Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods
Abstract
:1. Introduction
2. Synthetic Routes and Fabrication Techniques for TiO2
2.1. Sol-Gel
2.2. Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD)
2.3. Sonochemical and Microwave-Assisted Methods
2.4. Hydrothermal Method
2.5. Oxidation Method
2.6. Spray Pyrolysis
2.7. The “Green” Route
2.8. Deposition Techniques: Spin Coating and Doctor Blade
3. Toxicity Assessment of TiO2 NPs and Films
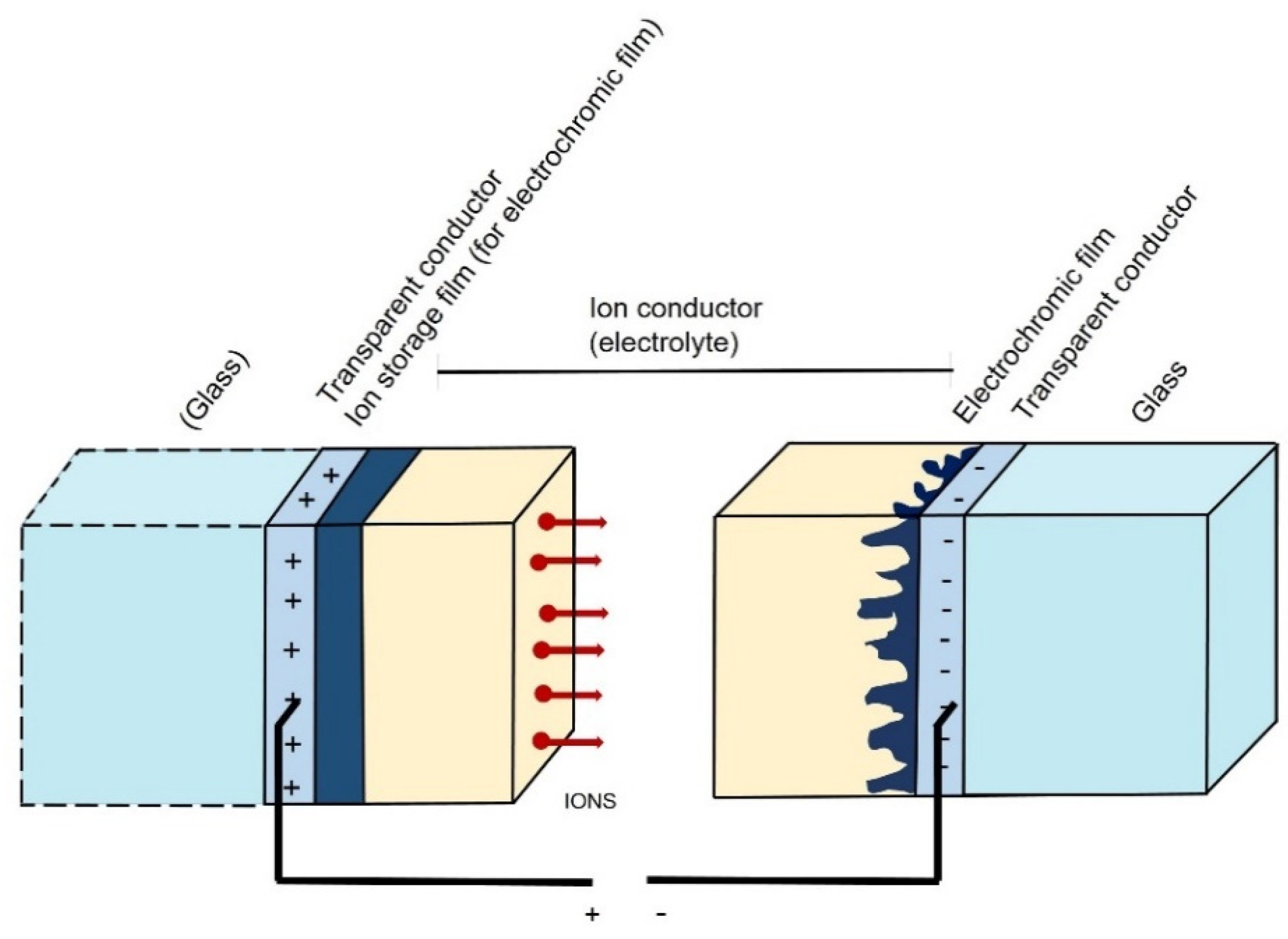
4. TiO2 in the Design of Chromogenic Devices—General Considerations
5. TiO2 in Photoelectrochromic (PEC) and Photovoltachromic Devices
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.D.A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro 2011, 25, 231–241. [Google Scholar] [CrossRef]
- Szymańska, R.; Kołodziej, K.; Ślesak, I.; Zimak-Piekarczyk, P.; Orzechowska, A.; Gabruk, M.; Żądło, A.; Habina, I.; Knap, W.; Burda, K.; et al. Titanium dioxide nanoparticles (100–1000 mg/L) can affect vitamin E response in Arabidopsis thaliana. Environ. Pollut. 2016, 213, 957–965. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. Chin. J. Catal. 2009, 30, 839–851. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Jameel, N.; Imad, H. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.D.; Stotland, M.E.J. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Tryk, D.A.; Nishimoto, S.; Murakami, T.; Fujishima, A. Superhydrophobic TiO2 Surfaces: Preparation, Photocatalytic Wettability Conversion, and Superhydrophobic−Superhydrophilic Patterning. J. Phys. Chem. C 2007, 111, 14521–14529. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Li, M.; Yin, J.J.; Wamer, W.G.; Lo, Y.M. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J. Food Drug Anal. 2014, 22, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium Dioxide (Anatase and Rutile): Surface Chemistry, Liquid–Solid Interface Chemistry, and Scientific Synthesis of Supported Catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef] [PubMed]
- Oi, L.E.; Choo, M.Y.; Lee, H.V.; Ong, H.C.; Abd Hamid, S.B.; Juan, J.C. Recent advances of titanium dioxide (TiO2) for green organic synthesis. Rsc. Adv. 2016, 6, 108741–108754. [Google Scholar] [CrossRef]
- Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Okada, I.; Yoshikawa, S. Formation of titania nanotubes and applications for dye-sensitized solar cells. J. Elelectrochem. Soc. 2003, 150, G488–G493. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, S.H.; Kang, M.S.; Kim, J.Y.; Kim, H.S.; Hyeon, T.; Sung, Y.E. Nanorod-Based Dye-Sensitized Solar Cells with Improved Charge Collection Efficiency. Adv. Mater. 2007, 20, 54–58. [Google Scholar] [CrossRef]
- De Matteis, V.; Rinaldi, R. Toxicity Assessment in the Nanoparticle Era. In Cellular and Molecular Toxicology of Nanoparticles. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Ahn, K.H.; Park, Y.B.; Park, D.W. Kinetic and mechanistic study on the chemical vapor deposition of titanium dioxide thin films by in situ FT-IR using TTIP. Surface Coat. Technol. 2003, 171, 198–204. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X. Growth of Rutile Titanium Dioxide Nanowires by Pulsed Chemical Vapor Deposition. Cryst. Growth Des. 2011, 949–954. [Google Scholar] [CrossRef]
- Fictorie, C.P.; Evans, J.F.; Gladfelter, W.L. Kinetic and mechanistic study of the chemical vapor deposition of titanium dioxide thin films using tetrakis (isopropoxo) titanium (IV). J. Vac. Sci. Technol. A 1998, 12, 1108–1113. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2018; pp. 151–175. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, C.; Fukunaga, N.; Nogami, G. Titanium dioxide thin film deposited by spray pyrolysis of aqueous solution. Thin Solid Film. 2005, 6–8. [Google Scholar] [CrossRef]
- Okuya, M.; Nakade, K.; Kaneko, S. Porous TiO2 thin films synthesized by a spray pyrolysis deposition (SPD) technique and their application to dye-sensitized solar cells. Solar Energy Mater. Solar Cells 2002, 70, 425–435. [Google Scholar] [CrossRef]
- Conde-gallardo, A.; Guerrero, M.; Castillo, N.; Soto, A.B. TiO2 anatase thin films deposited by spray pyrolysis of an aerosol of titanium diisopropoxide. Thin Solid Film. 2005, 473, 68–73. [Google Scholar] [CrossRef]
- Kursawe, M.; Anselmann, R.; Hilarius, V.; Pfaff, G.; Kgaa, M.; Optics, D.P.; Strasse, M. Nano-Particles by Wet Chemical Processing in Commercial Applications. J. Sol-Gel Sci. Technol. 2005, 71–74. [Google Scholar] [CrossRef]
- Gupta, S.K.; Desai, R.; Jha, P.K.; Kirin, D.; Wiley, J. Titanium dioxide synthesized using titanium chloride: Size effect study using Raman spectroscopy and photoluminescence. J. Raman Spectrosc. 2010, 2009, 350–355. [Google Scholar] [CrossRef]
