Carbon-Based Catalysts for Biodiesel Production—A Review
Abstract
:1. Introduction
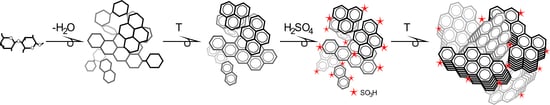
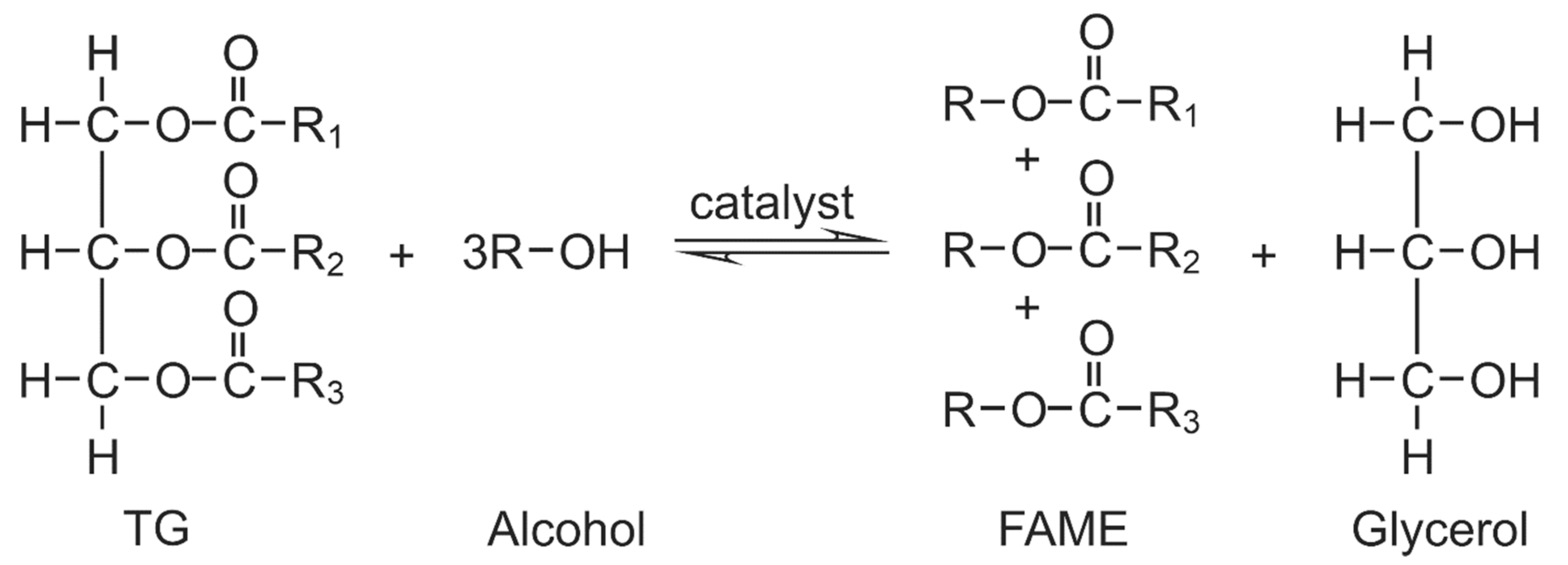
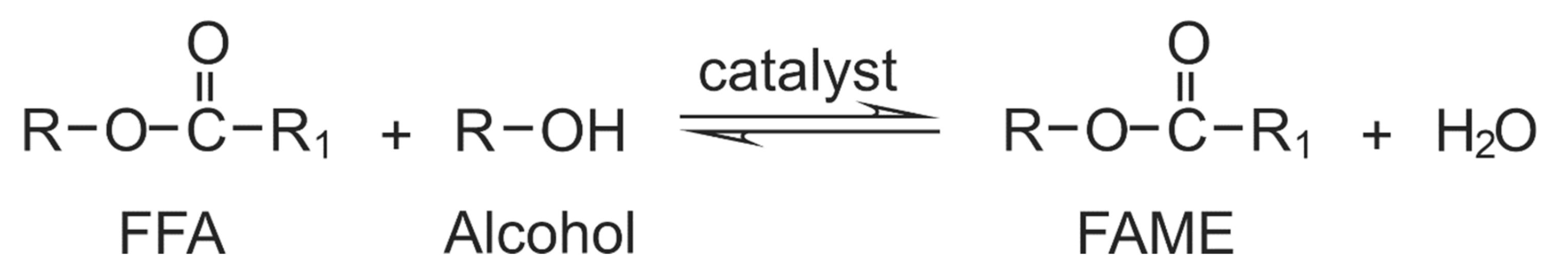
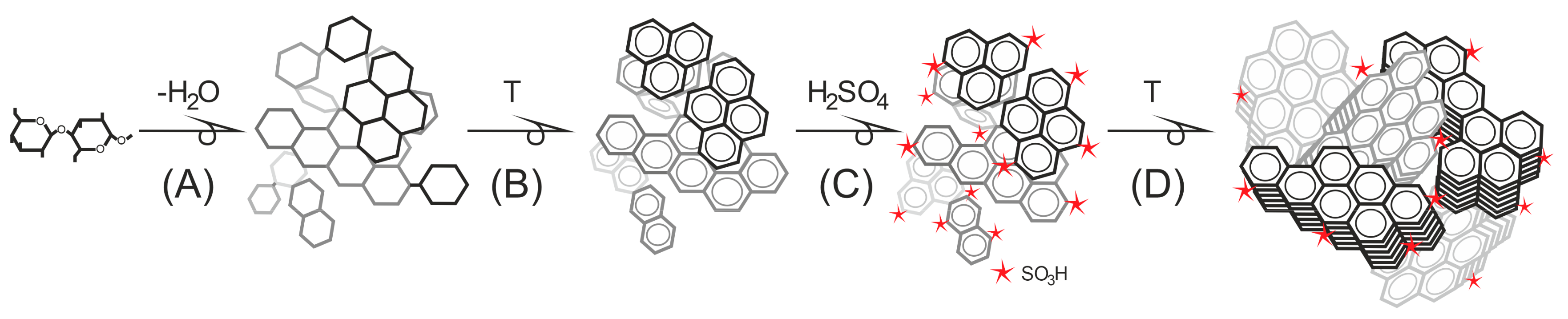
2. Carbohydrate Precursors
3. Biomass Precursors
4. Economic and Operating Condition Assessment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review Journal Series #12109, Agricultural Research Division, Institute of Agriculture and Natural Resources, University of Nebraska–Lincoln. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Borges, M.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Xie, W.; Li, H. Alumina-supported potassium iodide as a heterogeneous catalyst for biodiesel production from soybean oil. J. Mol. Catal. A Chem. 2006, 255, 1–9. [Google Scholar] [CrossRef]
- Sulaiman, S. Overview of Catalysts in Biodiesel Production. J. Eng. Appl. Sci. 2016, 11, 439–448. [Google Scholar]
- Ullah, F.; Dong, L.S.; Bano, A.; Peng, Q.Q.; Huang, J. Current advances in catalysis toward sustainable biodiesel production. J. Energy Inst. 2016, 89, 282–292. [Google Scholar] [CrossRef]
- Renewable Energy Directive. Available online: https://ec.europa.eu/energy/en/topics/renewable-energy/renewable-energy-directive/overview#content-heading-0 (accessed on 5 December 2019).
