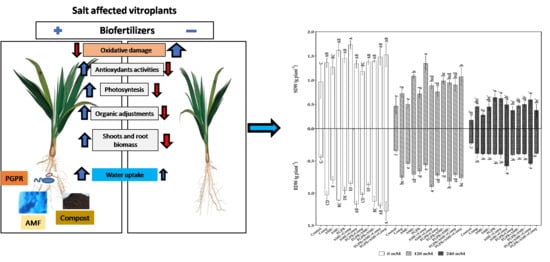
Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Experimental Design and Growth Conditions
- (1)
- Control plants without any biofertilizer (Control);
- (2)
- Plants amended with compost (Comp);
- (3)
- Plants inoculated with the native AMF consortium (AMF1);
- (4)
- Plants inoculated with the exotic AMF strain (AMF2);
- (5)
- Plants inoculated with the PGPR consortia (PGPR);
- (6)
- AMF1+Comp,
- (7)
- AMF2+Comp,
- (8)
- PGPR+Comp,
- (9)
- PGPR+AMF1,
- (10)
- PGPR+AMF2,
- (11)
- PGPR+AMF1+Comp,
- (12)
- PGPR+AMF2+Comp.
2.3. Data Collection and Analyses
2.3.1. Mycorrhizal Analysis
2.3.2. Assessment of Date Palm Growth and Development
2.3.3. Physiological Parameters
Chlorophyll Fluorescence, Gas Exchange and Leaf Water Potential
Photosynthetic Pigment Quantification
2.3.4. Biochemical Traits
Total Soluble Sugars Quantification
Total Soluble Proteins and Antioxidant Enzyme Activities
2.4. Soil Analyses
2.5. Statistical Analysis
3. Results
3.1. Salt Stress and Compost Reduced AMF Root Colonization
3.2. Biofertilizers Improved Date Palm Growth Parameters under Salt Stress
3.3. Biofertilizers Promoted Date Palm Chlorophyll Fluorescence, Gas Exchange and Leaf Water Potential under Salt Stress
3.4. Biofertilizers Stimulated Date Palm Chlorophyll and Carotenoid Production under Salt Stress
3.5. Biofertilizers Increased Date Palm Biochemical Parameters under Salt Stress
3.6. Biofertilizers Boosted Date Palm Antioxidant Enzyme Activities under Salt Stress
3.7. Biofertilizers Improved Soil Physical and Chemical Traits under Salt Stress
3.8. The Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gros-Balthazard, M.; Newton, C.; Ivorra, S.; Pintaud, J.-C.; Terral, J.-F. Origines et domestication du palmier dattier (Phoenix dactylifera L.). Rev. Ethnoécologie 2013, 1–15. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Organization of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 24 April 2019).
- Arias, E.; Hodder, A.J.; Oihabi, A. FAO support to date palm development around the world: 70 years of activity. Emirates J. Food Agric. 2016, 28, 1–11. [Google Scholar] [CrossRef]
- Meddich, A.; Oufdou, K.; Boutasknit, A.; Raklami, A.; Tahiri, A.; Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Mitsui, T.; Wahbi, S.; et al. Use of organic and biological fertilizers as strategies to improve crop biomass, yields and physicochemical Parameters of soil. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, China, 2020; pp. 247–288. ISBN 9789811386602. [Google Scholar]
- Ait-El-Mokhtar, M.; Boutasknit, A.; Ben-Laouane, R.; Anli, M.; El Amerany, F.; Toubali, S.; Lahbouki, S.; Wahbi, S.; Meddich, A. Vulnerability of oasis agriculture to climate change in morocco. In Impacts of Climate Change on Agriculture and Aquaculture; IGI Global: Hershey, PA, USA, 2020; pp. 76–106. ISBN 9781799833437. [Google Scholar]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Meddich, A.; Ait El Mokhtar, M.; Bourzik, W.; Mitsui, T.; Baslam, M.; Hafi, M. Optimizing growth and tolerance of date palm (Phoenix dactylifera L.) to drought, salinity, and vascular fusarium-induced wilt (Fusarium oxysporum) by application of arbuscular mycorrhizal fungi (AMF). In Root Biology; Giri, B., Prasad, R., Varma, A., Eds.; Springer: New York, NY, USA, 2018; pp. 239–258. ISBN 978-3-319-75909-8. [Google Scholar]