- Kontos, A.I.; Kontos, A.G.; Tsoukleris, D.S.; Bernard, M.C.; Spyrellis, N.; Falaras, P. Nanostructured TiO2 films for DSSCS prepared by combining doctor-blade and sol–gel techniques. J. Mater. Proc. Technol. 2008, 196, 243–248. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic Materials and Devices; Mortimer, R.J., Rosseinsky, D.R., Monk, P.M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527679850. [Google Scholar]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Brinker, C.J.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Oskam, G.; Nellore, A.; Penn, R.L.; Searson, P.C. The growth kinetics of TiO2 nanoparticles from titanium (IV) alkoxide at high water/titanium ratio. J. Phys. Chem. B 2003, 107, 1734–1738. [Google Scholar] [CrossRef]
- Mehrotra, R.C.; Singh, A. Recent trends in metal alkoxide chemistry. Prog. Inorg. Chem. 1997, 46, 239–454. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z.J. The evolution of “sol–gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey Brinker, C.; Scherer, G.W. (Eds.) Sol–Gel Science, the Physics and Chemistry of Sol–Gel Processing; Academic Press: Cambridge, MA, USA, 1990; 924p, ISBN 0-12-134970-5. [Google Scholar]
- Brinker, C.J.; Hurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashley, C.S. Review of sol-gel thin film formation. J. Non-Cryst. Solids 1992, 147–148, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, M.; Doeuff, S.; Sanchez, C.; Livage, J. Sol-gel synthesis of electrochromic films. Mater. Sci. Eng. B 1989, 3, 203–207. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Dinh, N.N.; Oanh, N.T.T.; Long, P.D.; Bernard, M.C.; Goff, A.H. Le Electrochromic properties of TiO2 anatase thin films prepared by a dipping sol-gel method. Thin Solid Film. 2003, 423, 70–76. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.; Xu, G.; Tan, S.; Zhang, J. Amorphous titanium dioxide film with improved electrochromism in near-infrared region. Opt. Mater. 2019, 89, 191–196. [Google Scholar] [CrossRef]
- Wu, L.; Yang, D.; Fei, L.; Huang, Y.; Wu, F.; Sun, Y.; Shi, J.; Xiang, Y. Dip-Coating Process Engineering and Performance Optimization for Three-State Electrochromic Devices. Nanoscale Res. Lett. 2017, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Muthee, D.K.; Dejene, B.F. The effect of tetra isopropyl orthotitanate (TIP) concentration on structural, and luminescence properties of titanium dioxide nanoparticles prepared by sol-gel method. Mater. Sci. Semicond. Process. 2020, 106, 104783. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, I.; Yadav, H.; Park, J.; Jo, J.W.; Yim, S.; Lee, J.J. Non-hydrolytic sol-gel route to synthesize TiO2 nanoparticles under ambient condition for highly efficient and stable perovskite solar cells. Sol. Energy 2019, 185, 307–314. [Google Scholar] [CrossRef]
- Nateq, M.H.; Ceccato, R. Sol-Gel Synthesis of TiO2 Nanocrystalline Particles with Enhanced Surface Area through the Reverse Micelle Approach. Adv. Mater. Sci. Eng. 2019, 1567824. [Google Scholar] [CrossRef] [Green Version]
- Gurav, A.S.; Kodas, T.T.; Wang, L.M.; Kauppinen, E.I.; Joutsensaari, J. Generation of nanometer-size fullerene particles via vapor condensation. Chem. Phys. Lett. 1994, 218, 304–308. [Google Scholar] [CrossRef]
- Maria Benelmekki, A.E. Nanostructured thin films–background, preparation and relation to the technological revolution of the 21st century. In Frontiers of Nanoscience; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 1–34. [Google Scholar]
- Peter, M. Martin Deposition Technologies: An Overview. In Handbook of Deposition Technologies for Films and Coatings (Third Edition) Science, Applications and Technology; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 1–31. [Google Scholar]
- Shamim Ahmad Organic semiconductors for device applications: Current trends and future prospects. J. Polym. Eng. 2014, 34, 279–338. [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Seifried, S.; Winterer, M.; Hahn, H. Nanocrystalline Titania films and particles by chemical vapor synthesis. Chem. Vap. Depos. 2000, 6, 239–244. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Reucroft, P.J.; Yang, F.; Dozier, A. Growth of TiO2 Nanorods by Metalorganic Chemical Vapor Deposition. J. Cryst. Growth 2003, 256, 83–88. [Google Scholar] [CrossRef]
- Wu, J.J.; Yu, C.C. Aligned TiO2 Nanorods and Nanowalls. J. Phys. Chem. B 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Macwan, D.P.; Balasubramanian, C.; Dave, P.N.; Chaturvedi, S. Thermal plasma synthesis of nanotitania and its characterization. J. Saudi Chem. Soc. 2014, 18, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Shih, H.C.; Wu, W.T.; Tseng, Y.K.; Chen, I.C. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J. Cryst. Growth 2005, 281, 384–390. [Google Scholar] [CrossRef]
- Wu, J.M.; Shih, H.C.; Wu, W.T. Electron field emission from single crystalline TiO2 nanowires prepared by thermal evaporation. Chem. Phys. Lett. 2005, 413, 490. [Google Scholar] [CrossRef]
- Qiang Zhang, C.L. High Temperature Stable Anatase Phase Titanium Dioxide Films Synthesized by Mist Chemical Vapor Deposition. Nanomaterials 2020, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Milton Ohring Thin-Film Evaporation Processes. In Materials Science of Thin Films (Second Edition) Deposition and Structure; Elsevier: Amsterdam, The Netherlands, 2002; pp. 95–144.