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Lou, W.-Y.; Zong, M.-H.; Duan, Z.-Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Munack, A. Books: Biodiesel—A comprehensive handbook. Martin Mittelbach, Claudia Remschmidt (Ed.). Biotechnol. J. 2006, 1, 102. [Google Scholar] [CrossRef]
- Melero, J.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, S.; Gupta, M.N. Biodiesel Preparation by Lipase-Catalyzed Transesterification of Jatropha Oil. Energy Fuels 2004, 18, 154–159. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, C.A.C.; Ukogu, K.; Ibemesi, J.A. Catalysis in Biodiesel Production by Transesterification Processes—An Insight. J. Chem. 2010, 7, 1120–1132. [Google Scholar] [CrossRef]
- Thangaraj, B.; Ramachandran, K.; Raj, S. Homogeneous Catalytic Transesterification of Renewable Azadirachta indica (Neem) Oil and Its Derivatives to Biodiesel Fuel via Acid/Alkaline Esterification Processes. Int. J. Renew. Energy Biofuels 2014, 2014, 11. [Google Scholar] [CrossRef]
- Ghadge, S.; Raheman, H. Biodiesel Production from Mahua (Madhuca indica) Oil Having High Free Fatty Acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.Y.; Periasamy, L.A.P.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E.C. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Mclaughlin, D.W. Land, Food, and Biodiversity. Conserv. Biol. 2011, 25, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, F.; Beukema, H.; Burgess, N.D.; Parish, F.; Bruhl, C.A.; Donald, P.F.; Murdiyarso, D.; Phalan, B.; Reijnders, L.; Struebig, M.; et al. Biofuel plantations on forested lands: Double jeopardy for biodiversity and climate. Conserv. Biol. 2009, 23, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Arzamendi, G.; Campo, I.; Arguiñarena, E.; Sánchez, M.; Montes, M.; Gandía, L.M. Synthesis of biodiesel with heterogeneous NaOH/alumina catalysts: Comparison with homogeneous NaOH. Chem. Eng. J. 2007, 134, 123–130. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Vitiello, R.; Li, C.Z.; Russo, V.; Tesser, R.; Turco, R.; Di Serio, M. Catalysis for esterification reactions: A key step in the biodiesel production from waste oils. Rend. Lincei Sci. Fis. Nat. 2017, 28, 117–123. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601–607. [Google Scholar] [CrossRef]