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, P.; Wu, Q.H.; Zou, Y.N.; Bao, Q.; Wu, Q.S. Arbuscular mycorrhizas improve plant growth and soil structure in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2017, 63, 491–500. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Centritto, M.; Lauteri, M.; Monteverdi, M.C.; Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 2009, 60, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.D.; Berta, G.; Lingua, G. Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Chandra, T.; Mishra, S.; Parmar, S.; Shaw, B.P.; Nilawe, P.D.; Chauhan, N.K.; Sahoo, S.; Panda, S.K. Identification and characterization of drought responsive miRNAs in a drought tolerant upland rice cultivar KMJ 1-12-3. Plant Physiol. Biochem. 2019, 137, 62–74. [Google Scholar] [CrossRef]
- Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm. Phoenix Dactylifera Trees 2014, 28, 161–172. [Google Scholar] [CrossRef]
- Liu, J.; Chu, J.; Ma, C.; Jiang, Y.; Ma, Y.; Xiong, J.; Cheng, Z.-M. Overexpression of an ABA-dependent grapevine bZIP transcription factor, VvABF2, enhances osmotic stress in Arabidopsis. Plant Cell Rep. 2019, 38, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role ofarbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Ahmad, P.; Jamsheed, S.; Hameed, A.; Rasool, S.; Sharma, I.; Azooz, M.M.; Hasanuzzaman, M. Drought Stress Induced Oxidative Damage and Antioxidants in Plants; Elsevier Inc.: Oxford, UK, 2014; ISBN 9780127999630. [Google Scholar]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (In)organic adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef]
- Boutaj, H.; Meddich, A.; Chakhchar, A.; Wahbi, S.; El Alaoui-Talibi, Z.; Douira, A.; Filali-Maltouf, A.; El Modafar, C. Arbuscular mycorrhizal fungi improve mineral nutrition and tolerance of olive tree to Verticillium wilt. Arch. Phytopathol. Plant Prot. 2020, 53, 673–689. [Google Scholar] [CrossRef]
- Boutaj, H.; Chakhchar, A.; Meddich, A.; Wahbi, S.; El Alaoui-Talibi, Z.; Douira, A.; Filali-Maltouf, A.; El Modafar, C. Bioprotection of olive tree from Verticillium wilt by autochthonous endomycorrhizal fungi. J. Plant Dis. Prot. 2020, 127, 349–357. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef]
- Anli, M.; Symanczik, S.; El Abbassi, A.; Ait-El-Mokhtar, M.; Boutasknit, A.; Ben-Laouane, R.; Toubali, S.; Baslam, M.; Mäder, P.; Hafidi, M.; et al. Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera “Boufgouss”. Plant Biosyst. An. Int. J. Deal. Asp. Plant Biol. 2020, 1–14. [Google Scholar] [CrossRef]
- Boutasknit, A.; Anli, M.; Tahiri, A.; Raklami, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Ait Rahou, Y.; Boutaj, H.; Oufdou, K.; Wahbi, S.; et al. Potential effect of horse manure-green waste and olive pomace-green waste composts on physiology and yield of garlic (Allium sativum L.) and soil fertility. Gesunde Pflanz. 2020, 72, 285–295. [Google Scholar] [CrossRef]
- Raklami, A.; El Gharmali, A.; Ait Rahou, Y.; Oufdou, K.; Meddich, A. Compost and mycorrhizae application as a technique to alleviate Cd and Zn stress in Medicago sativa. Int. J. Phytoremediat. 2020, 1–12. [Google Scholar] [CrossRef]