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302. [Google Scholar] [CrossRef]
- Donald, M. Mattox Atomistic Film Growth and Some Growth-Related Film Properties. In Handbook of Physical Vapor Deposition (PVD) Processing (Second Edition); William Andrew: Norwich, NY, USA, 2010; pp. 333–398. [Google Scholar]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. Aims Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Wasa, K.; Kitabatake, M.; Adachi, H. Thin Film Materials Technology: Sputtering of Control Compound Materials; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Wasa, K.; Hayakawa, S. Handbook of Sputter Deposition Technology; Noyes Publications; William Andrew: Norwich, NY, USA, 1992. [Google Scholar]
- Seyfert, U.; Heisig, U.; Teschner, G.; Strümpfel, J. 40 Years of Industrial Magnetron Sputtering in Europe. Svc. Bull. Fall 2015, 22–26. [Google Scholar]
- Sorar, I.; Pehlivan, E.; Niklasson, G.A.; Granqvist, C.G. Electrochromism of DC magnetron sputtered TiO2 thin films: Role of deposition parameters. Sol. Energy Mater. Sol. Cells 2013, 115, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Sorar, I.; Pehlivan, E.; Niklasson, G.A.; Granqvist, C.G. Electrochromism of DC magnetron-sputtered TiO2: Role of film thickness. Appl. Surf. Sci. 2014, 318, 24–27. [Google Scholar] [CrossRef]
- Gregory Chatel Sonochemistry in nanocatalysis: The use of ultrasound from the catalyst synthesis to the catalytic reaction. Curr. Opin. Green Sustain. Chem. 2019, 15, 1–6. [CrossRef]
- Kasap, S.; Tel, H.; Piskin, S. Preparation of TiO2 nanoparticles by sonochemical method, isotherm, thermodynamic and kinetic studies on the sorption of strontium. J. Radioanal. Nucl. Chem. 2011, 289, 489–495. [Google Scholar] [CrossRef]
- Okitsu, K.; Yue, A.; Tanabe, S.; Matsumoto, H.; Yobiko, Y. Formation of colloidal gold nanoparticles in an ultrasonic field: Control of rate of gold (iii) reduction and size of formed gold particles. Langmuir ACS J. Surf. Colloids 2001, 17, 7717–7720. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Phil. Trans. Roy. Soc. A 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Gupta, M.; Eugene, W.W.L. Microwaves—Theory. In Microwaves and Metals; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2011; pp. 25–41. [Google Scholar]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of inorganic solids using microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, S.; Komarneni, S.; Mariani, E.; Villa, C. Rapid microwave–hydrothermal synthesis of anatase form of titanium dioxide. J. Am. Ceram. Soc. 2005, 88, 3238. [Google Scholar] [CrossRef]
- Cabello, G.; Davoglio, R.A.; Pereira, E.C. Microwave-assisted synthesis of anatase-TiO2 nanoparticles with catalytic activity in oxygen reduction. J. Electroanal. Chem. 2017, 794, 36–42. [Google Scholar] [CrossRef]
- Delgado, L.P.; Figueroa-Torres, M.Z.; Ceballos-Chuc, M.C.; García-Rodríguez, R.; Alvarado-Gil, J.J.; Oskam, G.; Rodriguez-Gattorno, G. Tailoring the TiO2 phases through microwave-assisted hydrothermal synthesis: Comparative assessment of bactericidal activity. Mater. Sci. Eng. C 2020, 117, 111290. [Google Scholar] [CrossRef]
- Reeja-Jayan, B.; Harrison, K.L.; Yang, K.; Wang, C.L.; Yilmaz, A.E.; Manthiram, A. Microwave-assisted Low-temperature Growth of Thin Films in Solution. Sci. Rep. 2012, 2, 1003. [Google Scholar] [CrossRef]
- Dahiya, M.S.; Tomer, V.K.; Duhan, S. Metal–ferrite nanocomposites for targeted drug delivery. In Delivery, Applications of Nanocomposite Materials in Drug Biomaterials, Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 737–760. [Google Scholar]
- Reddy, K.M.; Guin, D.; Manorama, S.V.; Reddy, A.R. Selective synthesis of nanosized TiO2 by hydrothermal route: Characterization, structure property relation, and photochemical application. J. Mater. Res. 2004, 19, 2567–2575. [Google Scholar] [CrossRef]
- Kim, C.S.; Moon, B.K.; Park, J.H.; Choi, B.C.; Seo, H.J. Solvothermal synthesis of nanocrystalline TiO2 in toluene with surfactant. J. Cryst. Growth 2003, 257, 309–315. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, J.; Jiang, L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angew. Chem. Int. Edition. 2005, 44, 5115–5118. [Google Scholar] [CrossRef]
- Stride, J.A.; Tuong, N.T. Controlled synthesis of titanium dioxide nanostructures. Solid State Phenom. 2010, 162, 261–274. [Google Scholar] [CrossRef]
- Maheswari, P.; Ponnusamy, S.; Harish, S.; Ganesh, M.R.; Hayakawa, Y. Hydrothermal synthesis of pure and bio modified TiO2: Characterization, evaluation of antibacterial activity against gram positive and gram negative bacteria and anticancer activity against KB Oral cancer cell line. Arab. J. Chem. 2020, 13, 3484–3497. [Google Scholar] [CrossRef]
- Beyer, J.; Mamakhel, A.; Søndergaard-Pedersen, F.; Yu, J.; Iversen, B.B. Continuous flow hydrothermal synthesis of phase pure rutile TiO2 nanoparticles with a rod-like morphology. Nanoscale 2020, 12, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Malekshahi Byranvand, M.; Nemati Kharat, A.; Fatholahi, L.; Malekshahi Beiranvand, Z. A Review on Synthesis of Nano-TiO2 via Different Methods. J. Nanostruct. 2013, 3, 1–9. [Google Scholar]
- Xiaobo, C. Titanium dioxide nanomaterials and their energy applications. Chin. J. Catal. 2009, 30, 839–851. [Google Scholar]
- Rahmat, S.T.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Synthesis of rutile TiO2 nanowires by thermal oxidation of titanium inthe presence of KOH and their ability to photoreduce Cr(VI) ions. J. Alloy Compd. 2020, 152094. [Google Scholar] [CrossRef]
- Arcadipane, E.; Sanz, R.; Amiard, G.; Boninelli, S.; Impellizzeri, G.; Privitera, V.; Bonkerud, J.; Bhoodoo, C.; Vines, L.; Svensson, B.G.; et al. Single-crystal TiO2 nanowires by seed assisted thermal oxidation of Ti foil: Synthesis and photocatalytic properties. RSC Adv. 2016, 6, 55490–55498. [Google Scholar] [CrossRef] [Green Version]
- Gavrilović, T.V.; Jovanović, D.J.; Dramićanin, M.D. Synthesis of Multifunctional Inorganic Materials: From Micrometer to Nanometer Dimensions. In Nanomaterials for Green Energy-Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–81. [Google Scholar]
- Perednis, D.; Gauckler, L.J. Thin Film Deposition Using Spray Pyrolysis. J. Electroceram. 2005, 103–111. [Google Scholar] [CrossRef]
- Ramadhan, Z.R.; Yun, C.; Park, B.I.; Yu, S.; Kang, M.H.; Kim, S.; Lim, D.; Choi, B.H.; Han, J.W.; Kim, Y.H. High performance electrochromic devices based on WO3eTiO2 nanoparticles synthesized by flame spray pyrolysis. Opt. Mater. 2019, 89, 559–562. [Google Scholar] [CrossRef]
- Wang, W.N.; Lenggoro, I.W.; Terashi, Y.; Kim, T.O.; Okuyama, K. One-step synthesis of titanium oxide nanoparticles by spray pyrolysis of organic precursors. Mater. Sci. Eng. B 2005, 23, 194–202. [Google Scholar] [CrossRef]
- Abou-Helal, M.O.; Seeber, W.T. Preparation of TiO2 thin films by spray pyrolysis to be used as a photocatalyst. Appl. Surf. Sci. 2002, 195, 53–62. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, Y.; Hong, K.; Wang, G. Growth and mechanism of titania nanowires. Solid State Commun. 2003, 126, 545–549. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Gowri, S. Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011, 8, 447–451. [Google Scholar]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Rahuman, A.A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.D.; Lekshmi, M.; Madhumitha, G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta Part A 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Renata Dobrucka Synthesis of Titanium Dioxide Nanoparticles Using Echinacea purpurea Herba. Iran. J. Pharm. Res. 2017, 16, 756–762.
- Angus Rockett Chemical Vapor Deposition. In The Materials Science of Semiconductors; Springer: Boston, MA, USA, 2008.
