; McEntire, B.J.; et al. Incorporating Si 3 N 4 into PEEK to Produce Antibacterial, Osteocondutive, and Radiolucent Spinal Implants. Macromol. Biosci. 2018, 18, 1800033. [Google Scholar] [CrossRef]
- Baghdan, E.; Raschpichler, M.; Lutfi, W.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Nano spray dried antibacterial coatings for dental implants. Eur. J. Pharm. Biopharm. 2019, 139, 59–67. [Google Scholar] [CrossRef]
- Rojo, L.; Deb, S. Polymer Therapeutics in Relation to Dentistry. Front. Oral Biol. 2015, 17, 13–21. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [Green Version]
- Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: Antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Garchitorena, M. Bioactive Glasses in Restorative Dentistry. J. Clin. Periodontol. 2016, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, Q.; Goldmann, W.H.; Maqbool, M.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of lawsone loaded bioactive glass (BG)/chitosan composite on polyetheretherketone (PEEK)/BG layers as antibacterial and bioactive coating. J. Biomed. Mater. Res. Part A 2018, 106, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Dziadek, M.; Schietse, J.; Boone, M.; Declercq, H.A.; Coenye, T.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Buchweitz, M.; et al. Pectin-bioactive glass self-gelling, injectable composites with high antibacterial activity. Carbohydr. Polym. 2019, 205, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ding, Y.; Yu, S.; Yao, Q.; Boccaccini, A.R. Multifunctional Chitosan-45S5 Bioactive Glass-Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Microsphere Composite Membranes for Guided Tissue/Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 20845–20854. [Google Scholar] [CrossRef]
- Zhou, T.; Sui, B.; Mo, X.; Sun, J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: Fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3495–3507. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, M.; Miguel, M.; Aleixandre, A. The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of Quercitrin as a Potential Therapeutic Agent for Periodontal Applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration: Effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.L.; Ao, H.Y.; Ma, R.; Lin, W.T.; Tang, T.T. In vivo effect of quaternized chitosan-loaded polymethylmethacrylate bone cement on methicillin-resistant Staphylococcus epidermidis infection of the tibial metaphysis in a rabbit model. Antimicrob. Agents Chemother. 2014, 58, 6016–6023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef]
- Lewis, G. Properties of nanofiller-loaded poly (methyl methacrylate) bone cement composites for orthopedic applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1260–1284. [Google Scholar] [CrossRef]
- Wang, M.; Sa, Y.; Li, P.; Guo, Y.; Du, Y.; Deng, H.; Jiang, T.; Wang, Y. A versatile and injectable poly(methyl methacrylate) cement functionalized with quaternized chitosan-glycerophosphate/nanosized hydroxyapatite hydrogels. Mater. Sci. Eng. C 2018, 90, 264–272. [Google Scholar] [CrossRef]
- Wang, M.; Feng, X.; Wang, T.; Gao, Y.; Wang, Y.; Sa, Y.; Jiang, T. Synthesis and characterization of an injectable and self-curing poly(methyl methacrylate) cement functionalized with a biomimetic chitosan-poly(vinyl alcohol)/nano-sized hydroxyapatite/silver hydrogel. RSC Adv. 2016, 6, 60609–60619. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Escobar, J.A.D.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Osseointegration of antimicrobial acrylic bone cements modified with graphene oxide and chitosan. Appl. Sci. 2020, 10, 6528. [Google Scholar] [CrossRef]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, L.; Vázquez, B.; Román, J.S.; Deb, S. Eugenol functionalized poly(acrylic acid) derivatives in the formation of glass-ionomer cements. Dent. Mater. 2008, 24, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F.S.; Donegá, J.; Benassi, A.M.; Bocangel, J.A.J.S. Preliminary study on chitosan modified glass ionomer restoratives. Dent. Mater. 2007, 23, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Silva, R.; de Oliveira, B.M.B.; Fernandes, P.H.M.; Shimohara, L.Y.; Pereira, F.V.; Borges, A.F.S.; Buzalaf, M.A.R.; Pascotto, R.C.; Sidhu, S.K.; de Lima Navarro, M.F. Effects of the reinforced cellulose nanocrystals on glass-ionomer cements. Dent. Mater. 2019, 35, 564–573. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Neo, J.; Esguerra, R.J.; Fawzy, A.S. Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J. Biomater. Appl. 2015, 30, 409–419. [Google Scholar] [CrossRef]
- Debnath, A.; Kesavappa, S.B.; Singh, G.P.; Eshwar, S.; Jain, V.; Swamy, M.; Shetty, P. Comparative evaluation of antibacterial and adhesive properties of chitosan modified glass ionomer cement and conventional glass ionomer cement: An in vitro study. J. Clin. Diagn. Res. 2017, 11, ZC75–ZC78. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Meera Priyadarshini, B.; Neo, J.; Fawzy, A.S. Characterization of Chitosan/TiO2 Nano-Powder Modified Glass-Ionomer Cement for Restorative Dental Applications. J. Esthet. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Q.; Fan, C.; Ren, H.; Xu, S.; Hu, F.; Wang, L.; Yang, K.; Ji, Q. Characteristics of chitosan-modified glass ionomer cement and their effects on the adhesion and proliferation of human gingival fibroblasts: An in vitro study. J. Mater. Sci. Mater. Med. 2019, 30, 39. [Google Scholar] [CrossRef]
- Wu, T.; Hua, X.; He, Z.; Wang, X.; Yu, X.; Ren, W. The bactericidal and biocompatible characteristics of reinforced calcium phosphate cements. Biomed. Mater. 2012, 7, 045003. [Google Scholar] [CrossRef]
- Moncif, N.; Gourri, E.L.H.; Elouahli, A.B.A.; Ezzahmouly, M.; Nayme, K.; Timinouni, M.; Hatim, Z. Characterization of bio-composite apatite/chitosan cement and its antibacterial activity. Orient. J. Chem. 2018, 34, 1765–1773. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-del-Campo, M.; Fernández-Villa, D.; Cabrera-Rueda, G.; Rojo, L. Antibacterial Bio-Based Polymers for Cranio-Maxillofacial Regeneration Applications. Appl. Sci. 2020, 10, 8371. https://doi.org/10.3390/app10238371
Martín-del-Campo M, Fernández-Villa D, Cabrera-Rueda G, Rojo L. Antibacterial Bio-Based Polymers for Cranio-Maxillofacial Regeneration Applications. Applied Sciences. 2020; 10(23):8371. https://doi.org/10.3390/app10238371
Chicago/Turabian StyleMartín-del-Campo, Marcela, Daniel Fernández-Villa, Gabriela Cabrera-Rueda, and Luis Rojo. 2020. "Antibacterial Bio-Based Polymers for Cranio-Maxillofacial Regeneration Applications" Applied Sciences 10, no. 23: 8371. https://doi.org/10.3390/app10238371