- Raklami, A.; Tahiri, A.; Bechtaoui, N.; Abdelhay, E.G.; Pajuelo, E.; Baslam, M.; Meddich, A.; Oufdou, K. Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J. Environ. Sci. 2020, 99, 210–221. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago sativa to salt stress. Gesunde Pflanz. 2019, 71, 135–146. [Google Scholar] [CrossRef]
- Meddich, A.; Elouaqoudi, F.; Khadra, A.; Bourzik, W. Valorization of green and industrial waste by composting process. J. Rev. Compos. Adv. Mater. 2016, 26, 451–469. [Google Scholar]
- Trouvelot, A.; Kough, J.L.; Gianinazzi, V. Mesure de taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetic Aspects of Mycorhizical; Gianinazzi, P., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T.D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Duarte, B.; Goessling, J.W.; Marques, J.C.; Caçador, I. Ecophysiological constraints of Aster tripolium under extreme thermal events impacts: Merging biophysical, biochemical and genetic insights. Plant Physiol. Biochem. 2015, 97, 217–228. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Bergmeyer, H.; Bernt, E. Determination of glucose with glucose-oxidase and peroxidase. In Methods of Enzymatic Analysis; Bergmeyer, H., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 123–129. [Google Scholar]
- Moore, B.M.; Flurkey, W.H. Sodium dodecyl sulfate activation of a plant polyphenoloxidase. J. Biol. Chem. 1990, 265, 4982–4989. [Google Scholar]
- Aubert, G. Méthodes D’analyses des Sols; Centre National de documentation Pédagogique, Centre Régional de Documentation Pédagogique: Marseille, France, 1978; p. 191. [Google Scholar]
- Olsen, S.; Sommers, L. Methods of soil analysis. Part 2. Chemical and microbiological properties of phosphorus. ASA Monograp. 1982, 9, 403–430. [Google Scholar]
- Visen, A.; Bohra, M.; Singh, P.N.; Srivastava, P.C.; Kumar, S.; Sharma, A.K.; Chakraborty, B. Two pseudomonad strains facilitate AMF mycorrhization of litchi (Litchi chinensis Sonn.) and improving phosphorus uptake. Rhizosphere 2017, 3, 196–202. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paymaneh, Z.; Sarcheshmehpour, M.; Bukovská, P.; Jansa, J. Could indigenous arbuscular mycorrhizal communities be used to improve tolerance of pistachio to salinity and/or drought? Symbiosis 2019, 79, 269–283. [Google Scholar] [CrossRef]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef]
- Antoun, H. Plant-growth-promoting rhizobacteria. In Brenner’s Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 353–355. ISBN 9780080961569. [Google Scholar]
- Giovannetti, M.; Fortuna, P.; Citernesi, A.S.; Morini, S.; Nuti, M.P. The occurrence of anastomosis formation and nuclear exchange in intact arbuscular mycorrhizal networks. New Phytol. 2001, 151, 717–724. [Google Scholar] [CrossRef]
- Bharti, N.; Barnawal, D.; Wasnik, K.; Tewari, S.K.; Kalra, A. Co-inoculation of Dietzia natronolimnaea and Glomus intraradices with vermicompost positively influences Ocimum basilicum growth and resident microbial community structure in salt affected low fertility soils. Appl. Soil Ecol. 2016, 100, 211–225. [Google Scholar] [CrossRef]