- Donald, M. Mattox Handbook of Physical Vapor Deposition (PVD) Processing; Noyes Publications; William Andrew: Westwood, NJ, USA, 1998; ISBN 978-0-81-552037-5. [Google Scholar]
- De Matteis, V.; Cannavale, A.; Blasi, L.; Quarta, A.; Gigli, G. Chromogenic device for cystic fibrosis precocious diagnosis: A “point of care” tool for sweat test. Sens. Actuators B Chem. 2016, 225, 474–480. [Google Scholar] [CrossRef]
- Asgharzadehahmadi, S.; Raman, A.A.; Parthasarathy, R.; Sajjadi, B. Sonochemical reactors: Review on features, advantages and limitations. Renew. Sustain. Energy Rev. 2016, 63, 302–314. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, F.; Jin, C.; Liang, H.; Zhong, X.; Yang, Y. Mitochondrial Injury Induced by Nanosized Titanium Dioxide in A549 Cells and Rats. Environ. Toxicol. Pharmacol. 2013, 36, 66–72. [Google Scholar] [CrossRef]
- Ulrich Schubert, N.H. Synthesis of Inorganic Materials, 4th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; ISBN 978-3-527-34457-4. [Google Scholar]
- Su, Z.; Zhang, L.; Jiang, F.; Hong, M. Formation of crystalline TiO2 by anodic oxidation of titanium. Prog. Nat. Sci. Mater. Int. 2013, 23, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Varghese, O.K.; Gong, D.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2003, 18, 156–165. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef] [PubMed]
- Mihelčič, M.; Jerman, I.; Orel, B. Preparation of electrochromic Ni1−xO and TiO2 coatings from pigment dispersions and their application in electrochromic foil based devices. Prog. Org. Coat. 2013, 76, 1752–1755. [Google Scholar] [CrossRef]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Mennig, M. (Eds.) Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Sarangika, H.N.M.; Dissanayake, M.A.K.L.; Senadeera, G.K.R.; Karunarathne, W.G.M.D. Low cost quasi solid state electrochromic devices based on F-doped tin oxide and TiO2. Mater. Today Proc. 2019, 23, 100–104. [Google Scholar] [CrossRef]
- Dinh, N.N.; Quyen, N.M.; Zikova, M.; Truong, V.V. Highly-efficient electrochromic performance of nanostructured TiO2 films made by doctor blade technique. Sol. Energy Mater. Sol. Cells 2011, 95, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Morphomechanical and organelle perturbation induced by silver nanoparticle exposure. J. Nanopart Res. 2018, 20, 273. [Google Scholar] [CrossRef]
- Mühlebach, S.; Borchard, G.; Yildiz, S. Regulatory challenges and approaches to characterize nanomedicines and their follow-on similars. Nanomedicine 2015, 10, 659–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 2008, 5, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary Responses of Mice, Rats, and Hamsters to Subchronic Inhalation of Ultrafine Titanium Dioxide Particles. Toxicol. Sci. 2004, 357, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Lee, B.W.; Ogami, A.; Oyabu, T.; Myojo, T. Inhalation of titanium dioxide (P25) nanoparticles to rats and changes in surfactant protein (SP-D) levels in bronchoalveolar lavage fluid and serum. Toxicology 2019, 13, 1396–1408. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Zheng, L.; Wang, S.; Wang, Y.; Zhao, X.; Duan, Y.; Cui, Y.; Zhou, M.; Cai, J.; et al. P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol. Trace Elem. Res. 2011, 140, 186–197. [Google Scholar] [CrossRef]
- Mohamed, H.R. Estimation of TiO2 nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice. Food Chem. Toxicol. 2015, 83, 76–83. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fan, J.; Qin, X.; Wang, T.; Zhang, Y.; Zhang, J.; Qiu, Y.; Qiu, J.; et al. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch. Toxicol. 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S.; et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.M.; de Azevedo, W.M.; Dagli, M.L.; Toma, S.H.; de Arruda Leite, A.Z.; Lordello, M.L.; Nishitokukado, I.; Ortiz-Agostinho, C.L.; Duarte, M.I.; Ferreira, M.A.; et al. Titanium dioxide induced inflammation in the small intestine. World J. Gastroenterol. 2012, 18, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Sanchez, W.Y.; Roberts, M.S. Are commercially available nanoparticles safe when applied to the skin? J. Biomed. Nanotechnol. 2010, 6, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 9282–9297. [Google Scholar] [CrossRef]
- Schulz, J.; Hohenberg, H.; Pflücker, F.; Gärtner, E.; Will, T.; Pfeiffer, S.; Wepf, R.; Wendel, V.; Gers-Barlag, H.; Wittern, K.P. Distribution of sunscreens on skin. Adv. Drug Deliv. Rev. 2002, 54, 157–163. [Google Scholar] [CrossRef]