- Venkat Reddy, C.R.; Oshel, R.; Verkade, J.G. Room-Temperature Conversion of Soybean Oil and Poultry Fat to Biodiesel Catalyzed by Nanocrystalline Calcium Oxides. Energy Fuels 2006, 20, 1310–1314. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 9. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Casale, L.; D’Angelo, A.; Trifuoggi, M.; Santacesaria, E. Heterogeneous Catalysis in Biodiesel Production: The Influence of Leaching. Top. Catal. 2010, 53, 811–819. [Google Scholar] [CrossRef]
- D’Cruz, A.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K. Synthesis of Biodiesel from Canola Oil Using Heterogeneous Base Catalyst. J. Am. Oil Chem. Soc. 2007, 84, 937–943. [Google Scholar] [CrossRef]
- Descorme, C.; Gallezot, P.; Geantet, C.; George, C. Heterogeneous Catalysis: A Key Tool toward Sustainability. ChemCatChem 2012, 4, 1897–1906. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic applications in the production of biodiesel from vegetable oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Nata, I.F.; Putra, M.D.; Irawan, C.; Lee, C.-K. Catalytic performance of sulfonated carbon-based solid acid catalyst on esterification of waste cooking oil for biodiesel production. J. Environ. Chem. Eng. 2017, 5, 2171–2175. [Google Scholar] [CrossRef]
- Okamura, M.; Takagaki, A.; Toda, M.; Kondo, J.N.; Domen, K.; Tatsumi, T.; Hara, M.; Hayashi, S. Acid-Catalyzed Reactions on Flexible Polycyclic Aromatic Carbon in Amorphous Carbon. Chem. Mater. 2006, 18, 3039–3045. [Google Scholar] [CrossRef]
- Hara, M. Biodiesel Production by Amorphous Carbon Bearing SO3H, COOH and Phenolic OH Groups, a Solid Bronsted Acid Catalyst. Top. Catal. 2010, 53, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Lotero, E.; Changqing, L.; Liu, Y.; Goodwin, J. A Novel Sulfonated Carbon Composite Solid Acid Catalyst for Biodiesel Synthesis. Catal. Lett. 2008, 123, 1–6. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Zhao, X.; Feng, P. Sulfonated ordered mesoporous carbon for catalytic preparation of biodiesel. Carbon 2008, 46, 1664–1669. [Google Scholar] [CrossRef]