- Mbarki, S.; Labidi, N.; Mahmoudi, H.; Jedidi, N.; Abdelly, C. Contrasting effects of municipal compost on alfalfa growth in clay and in sandy soils: N, P, K, content and heavy metal toxicity. Bioresour. Technol. 2008, 99, 6745–6750. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzad, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Kim, K.; Subramanian, P.; Senthilkumar, M.; Anandham, R.; Sa, T. Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agric. Ecosyst. Environ. 2016, 231, 233–239. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Mostofa, M.G.; Rahman, M.M.; Abdel-Farid, I.B.; Tran, L.-S.P. Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physio-biochemical characteristics of stressed plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Abdel Latef, A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Jamil, M.; Lee, K.J.; Kim, J.M.; Kim, H.-S.; Rha, E.S. Salinity reduced growth PS2 photochemistry and chlorophyll content in radish. Sci. Agric. 2007, 64, 111–118. [Google Scholar] [CrossRef]
- Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W. Comparative water relations of two contrasting date palm genotypes under salinity. Int. J. Agron. 2019, 2019, 4262013. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Copetta, A.; Bardi, L.; Bertolone, E.; Berta, G. Fruit production and quality of tomato plants (Solanum lycopersicum L.) are affected by green compost and arbuscular mycorrhizal fungi. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2011, 145, 106–115. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; Volume 1, pp. 1–376. ISBN 9781461485919. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Ben Achiba, W.; Gabteni, N.; Lakhdar, A.; Du Laing, G.; Verloo, M.; Jedidi, N.; Gallali, T. Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric. Ecosyst. Environ. 2009, 130, 156–163. [Google Scholar] [CrossRef]
- del Mar Montiel-Rozas, M.; López-García, Á.; Kjøller, R.; Madejón, E.; Rosendahl, S. Organic amendments increase phylogenetic diversity of arbuscular mycorrhizal fungi in acid soil contaminated by trace elements. Mycorrhiza 2016, 26, 575–585. [Google Scholar] [CrossRef]
- Gaiotti, F.; Marcuzzo, P.; Belfiore, N.; Lovat, L.; Fornasier, F.; Tomasi, D.; Bel, N.; Lovat, L.; Fornasier, F.; Tomasi, D. Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci. Hortic. 2017, 225, 88–95. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of salinity stress and microbial inoculations on glomalin production and plant growth parameters of snap bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef]





| Soil | Compost | |
|---|---|---|
| pH | 8.60 (0.08) | 7.74 (0.01) |
| EC (µS cm−2) | 190.00 (0.45) | 5460.00 (0.20) |
| NTK (%) | 0.11 (0.01) | 1.32 (0.01) |
| NH4+ (%) | - | 0.09 (0.01) |
| NO3−(%) | - | 0.31 (0.01) |
| TOC (%) | 0.59 (0.12) | 5.72 (0.45) |
| OM (%) | 1.02 (0.20) | 9.86 (0.78) |
| C/N | - | 7.49 |
| NH4+/NO3− | - | 0.29 |
| Polsen (ppm) | 11.00 (0.22) | 489.95 (20.29) |
| Na+ (ppm) | 990.00 (30.00) | 2110.00 (50.00) |
| K+ (ppm) | 890.00 (50.00) | 5590.00 (150.00) |
| Ca2+ (ppm) | 11,480.00 (480.00) | 37,380.00 (1840.00) |
| NaCl | Treatment | Leaf Number | Plant Height (cm) |
|---|---|---|---|
| 0 mM | Control | 5.33 (1.53) a | 33.50 (3.28) b |
| Comp | 6.67 (1.15) a | 42.00 (9.16) a | |
| AMF1 | 7.67 (0.58) a | 41.67 (3.51) a | |
| AMF2 | 5.33 (1.53) a | 42.63 (1.18) a | |
| PGPR | 5.67 (1.15) a | 46.07 (1.01) a | |
| AMF1+Comp | 7.33 (1.15) a | 47.27 (0.64) a | |
| AMF2+Comp | 7.00 (1.00) a | 45.67 (1.53) a | |
| PGPR+Comp | 6.67 (0.58) a | 44.17 (0.76) a | |
| PGPR+AMF1 | 6.33 (0.58) a | 45.93 (0.90) a | |