- Lademann, J.; Weigmann, H.J.; Rickmeyer, C.; Barthelmes, H.; Schaefer, H.; Mueller, G.; Sterry, W. Penetration of Titanium Dioxide Microparticles in a Sunscreen Formulation into the Horny Layer and the Follicular Orifice. Ski. Pharm. Appl. Ski. Physiol. 1999, 12, 247–256. [Google Scholar] [CrossRef]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on penetration of titanium dioxide (TiO(2)) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010, 35, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Bennat, C.; Müller-Goymann, C.C. Müller-Goymann Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int. J. Cosmet. Sci. 2001, 22, 271–283. [Google Scholar] [CrossRef]
- Sadrieh, N.; Wokovich, A.M.; Gopee, N.V.; Zheng, J.; Haines, D.; Parmiter, D.; Siitonen, P.H.; Cozart, C.R.; Patri, A.K.; McNeil, S.E.; et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 2010, 155, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo-Vila, M.; Moreno-Burriel, B.; Chinarro, E.; Jurado, J.R.; Casañ-Pastor, N.; Collazos-Castro, J.E. Titanium oxide as substrate for neural cell growth. J. Biomed. Mater. Res. A 2009, 90, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Brors, D.; Aletsee, C.; Schwager, K.; Mlynski, R.; Hansen, S.; Schäfers, M.; Ryan, A.F.; Dazert, S. Interaction of spiral ganglion neuron processes with alloplastic materials in vitro(1). Hear. Res. 2002, 167, 110–121. [Google Scholar] [CrossRef]
- Buchloh, S.; Stieger, B.; Meier, P.J.; Gauckler, L. Hepatocyte performance on different crystallographic faces of rutile. Biomaterials 2003, 24, 2605–2610. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, B.; López-Huerta, F.; Vega, R.; Hernández-Torres, J.; García-González, L.; Salceda, E.; Herrera-May, A.L.; Soto, E. Cytotoxicity Evaluation of Anatase and Rutile TiO2 Thin Films on CHO-K1 Cells in Vitro. Materials 2016, 9, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavale, A.; Ayr, U.; Fiorito, F.; Martellotta, F. Smart electrochromic windows to enhance building energy efficiency and visual comfort. Energies 2020, 13, 1449. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G.; Lansåker, P.C.; Mlyuka, N.R.; Niklasson, G.A.; Avendaño, E. Progress in chromogenics: New results for electrochromic and thermochromic materials and devices. Sol. Energy Mater. Sol. Cells 2009, 93, 2032–2039. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 0444899308. [Google Scholar]
- Cao, X.; Chang, T.; Shao, Z.; Xu, F.; Luo, H.; Jin, P. Challenges and Opportunities toward Real Application of VO2-Based Smart Glazing. Matter 2020, 2, 862–881. [Google Scholar] [CrossRef]
- Karlessi, T.; Santamouris, M. Improving the performance of thermochromic coatings with the use of UV and optical filters tested under accelerated aging conditions. Int. J. Low-Carbon Technol. 2015, 10, 45–61. [Google Scholar] [CrossRef]
- Cipolloni, M.; Heynderickx, A.; Maurel, F.; Perrier, A.; Jacquemin, D.; Siri, O.; Ortica, F.; Favaro, G. Multiswitchable acidichromic and photochromic bisdiarylethene. An experimental and theoretical study. J. Phys. Chem. C 2011, 115, 23096–23106. [Google Scholar] [CrossRef]
- Arbab, S.; Matusiak, B.S. Toward colour rendering method of window glass. Color Res. Appl. 2019, 44, 34–43. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Wen, R.T.; Arvizu, M.A.; Niklasson, G.A.; Granqvist, C.G. Electrochromics for energy efficient buildings: Towards long-term durability and materials rejuvenation. Surf. Coat. Technol. 2015, 278, 121–125. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Film. 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Bayrak Pehlivan, İ.; Niklasson, G.A. Electrochromics on a roll: Web-coating and lamination for smart windows. Surf. Coat. Technol. 2018, 336, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G.; Azens, A.; Heszler, P.; Kish, L.B.; Österlund, L. Nanomaterials for benign indoor environments: Electrochromics for “smart windows”, sensors for air quality, and photo-catalysts for air cleaning. Sol. Energy Mater. Sol. Cells 2007, 91, 355–365. [Google Scholar] [CrossRef]
- Hossain, K.; Rameeja, S. Importance of Nanotechnology in Civil Engineering. Eur. J. Sustain. Dev. 2015, 4, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, D.; Diamantopoulou, A.; Pantazopoulos, P.A.; Palles, D.; Sakellis, E.; Boukos, N.; Stefanou, N.; Likodimos, V. Nanographene oxide-TiO2 photonic films as plasmon-free substrates for surface-enhanced Raman scattering. Nanoscale 2019, 11, 21542–21553. [Google Scholar] [CrossRef]
- Nunes, D.; Freire, T.; Barranger, A.; Matias, M.; Pereira, S.; Pimentel, A.; Cordeiro, N.J.A.; Fortunato, E.; Martins, R. TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Appl. Sci. 2020, 10, 1200. [Google Scholar] [CrossRef] [Green Version]
- Pigeot-Rémy, S.; Gregori, D.; Hazime, R.; Hérissan, A.; Guillard, C.; Ferronato, C.; Cassaignon, S.; Colbeau-Justin, C.; Durupthy, O. Size and shape effect on the photocatalytic efficiency of TiO2 brookite. J. Mater. Sci. 2019, 54, 1213–1225. [Google Scholar] [CrossRef]