| PGPR+AMF2 | 6.00 (1.00) a | 46.97 (0.35) a | |
| PGPR+AMF1+Comp | 7.67 (0.58) a | 47.87 (0.81) a | |
| PGPR+AMF2+Comp | 6.67 (1.53) a | 48.10 (0.96) a | |
| 120 mM | Control | 4.00 (1.00) a | 29.00 (1.00) d |
| Comp | 4.33 (1.53) a | 31.33 (2.08) c,d | |
| AMF1 | 6.33 (0.58) a | 31.03 (1.55) c,d | |
| AMF2 | 4.67 (0.58) a | 32.50 (1.32) c | |
| PGPR | 5.33 (0.58) a | 30.23 (1.12) c,d | |
| AMF1+Comp | 6.00 (1.00) a | 43.5 (0.50) a | |
| AMF2+Comp | 5.67 (0.58) a | 43.17 (0.76) a | |
| PGPR+Comp | 5.00 (1.00) a | 40.50 (0.50) b | |
| PGPR+AMF1 | 6.00 (1.00) a | 40.20 (1.25) b | |
| PGPR+AMF2 | 5.67 (0.58) a | 43.50 (2.18) a | |
| PGPR+AMF1+Comp | 6.33 (1.00) a | 44.50 (0.50) a | |
| PGPR+AMF2+Comp | 5.33 (0.58) a | 43.83 (0.76) a | |
| 240 mM | Control | 3.33 (0.58) b | 23.33 (0.76) e |
| Comp | 3.33 (0.58) b | 29.33 (1.53) c | |
| AMF1 | 4.67 (0.58) a,b | 28.17 (0.76) c | |
| AMF2 | 3.33 (0.58) b | 27.47 (0.61) c,d | |
| PGPR | 4.67 (0.58) a,b | 25.37 (2.95) d,e | |
| AMF1+Comp | 5.67 (1.15) a,b | 34.23 (0.75) b | |
| AMF2+Comp | 5.33 (0.58) a,b | 34.60 (0.79) b | |
| PGPR+Comp | 4.33 (0.58) a,b | 36.63 (0.71) b | |
| PGPR+AMF1 | 4.67 (0.58) a,b | 36.77 (1.75) b | |
| PGPR+AMF2 | 4.67 (0.58) a,b | 36.37 (1.10) b | |
| PGPR+AMF1+Comp | 6.00 (1.00) a | 42.07 (0.90) a | |
| PGPR+AMF2+Comp | 4.67 (0.58) a,b | 41.36 (0.78) a |
| AMF | PGPR | AMF x PGPR | Compost | AMF x Compost | PGPR x Compost | AMF x PGPR x Compost | Salinity | AMF x Salinity | PGPR x Salinity | AMF x PGPR x Salinity | Compost x Salinity | AMF x Compost x Salinity | PGPR x Compost x Salinity | AMF x PGPR x Compost x Salinity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | *** | *** | ns | *** | ns | ** | ** | *** | * | * | ** | *** | ns | ** | *** |
| Leaves number | *** | ns | ns | ** | ns | ns | * | *** | ns | ns | ns | ns | ns | ns | ns |
| SDW | *** | * | * | *** | *** | *** | *** | *** | *** | ns | ns | ns | ns | ns | *** |
| RDW | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** | ** | *** |
| F% | *** | ** | *** | *** | *** | *** | *** | *** | *** | *** | ** | * | ns | *** | * |
| I% | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | ns | *** | *** | *** | ** |
| gs | *** | *** | ** | *** | ns | * | ns | *** | ns | ns | ns | ns | ns | ns | ** |
| Leaf water potential | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | *** | *** | * |
| Chl a | *** | *** | ns | *** | ns | * | ns | *** | ns | *** | ns | * | ns | ns | ns |
| Chl b | *** | *** | *** | *** | ns | * | ns | *** | ns | * | * | ns | ns | * | ns |
| Total chlorophyll | *** | *** | ns | *** | ns | ns | ns | *** | ns | *** | ns | * | ns | ns | ns |
| Carotenoids | ** | *** | ns | *** | ns | ns | ns | *** | ns | *** | ns | ns | ns | ns | ns |
| PPO | *** | *** | ** | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| POD | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | ns | *** | * | ns |
| CAT | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | ns | *** | *** | *** | ** |
| SOD | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** |
| Sugar content | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** | ns | *** | * | ** | *** |
| Proteins content | *** | *** | *** | *** | ns | * | ns | *** | *** | *** | ns | ns | ns | ns | ns |
| pH | *** | *** | *** | *** | * | *** | ns | *** | *** | *** | *** | *** | ns | ns | *** |