- Sharma, S.; Venkata Dhanunjaya Reddy, A.; Jayarambabu, N.; Vikram Manoj Kumar, N.; Saineetha, A.; Venkateswara Rao, K.; Kailasa, S. Synthesis and characterization of Titanium dioxide nanopowder for various energy and environmental applications. Mater. Today Proc. 2019, 3–6. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic oxides: A bandstructure approach. Sol. Energy Mater. Sol. Cells 1994, 32, 369–382. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic oxides: A unified view. Solid State Ion. 1994, 70–71, 678–685. [Google Scholar] [CrossRef]
- Jelle, B.P. Electrochromic Smart Windows for Dynamic Daylight and Solar Energy Control in Buildings. In Electrochromic Materials and Devices; Mortimer, R.J., Rosseinsky, D.R., Paul, M.S., Eds.; Monk Wiley-VCH: Hoboken, NJ, USA, 2015; pp. 419–502. [Google Scholar]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, T.; Sivakumar, R.; Zheng, D.; Xu, H.; Ilangovan, R.; Sanjeeviraja, C.; Lin, J. WO3/TiO2 hierarchical nanostructures for electrochromic applications. Mater. Sci. Semicond. Process. 2020, 2–7. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Y.M.; Cai, Y.; Yang, B.; Gu, C.; Zhang, S.X. Advances in nanomaterials for electrochromic devices. Chem. Soc. Rev. 2020, 49, 8687–8720. [Google Scholar] [CrossRef]
- Sung, Y.C.; Mamak, M.; Coombs, N.; Chopra, N.; Ozin, G.A. Electrochromic performance of viologen-modified periodic mesoporous nanocrystalline anatase electrodes. Nano Lett. 2004, 4, 1231–1235. [Google Scholar] [CrossRef]
- Vlachopoulos, N.; Nissfolk, J.; Möller, M.; Briançon, A.; Corr, D.; Grave, C.; Leyland, N.; Mesmer, R.; Pichot, F.; Ryan, M.; et al. Electrochemical aspects of display technology based on nanostructured titanium dioxide with attached viologen chromophores. Electrochim. Acta 2008, 53, 4065–4071. [Google Scholar] [CrossRef]
- Giannuzzi, R.; Manca, M.; De Marco, L.; Belviso, M.R.; Cannavale, A.; Sibillano, T.; Giannini, C.; Cozzoli, P.D.; Gigli, G. Ultrathin TiO2(B) nanorods with superior lithium-ion storage performance. ACS Appl. Mater. Interfaces 2014, 6, 1933–1943. [Google Scholar] [CrossRef]
- Patil, R.A.; Devan, R.S.; Liou, Y.; Ma, Y.R. Efficient electrochromic smart windows of one-dimensional pure brookite TiO2 nanoneedles. Sol. Energy Mater. Sol. Cells 2016, 147, 240–245. [Google Scholar] [CrossRef]
- Han, J.; Ko, K.W.; Sarwar, S.; Lee, M.S.; Park, S.; Hong, S.; Han, C.H. hwan Enhanced electrochromic properties of TiO2 nanocrystal embedded amorphous WO3 films. Electrochim. Acta 2018, 278, 396–404. [Google Scholar] [CrossRef]
- Barawi, M.; De Trizio, L.; Giannuzzi, R.; Veramonti, G.; Manna, L.; Manca, M. Dual Band Electrochromic Devices Based on Nb-Doped TiO2 Nanocrystalline Electrodes. ACS Nano 2017, 11, 3576–3584. [Google Scholar] [CrossRef]
- Barawi, M.; Veramonti, G.; Epifani, M.; Giannuzzi, R.; Sibillano, T.; Giannini, C.; Rougier, A.; Manca, M. A dual band electrochromic device switchable across four distinct optical modes. J. Mater. Chem. A 2018, 6, 10201–10205. [Google Scholar] [CrossRef]
- Maiorov, V.A. Electrochromic Glasses with Separate Regulation of Transmission of Visible Light and Near-Infrared Radiation (Review). Opt. Spectrosc. 2019, 126, 412–430. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Lee, J.Y. Plasmonic Oxygen-Deficient TiO2-x Nanocrystals for Dual-Band Electrochromic Smart Windows with Efficient Energy Recycling. Adv. Mater. 2020, 32, 2–9. [Google Scholar] [CrossRef]
- Qu, X.; Fu, Y.; Ma, C.; Yang, Y.; Shi, D.; Chu, D.; Yu, X. Bifunctional electrochromic-energy storage materials with enhanced performance obtained by hybridizing TiO2 nanowires with POMs. New J. Chem. 2020, 44, 15475–15482. [Google Scholar] [CrossRef]
- Cannavale, A.; Cossari, P.; Eperon, G.E.; Colella, S.; Fiorito, F.; Gigli, G.; Snaith, H.J.; Listorti, A. Forthcoming perspectives of photoelectrochromic devices: A critical review. Energy Environ. Sci. 2016, 9, 2682–2719. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1–38. [Google Scholar] [CrossRef]
- Ortica, F. The role of temperature in the photochromic behaviour. Dye Pigment 2012, 92, 807–816. [Google Scholar] [CrossRef]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A Low-Cost, High-Efficiency Solar-Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Huang, S.Y.; Schlichthörl, G.; Nozik, A.J.; Grätzel, M.; Frank, A.J. Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B 1997, 101, 2576–2582. [Google Scholar] [CrossRef]
- Parisi, M.L.; Maranghi, S.; Basosi, R. The evolution of the dye sensitized solar cells from Grätzel prototype to up-scaled solar applications: A life cycle assessment approach. Renew. Sustain. Energy Rev. 2014, 39, 124–138. [Google Scholar] [CrossRef]