| EC | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| TOC% | *** | *** | *** | *** | ns | ns | *** | *** | * | *** | *** | * | *** | *** | ns |
| OM % | *** | *** | *** | *** | ns | ns | *** | *** | * | *** | *** | * | *** | *** | ns |
| P | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| NaCl | Treatments | Chl a Content (mg g−1 FW) | Chl b Content (mg g−1 FW) | Total Chlorophyll Content (mg g−1 FW) | Carotenoids Content (mg g−1 FW) | Sugar Content (mg eq. Glucose g−1 FW) | Protein Content (mg eq. SAB g−1 FW) |
|---|---|---|---|---|---|---|---|
| 0 mM | Control | 13.94 (0.97) c | 4.17 (0.22) c | 18.12 (1.16) d | 72.33 (4.57) b,c | 53.00 (1.00) d | 3.17 (0.76) e |
| Comp | 15.13 (3.96) b,c | 5.90 (2.13) b,c | 21.03 (6.07) c,d | 75.78 (16.10) a,b,c | 73.67 (1.58) c | 3.43 (0.40) e | |
| AMF1 | 14.76 (2.06) b,c | 5.94 (0.92) b,c | 20.70 (2.04) c,d | 68.83 (3.88) c | 72.00 (1.00) c | 5.50 (0.87) c,d | |
| AMF2 | 14.38 (1.17) b,c | 6.58 (1.49) b,c | 20.96 (0.82) c,d | 69.97 (3.05) c | 73.00 (1.00) c | 5.60 (0.36) c,d | |
| PGPR | 19.17 (2.39) a,b | 6.84 (0.31) b,c | 26.02 (2.23) a,b,c | 92.45 (7.58) a,b,c | 71.67 (7.23) c | 5.33 (1.04) d | |
| AMF1+Comp | 19.28 (2.89) ab | 6.06 (1.15) b,c | 25.34 (4.05) b,c | 88.75 (7.76) a,b,c | 86.00 (1.00) a,b | 6.27 (0.87) b,c,d | |
| AMF2+Comp | 19.56 (2.63) ab | 6.30 (1.32) b,c | 25.86 (3.32) a,b,c | 93.19 (11.15) a,b,c | 86.33 (2.08) a,b | 6.37 (1.00) b,c,d | |
| PGPR+Comp | 19.93 (2.04) ab | 7.87 (1.30) b | 27.81 (3.26) ab | 94.68 (11.54) a,b,c | 86.67 (2.08) a,b | 7.20 (0.53) a,b | |
| PGPR+AMF1 | 19.93 (2.29) ab | 8.62 (1.80) a,b | 28.56 (2.05) a,b | 96.79 (13.31) a,b,c | 85.33 (1.53) b | 7.03 (0.15) a,b,c | |
| PGPR+AMF2 | 20.12 (2.20) a,b | 8.91 (1.66) a,b | 29.04 (1.99) a,b | 97.62 (13.60) a,b,c | 86.33 (1.53) a,b | 7.50 (0.50) a,b | |
| PGPR+AMF1+Comp | 21.09 (2.66) a | 10.78 (1.32) a | 31.88 (2.07) a | 101.20 (18.22) a,b | 92.00 (1.00) a | 7.77 (0.38) a,b | |
| PGPR+AMF2+Comp | 21.44 (2.99) a | 10.74 (1.30) a | 32.19 (2.53) a | 103.40 (21.69) a | 91.00 (1.00) a,b | 8.27 (0.25) a | |
| 120 mM | Control | 10.43 (0.68) d | 3.24 (0.46) d | 13.67 (1.01) e | 55.64 (2.46) c | 50.00 (2.00) h | 2.60 (0.63) f |
| Comp | 13.20 (0.15) c | 2.60 (0.37) d | 15.08 (0.28) d | 62.60 (2.94) b | 61.33 (1.53) g | 2.97 (0.15) f | |
| AMF1 | 13.26 (0.09) c | 2.88 (0.34) d | 16.14 (0.28) d | 64.48 (4.82) b | 70.33 (1.53) de | 4.23 (0.40) e | |
| AMF2 | 13.22 (0.16) c | 2.49 (0.67) d | 15.72 (0.67) d | 64.96 (5.12) b | 69.00 (2.00) e | 4.83 (0.35) de | |
| PGPR | 13.39 (0.18) c | 3.05 (0.40) d | 16.44 (0.29) d | 67.80 (4.30) b | 60.33 (1.53) g | 4.77 (0.21) d,e | |
| AMF1+Comp | 13.29 (0.08) b,c | 2.76 (0.14) d | 16.06 (0.08) d | 66.66 (5.09) b | 74.33 (3.06) b,c | 5.50 (0.50) b,c,d | |
| AMF2+Comp | 14.58 (1.45) a,b | 4.31 (0.88) c | 16.44 (0.59) c | 70.60 (3.18) b | 75.67 (0.58) a,b,c | 5.67 (0.49) b,c,d | |
| PGPR+Comp | 14.53 (0.95) a,b | 4.50 (0.66) c | 19.04 (0.31) c | 71.24 (2.77) b | 64.67 (1.53) f | 6.10 (0.36) b,c | |
| PGPR+AMF1 | 14.32 (0.25) a,b,c | 5.61 (1.07) b | 19.93 (0.91) b,c | 67.53 (4.10) b | 72.00 (2.00) c,d,e | 5.17 (0.76) c,d,e | |
| PGPR+AMF2 | 14.46 (0.27) a,b,c | 5.96 (1.37) b | 20.42 (1.14) b | 69.00 (4.72) b | 73.00 (1.00) c,d | 6.30 (0.82) b | |