- Gregg, B.A. Photoelectrochromic applications. Endeavour 1997, 21, 52–55. [Google Scholar] [CrossRef]
- Li, Y.; Hagen, J.; Haarer, D. Novel photoelectrochromic cells containing a polyaniline layer and a dye-sensitized nanocrystalline TiO2 photovoltaic cell. Synth. Met. 1998, 94, 273–277. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, K.M.; Huang, J.H.; Justin Thomas, K.R.; Lin, J.T.; Ho, K.C. A novel photoelectrochromic device with dual application based on poly(3,4-alkylenedioxythiophene) thin film and an organic dye. J. Power Sources 2008, 185, 1505–1508. [Google Scholar] [CrossRef]
- Hauch, A.; Georg, A.; Baumgärtner, S.; Opara Krašovec, U.; Orel, B. New photoelectrochromic device. Electrochim. Acta 2001, 46, 2131–2136. [Google Scholar] [CrossRef]
- Hauch, A.; Georg, A.; Opara Krašovec, U.; Orel, B. Comparison of photoelectrochromic devices with different layer configurations. J. Electrochem. Soc. 2002, 149, H159–H163. [Google Scholar] [CrossRef]
- Georg, A.; Georg, A.; Opara Krašovec, U. Photoelectrochromic window with Pt catalyst. Thin Solid Film. 2006, 502, 246–251. [Google Scholar] [CrossRef]
- Liao, J.Y.; Ho, K.C. A photoelectrochromic device using a PEDOT thin film. J. New Mater. Electrochem. Syst. 2005, 8, 37–47. [Google Scholar]
- Krašovec, U.O.; Georg, A.; Georg, A.; Wittwer, V.; Luther, J.; Topič, M. Performance of a solid-state photoelectrochromic device. Sol. Energy Mater. Sol. Cells 2004, 84, 369–380. [Google Scholar] [CrossRef]
- Wu, J.J.; Hsieh, M.D.; Liao, W.P.; Wu, W.T.; Chen, J.S. Fast-switching photovoltachromic cells with tunable transmittance. ACS Nano 2009, 3, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Cannavale, A.; Manca, M.; Malara, F.; De Marco, L.; Cingolani, R.; Gigli, G. Highly efficient smart photovoltachromic devices with tailored electrolyte composition. Energy Environ. Sci. 2011, 4, 2567–2574. [Google Scholar] [CrossRef]
- Cannavale, A.; Manca, M.; De Marco, L.; Grisorio, R.; Carallo, S.; Suranna, G.P.; Gigli, G. Photovoltachromic device with a micropatterned bifunctional counter electrode. ACS Appl. Mater. Interfaces 2014, 6, 2415–2442. [Google Scholar] [CrossRef]
- Malara, F.; Cannavale, A.; Carallo, S.; Gigli, G. Smart windows for building integration: A new architecture for photovoltachromic devices. ACS Appl. Mater. Interfaces 2014, 6, 9290–9297. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Development of photoelectrochromic devices for dynamic solar control in buildings. Sol. Energy Mater. Sol. Cells 2010, 94, 2304–2313. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Partly covered photoelectrochromic devices with enhanced coloration speed and efficiency. Sol. Energy Mater. Sol. Cells 2012, 96, 86–92. [Google Scholar] [CrossRef]
- Theodosiou, Κ.; Dokouzis, A.; Antoniou, I.; Leftheriotis, G. Gel electrolytes for partly covered photoelectrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 202, 110124. [Google Scholar] [CrossRef]
- Dokouzis, A.; Theodosiou, K.; Leftheriotis, G. Assessment of the long-term performance of partly covered photoelectrochromic devices under insolation and in storage. Sol. Energy Mater. Sol. Cells 2018, 182, 281–293. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Novel Photoelectrochemical Cell with Mesoscopic Electrodes Sensitized by Lead-halide Compounds (11). Meet. Abstr. 2008, MA2008-02, 27. [Google Scholar]
- You, J.; Hong, Z.; Yang, Y.M.; Chen, Q.; Cai, M.; Song, T.; Chen, C.; Lu, S.; Liu, Y.; Zhou, H.; et al. Low-Temperature Solution-Processed Perovskite Solar Cells with High E ffi ciency and Flexibility. ACS Nano 2014, 8, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982. [Google Scholar] [CrossRef]
- Park, N.-G.; Grätzel, M.; Miyasaka, T.; Zhu, K.; Emery, K. Towards stable and commercially available perovskite solar cells. Nat. Energy 2016, 1, 16152. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Neutral color semitransparent microstructured perovskite solar cells. ACS Nano 2014, 8, 591–598. [Google Scholar] [CrossRef]
- Hörantner, M.T.; Nayak, P.K.; Mukhopadhyay, S.; Wojciechowski, K.; Beck, C.; McMeekin, D.; Kamino, B.; Eperon, G.E.; Snaith, H.J. Shunt-Blocking Layers for Semitransparent Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Cannavale, A.; Eperon, G.E.; Cossari, P.; Abate, A.; Snaith, H.J.; Gigli, G. Perovskite photovoltachromic cells for building integration. Energy Environ. Sci. 2015, 8, 1578–1584. [Google Scholar] [CrossRef]
- Hočevar, M.; Opara Krašovec, U. A photochromic single glass pane. Sol. Energy Mater. Sol. Cells 2018, 186, 111–114. [Google Scholar] [CrossRef]
- Sarwar, S.; Park, S.; Dao, T.T.; Lee, M.; Ullah, A.; Hong, S.; Han, C.H. Scalable photoelectrochromic glass of high performance powered by ligand attached TiO2 photoactive layer. Sol. Energy Mater. Sol. Cells 2020, 210, 110498. [Google Scholar] [CrossRef]