| PGPR+AMF1+Comp | 14.57 (0.52) a,b | 7.50 (0.52) a | 22.07 (0.29) a | 79.15 (2.80) a | 79.00 (1.00) a | 6.43 (0.40) b | |
| PGPR+AMF2+Comp | 14.73 (0.13) a | 7.83 (0.46) a | 22.07 (0.36) a | 80.68 (2.38) a | 77.00 (1.00) a,b | 7.27 (0.25) a | |
| 240 mM | Control | 5.66 (0.38) f | 2.08 (0.18) e | 7.74 (0.32) g | 47.49 (12.56) a | 38.67 (0.58) f | 2.13 (0.32) c |
| Comp | 6.71 (0.44) e | 3.26 (0.13) d | 9.98 (0.50) f | 51.94 (12.19) a | 58.00 (1.00) c,d | 2.47 (0.35) b,c | |
| AMF1 | 7.03 (0.38) de | 3.34 (0.16) d | 10.37 (0.47) f | 49.39 (0.71) a | 60.00 (1.00) c,d | 3.70 (0.62) a,b,c | |
| AMF2 | 7.13 (0.37) de | 3.47 (0.17) d | 10.60 (0.48) f | 49.57 (0.74) a | 60.33 (2.08) c | 3.83 (0.76) a,b,c | |
| PGPR | 7.76 (0.20) c | 4.34 (0.18) c | 12.11 (0.75) de | 51.94 (0.48) a | 52.00 (1.00) e | 3.73 (0.38) a,b,c | |
| AMF1+Comp | 7.47 (0.13) c,d | 4.22 (0.66) c | 11.69 (0.74) e | 51.67 (0.49) a | 68.33 (1.53) b | 4.17 (1.26) a,b | |
| AMF2+Comp | 7.77 (0.27) c | 5.02 (0.60) b | 12.80 (0.69) c,d,e | 52.41 (0.57) a | 69.67 (1.15) a,b | 4.33 (0.76) a | |
| PGPR+Comp | 7.93 (0.30) b,c | 5.27 (0.44) b | 13.21 (0.69) c,d | 52.87 (0.68) a | 60.67 (1.53) c | 4.47 (0.90) a | |
| PGPR+AMF1 | 8.03 (0.32) a,b,c | 5.51 (0.27) b | 13.54 (0.58) c | 53.26 (0.43) a | 57.33 (1.15) d | 3.17 (0.76) a,b,c | |
| PGPR+AMF2 | 8.12 (0.31) a,b,c | 5.77 (0.37) a,b | 13.89 (0.68) b,c | 54.54 (0.42) a | 60.00 (1.00) c,d | 3.20 (0.26) a,b,c | |
| PGPR+AMF1+Comp | 8.51 (0.41) a,b | 6.34 (0.49) a | 14.86 (0.81) a,b | 55.86 (0.67) a | 72.00 (1.00) a | 4.47 (0.55) a | |
| PGPR+AMF2+Comp | 8.66 (0.41) a | 6.47 (0.68) a | 15.13 (1.09) a | 56.43 (0.42) a | 71.00 (1.00) a | 4.07 (0.12) a,b |
| NaCl | Treatments | pH | EC (µs · cm−2) | TOC (%) | OM (%) | POlsen (ppm) |
|---|---|---|---|---|---|---|
| 0 mM | Control | 8.47 (0.08) c,d | 334.00 (10.00) h | 0.50 (0.12) f | 0.87 (0.21) f | 13.68 (1.06) f |
| Comp | 8.42 (0.03) d,e | 742.00 (12.00) e | 1.47 (0.06) b | 2.54 (0.10) b | 162.42 (3.32) c | |
| AMF1 | 8.60 (0.02) a,b | 410.00 (5.00) f | 1.06 (0.06) c,d | 1.82 (0.10) c,d | 38.71 (2.03) e | |
| AMF2 | 8.45 (0.04) c,d | 430.00 (5.00) f | 1.00 (0.01) c,d | 1.74 (0.02) c,d | 41.05 (1.47) e | |
| PGPR | 8.61 (0.02) a,b | 387.00 (4.00) g | 0.71 (0.16) e | 1.23 (0.27) e | 55.47 (2.60) d | |
| AMF1+Comp | 8.37 (0.01) d,e | 1403.00 (20.00) a | 1.924 (0.10) a | 3.32 (0.18) a | 182.92 (8.34) ab | |
| AMF2+Comp | 8.33 (0.04) e | 1354.00 (9.00) b | 2.02 (0.09) a | 3.48 (0.15) a | 182.92 (8.34) ab | |
| PGPR+Comp | 8.13 (0.10) f | 1057.50 (24.50) c | 1.13 (0.06) c | 1.94 (0.10) c | 173.27 (4.49) b | |
| PGPR+AMF1 | 8.69 (0.03) a | 374.00 (10.00) g | 0.26 (0.10) g | 0.45 (0.18) g | 61.03 (166) d | |
| PGPR+AMF2 | 8.65 (0.04) a | 318.50 (5.50) h | 0.25 (0.04) g | 0.43 (0.08) g | 59.63 (1.56) d | |
| PGPR+AMF1+Comp | 8.53 (0.04) b,c | 794.00 (15.00) d | 0.88 (0.10) d | 1.52 (0.18) d | 192.48 (11.54) a | |
| PGPR+AMF2+Comp | 8.46 (0.01) c,d | 780.50 (1.50) d | 1.06 (0.07) c,d | 1.83 (0.13) c,d | 187.88 (10.75) a | |
| 120 mM | Control | 8.05 (0.08) e | 830.00 (15.00) b | 0.26 (0.10) e | 0.45 (0.18) e | 11.27 (1.09) g |
| Comp | 7.94 (0.01) f | 739.00 (14.00) c | 1.09 (0.10) c | 1.88 (0.18) c | 218.25 (5.18) a | |
| AMF1 | 8.32 (0.05) c | 634.50 (13.50) e | 0.68 (0.10) d | 1.17 (0.18) d | 51.15 (1.94) e | |
| AMF2 | 8.31 (0.02) c | 656.00 (9.00) d,e | 0.77 (0.12) d | 1.33 (0.20) d | 50.65 (1.80) e | |
| PGPR | 8.56 (0.03) a | 820.50 (4.5.) b | 0.54 (0.12) d | 0.93 (0.21) d | 74.86 (1.60) d | |
| AMF1+Comp | 8.38 (0.05) b,c | 640.00 (5.00) d,e | 1.20 (0.10) b,c | 2.06 (0.18) b,c | 187.40 (3.98) c | |
| AMF2+Comp | 8.32 (0.05) c | 641.50 (5.50) d,e | 1.23 (0.12) b,c | 2.13 (0.21) b,c | 187.46 (4.33) c | |
| PGPR+Comp | 8.51 (0.03) a | 598.00 (6.00) f | 1.20 (0.10) b,c | 2.06 (0.18) b,c | 219.65 (3.02) a | |
| PGPR+AMF1 | 8.43 (0.03) b | 899.50 (5.50) a | 0.57 (0.10) d | 0.99 (0.18) d | 39.01 (3.30) f | |
| PGPR+AMF2 | 8.41 (0.02) b | 885.50 (10.50) a | 0.75 (0.01) d | 1.29 (0.02) d | 50.78 (2.95) e | |
| PGPR+AMF1+Comp | 8.20 (0.01) d | 660.00 (15.00) d | 1.37 (0.16) a,b | 2.36 (0.27) a,b | 201.43 (3.76) b | |
| PGPR+AMF2+Comp | 8.24 (0.03) d | 653.00 (5.00) d,e | 1.51 (0.03) a | 2.61 (0.05) a | 193.04 (6.55) c | |
| 240 mM | Control | 8.15 (0.01) e | 1260.00 (4.00) b | 0.38 (0.08) e | 0.65 (0.14) e | 10.88 (0.46) g |
| Comp | 8.03 (0.04) f | 856.50 (2.50) i | 1.31 (0.04) b | 2.26 (0.07) b | 256.58 (6.86) a | |
| AMF1 | 8.26 (0.01) c,d | 1023.50 (0.50) e | 0.88 (0.04) c | 1.52 (0.06) c | 46.86 (1.62) f | |
| AMF2 | 8.20 (0.08) c,d,e | 992.50 (0.50) f | 0.95 (0.05) c | 1.64 (0.09) c | 46.25 (2.27) f | |
| PGPR | 8.47 (0.01) a | 1125.00 (1.00) d | 0.49 (0.02) d,e | 0.84 (0.03) d,e | 88.95 (2.42) e | |
| AMF1+Comp | 8.27 (0.05) c,d | 862.00 (3.00) i | 1.30 (0.05) b | 2.25 (0.09) b | 241.91 (3.56) b | |
| AMF2+Comp | 8.28 (0.09) c | 895.50 (0.50) g | 1.45 (0.06) a,b | 2.50 (0.10) a,b | 238.41 (2.84) b | |
| PGPR+Comp | 8.37 (0.01) b | 992.00 (6.00) f | 1.43 (0.07) a,b | 2.46 (0.12) a,b | 261.34 (3.20) a | |
| PGPR+AMF1 | 8.40 (0.01) b | 1161.50 (2.50) c | 0.52 (0.04) d | 0.90 (0.06) d | 50.48 (0.95) f | |
| PGPR+AMF2 | 8.36 (0.02) b | 1274.00 (10) a | 0.56 (0.03) d | 0.97 (0.05) d | 49.92 (3.70) f | |
| PGPR+AMF1+Comp | 8.18 (0.02) d,e | 891.00 (5.00) g | 1.48 (0.14) a | 2.55 (0.23) a | 219.92 (1.29) c | |
| PGPR+AMF2+Comp | 8.18 (0.02) d,e | 872.00 (7.00) h | 1.51 (0.09) a | 2.60 (0.16) a | 208.88 (3.31) d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toubali, S.; Tahiri, A.-i.; Anli, M.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Oufdou, K.; Ait-Rahou, Y.; Ben-Ahmed, H.; et al. Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress. Appl. Sci. 2020, 10, 8665. https://doi.org/10.3390/app10238665
Toubali S, Tahiri A-i, Anli M, Symanczik S, Boutasknit A, Ait-El-Mokhtar M, Ben-Laouane R, Oufdou K, Ait-Rahou Y, Ben-Ahmed H, et al. Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress. Applied Sciences. 2020; 10(23):8665. https://doi.org/10.3390/app10238665
Chicago/Turabian StyleToubali, Salma, Abdel-ilah Tahiri, Mohamed Anli, Sarah Symanczik, Abderrahim Boutasknit, Mohamed Ait-El-Mokhtar, Raja Ben-Laouane, Khalid Oufdou, Youssef Ait-Rahou, Hela Ben-Ahmed, and et al. 2020. "Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress" Applied Sciences 10, no. 23: 8665. https://doi.org/10.3390/app10238665
APA StyleToubali, S., Tahiri, A.-i., Anli, M., Symanczik, S., Boutasknit, A., Ait-El-Mokhtar, M., Ben-Laouane, R., Oufdou, K., Ait-Rahou, Y., Ben-Ahmed, H., Jemo, M., Hafidi, M., & Meddich, A. (2020). Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress. Applied Sciences, 10(23), 8665. https://doi.org/10.3390/app10238665