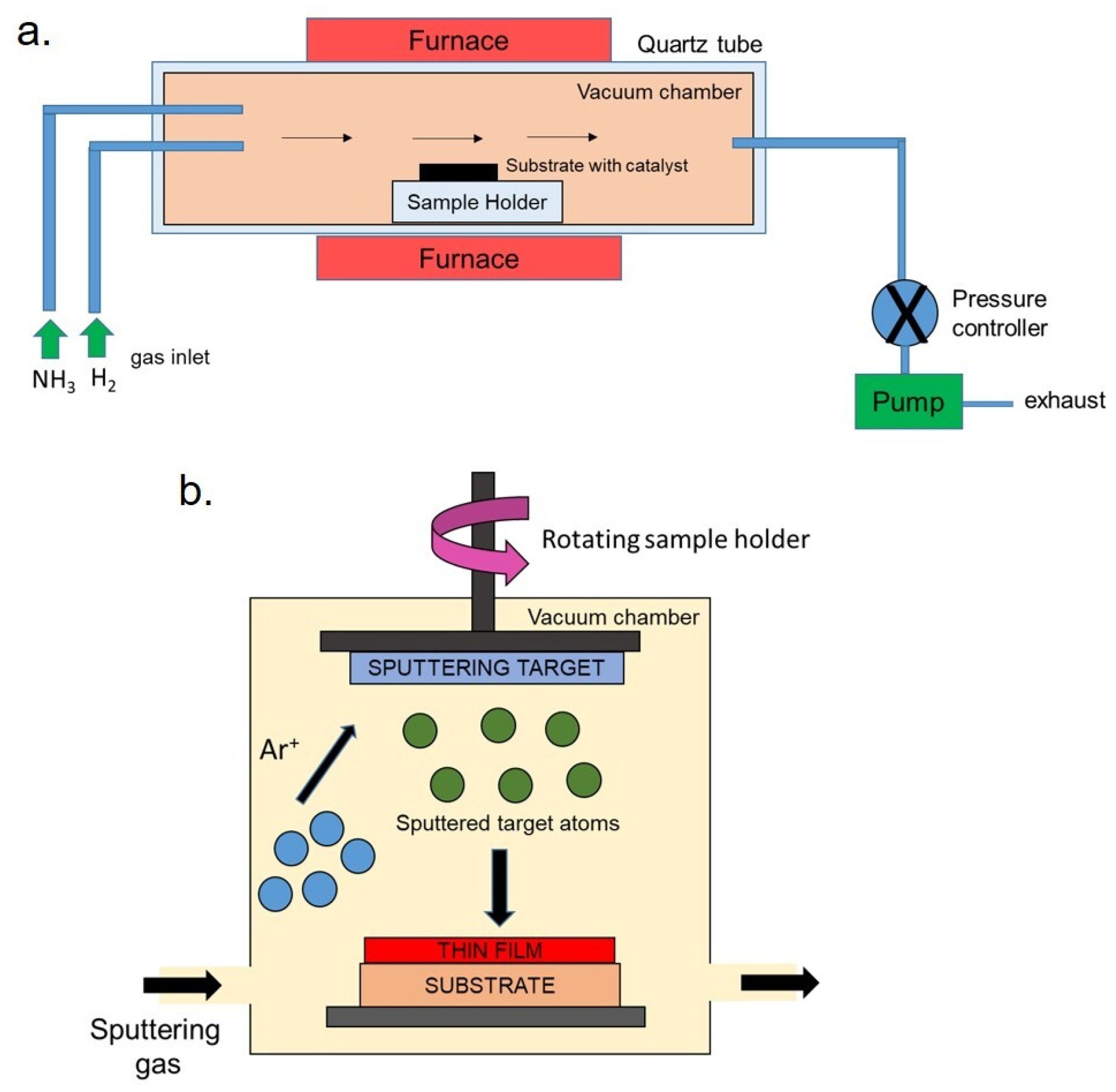
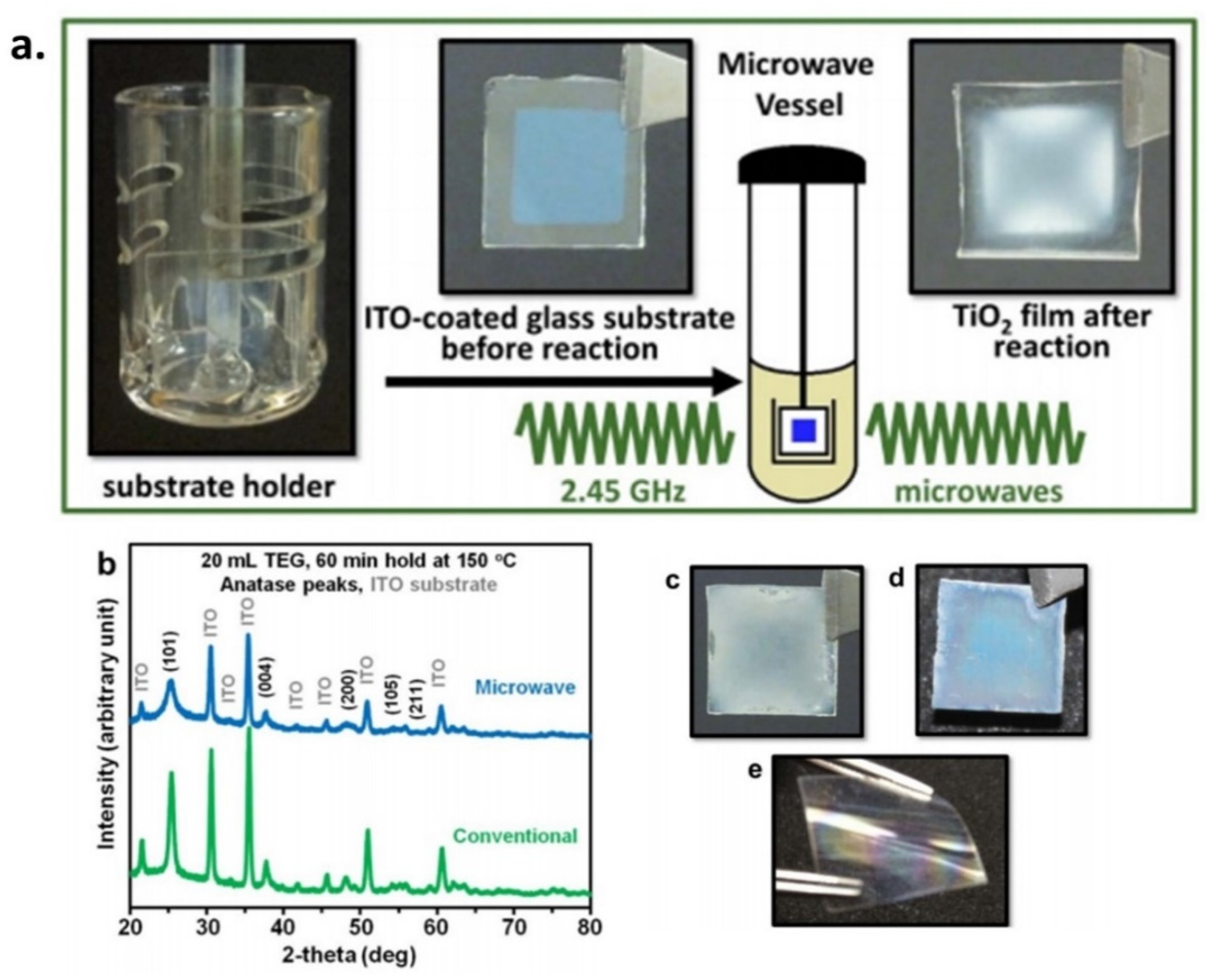
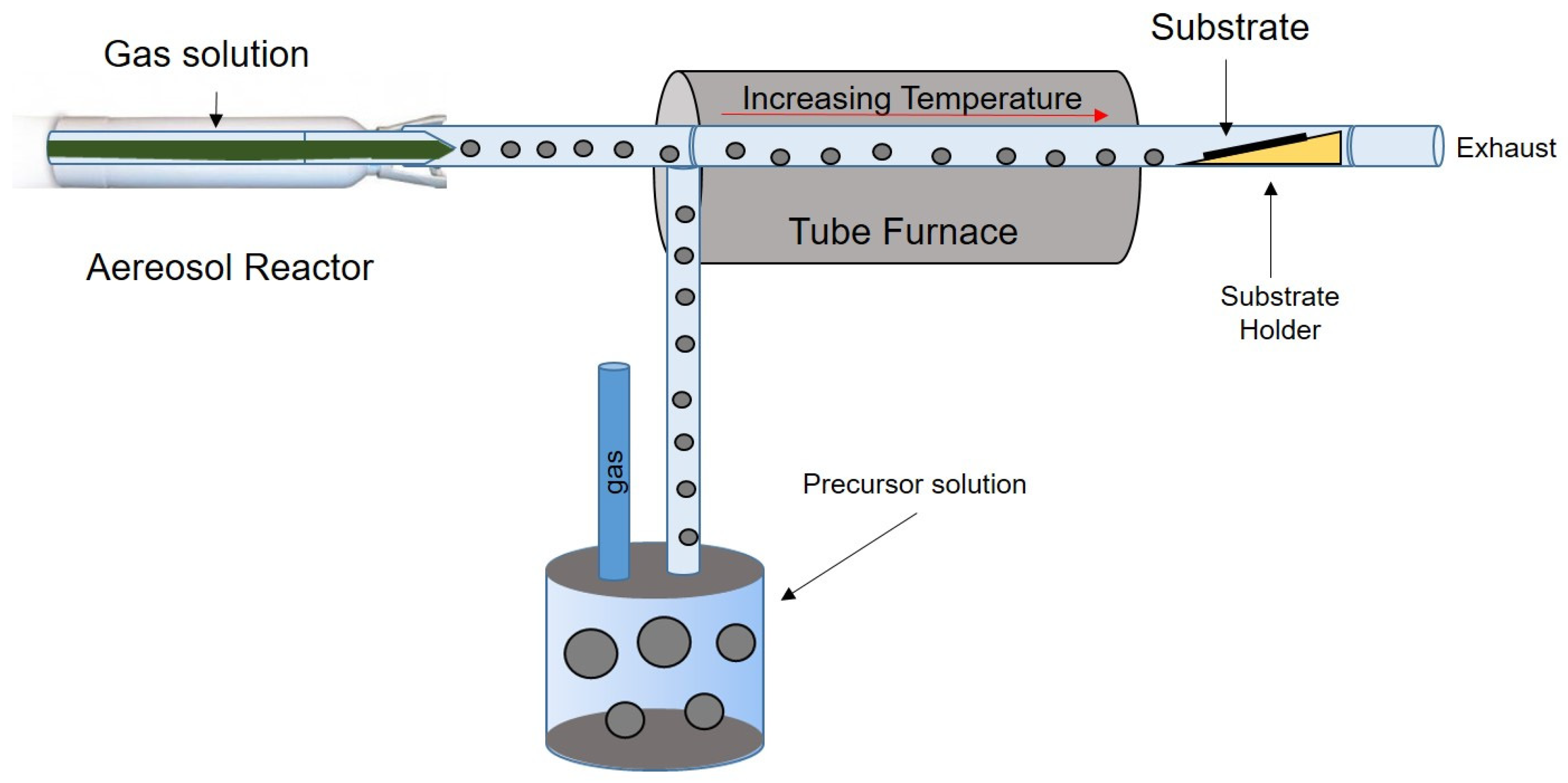
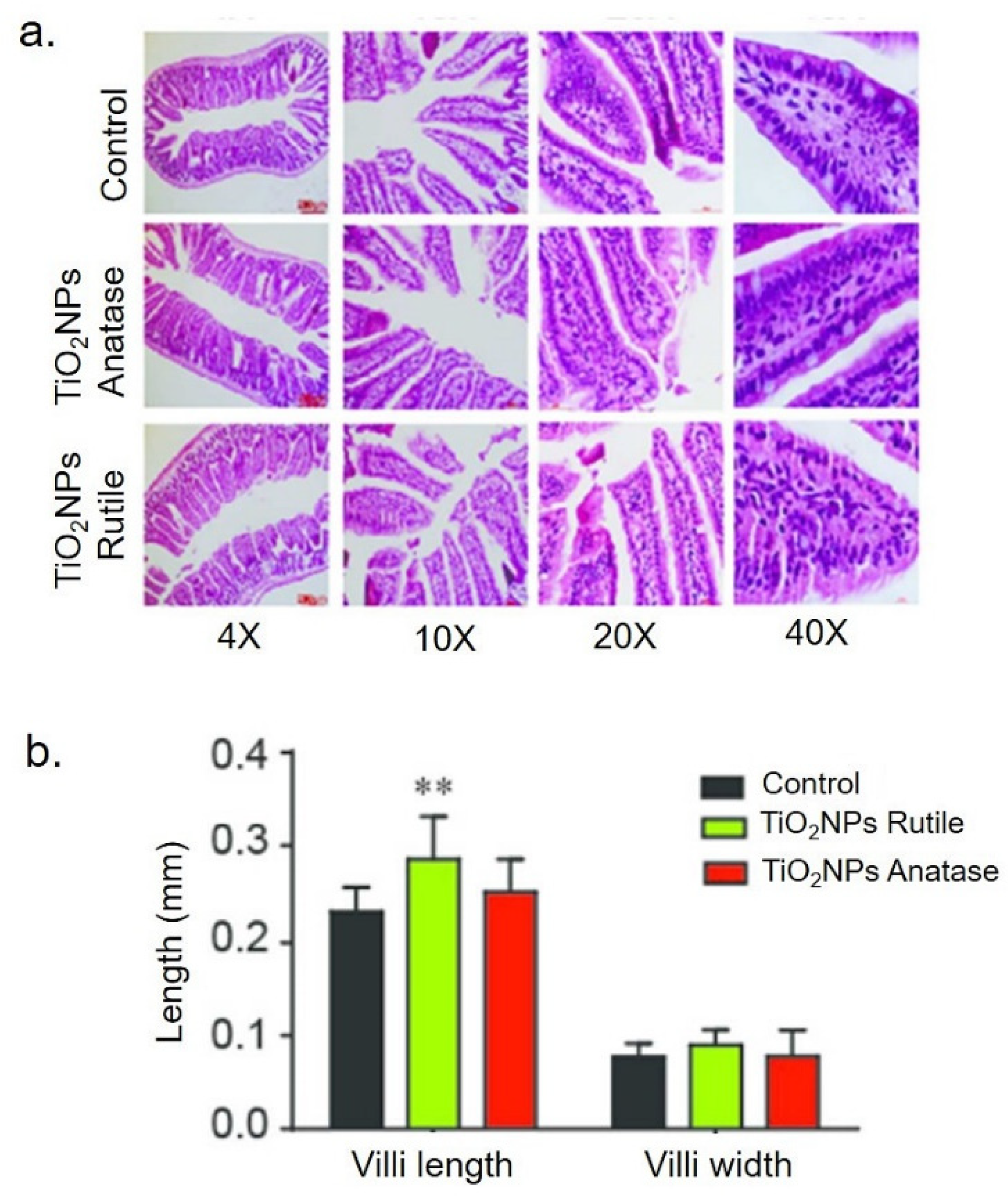



| Synthetic Methods | Advantages | Disadvantages | Morphology and Uses | References |
|---|---|---|---|---|
| Sol-gel |
|
|
| [36,37,38,42,43,45,46] |
| Chemical Vapor Deposition (CVD) |
|
|
| [24,48,54,55,102] |
| Physical Vapor Deposition(PVD) |
|
|
| [56,58,59,61,67,103,104] |
| Sonochemical and microwave-assisted methods |
|
|
| [69,76,78,81,105,106] |
| Hydrothermal method |
|
|
| [76,81,83,85,107] |
| Oxidation method |
|
|
| [87,90,108,109] |
| Spray pyrolysis |
|
|
| [26,93,110,111] |
| Green route |
|
|
| [112,113] |
| Reference | Fabrication Techniques for TiO2 | Role of TiO2 | Main Figures of Merit |
|---|---|---|---|
| Nunes et al. [159] | Hydrothermal synthesis assisted by microwave irradiation. | Electrochromic material | ΔR of 57%, 9% and 22% between colored and bleached states, at 250, 550 and 850 nm |
| Dhandayuthapani et al. [166] | Two step process by combining chemical bath deposition and nebulized spray deposition | Electrochromic material | ΔT of 78%; CE of 128.3 cm2/C |
| Choi et al. [168] | Spin-coated nanocrystalline films modified with the viologen from an aqueous solution | Electrochromic material | The absorbance difference at 608 nm between the bleached and colored state of meso-nc-TiO2 EC cell was about 0.43. |
| Giannuzzi et al. [170] | Surfactant-assisted nonaqueous sol−gel route | Electrochromic material | CE of 130 cm2/C at 800 nm; coloration time of 5 s |
| Patil et al. [171] | One dimensional brookite TiO2 nanoneedles coated on glass substrates | Electrochromic material | ΔT of 67% and ΔOD of 0.85 |
| Han et al. [172] | Dip-coating | Electrochromic material | CE of 68 cm2/C |
| Barawi et al. [173] | Screen-printing of a Nb-TiO2-containing viscous paste | Electrochromic material | ΔTNIR ≈ 53%, ΔTMAX = 67% at 2000 nm at 3 V. |
| Zhang et al. [176] | Modified nonaqueous fluoride-assisted one-pot method | Electrochromic material | ΔT of 95.4% at 633 nm, 98.7% at 800 nm, 90.5% at 1200 nm, and 76.2% at 1600 nm after 2000 cycles |
| Qu et al. [177] | Hydrothermal and layer-by-layer self-assembly combination methods | Electrochromic material and energy storage | CE of 150.34 cm2/C at 600 nm and volumetric capacitance of 172.3 F/cm−3 |
| Bechinger et al. [181] | Dye-sensitized Nanocrystalline TiO2. | Smart coloration according to photogenerated photovoltage | Full modulation in 100 s. Highly reversible after 107 cycles. |
| Hsu et al. [187] | Synthesis of TiO2 nanoparticles as reported for Gratzel cells [182]. | Solar energy conversion and photoelectrochromic behavior | ΔT of 33.7%; switching response of 3 s; JSC of 0.89 mA/cm2 and VOC of 0.57 V |
| Hauch et al. [188] | Doctor-blading technique using a sol–gel TiO2 paste | Smart coloration according to photogenerated photovoltage | ΔT from 64% to 23% in 2 min |
| Liao et al. [191] | Dip- coating of a TiO2-containing aqueous suspension, dried in air | Smart coloration according to photogenerated photovoltage | CE of 280 cm2/C, ΔT of 25% |
| Wu et al. [193] | Spin-coating of TiO2 P25 paste | Solar energy conversion and photoelectrochromic behavior | Bleaching time of 4 s at open circuit. Photovoltaic conversion efficiency (η) of 0.46. |
| Cannavale et al. [194] | Screen-printable paste containing 30 nm sized TiO2 colloids | Solar energy conversion and photoelectrochromic behavior | η of 6.55; ΔOD of 0.83 at 780 nm |
| Leftheriotis et al. [197] | Doctor blade technique | Smart coloration according to photogenerated photovoltage | ΔT of 36%; coloration time of 12 min. |
| Cannavale et al. [208] | Spin-coating of a mildly acidic solution of titanium isopropoxide in ethanol | Solar energy conversion and photoelectrochromic behavior | Average visible transmittance of 26%; η of 5.5% |
| Hočevar et al. [209] | Sol-gel processing | Smart coloration according to photogenerated photovoltage | ΔT of 41% in 15 min |
| Sarwar et al. [210] | Doctor blade technique | Smart coloration according to photogenerated photovoltage | ΔT of 40.8% at 550 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matteis, V.; Cannavale, A.; Ayr, U. Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods. Appl. Sci. 2020, 10, 8896. https://doi.org/10.3390/app10248896
De Matteis V, Cannavale A, Ayr U. Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods. Applied Sciences. 2020; 10(24):8896. https://doi.org/10.3390/app10248896
Chicago/Turabian StyleDe Matteis, Valeria, Alessandro Cannavale, and Ubaldo Ayr. 2020. "Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods" Applied Sciences 10, no. 24: 8896. https://doi.org/10.3390/app10248896