- Zong, M.-H.; Duan, Z.-Q.; Lou, W.-Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Novotny, E.H.; Hayes, M.H.B. Biochar from Biomass and Waste. Waste Biomass Valoriz. 2010, 1, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Arumugamurthy, S.S.; Sivanandi, P.; Pandian, S.; Choksi, H.; Subramanian, D. Conversion of a low value industrial waste into biodiesel using a catalyst derived from brewery waste: An activation and deactivation kinetic study. Waste Manag. 2019, 100, 318–326. [Google Scholar] [CrossRef]
- Shu, Q.; Nawaz, Z.; Gao, J.; Liao, Y.; Zhang, Q.; Wang, D.; Wang, J. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: Reaction and separation. Bioresour. Technol. 2010, 101, 5374–5384. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Severini, F.; Leahy, J.J.; Kwapinski, W. Heterogeneous Char Based Solid Acid Catalysts from Brown Bin Waste to Create a Green Process for the Production of Butyl Butyrate. Waste Biomass Valoriz. 2017, 8, 2431–2441. [Google Scholar] [CrossRef]
- Severini, F.; Flannelly, T.; O’Nolan, D.; Leahy, J.J.; Kwapinski, W. Development of heterogeneous acid catalysts produced from the carbonization of Miscanthus x giganteus for the esterification of butyric acid to butyl butyrate with n-butanol. J. Chem. Technol. Biotechnol. 2016, 91, 2076–2084. [Google Scholar] [CrossRef]
- Wang, Y.T.; Delbecq, F.; Kwapinski, W.; Len, C. Application of sulfonated carbon-based catalyst for the furfural production from D-xylose and xylan in a microwave-assisted biphasic reaction. Mol. Catal. 2017, 438, 167–172. [Google Scholar] [CrossRef]
- Cheng, F. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of Biochar-based Catalyst for Transesterification of Canola Oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A Gen. 2014, 469, 284–289. [Google Scholar] [CrossRef]
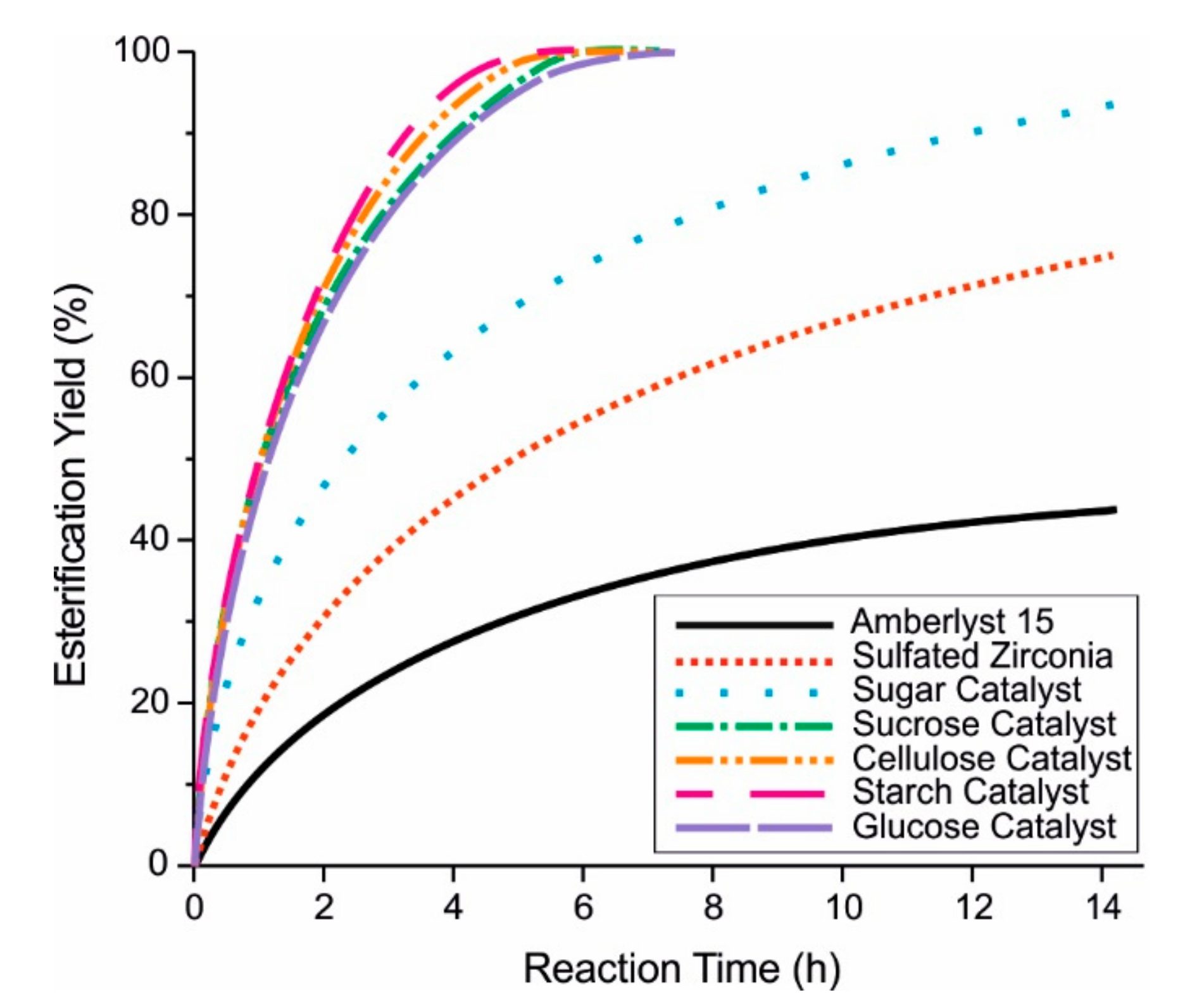

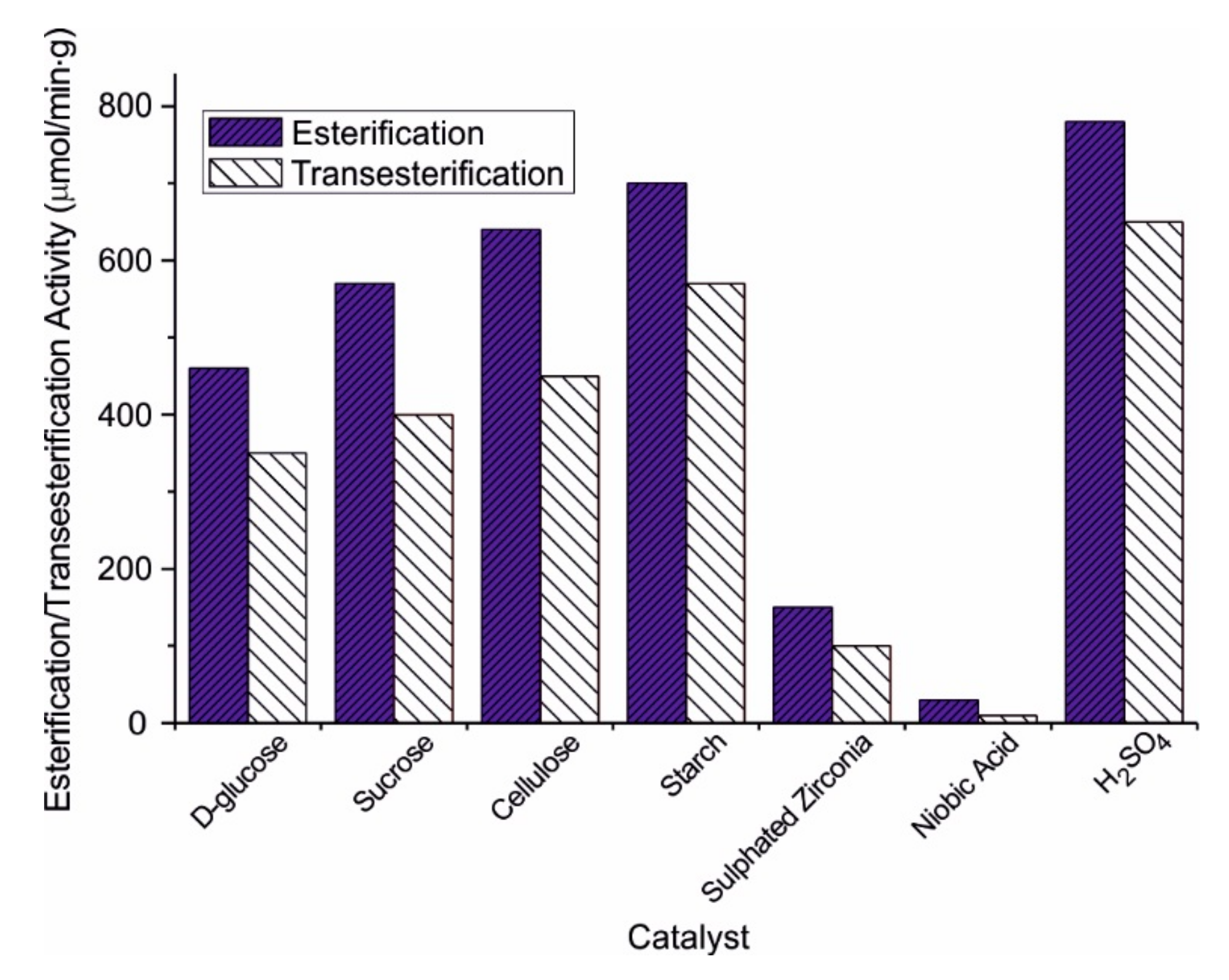

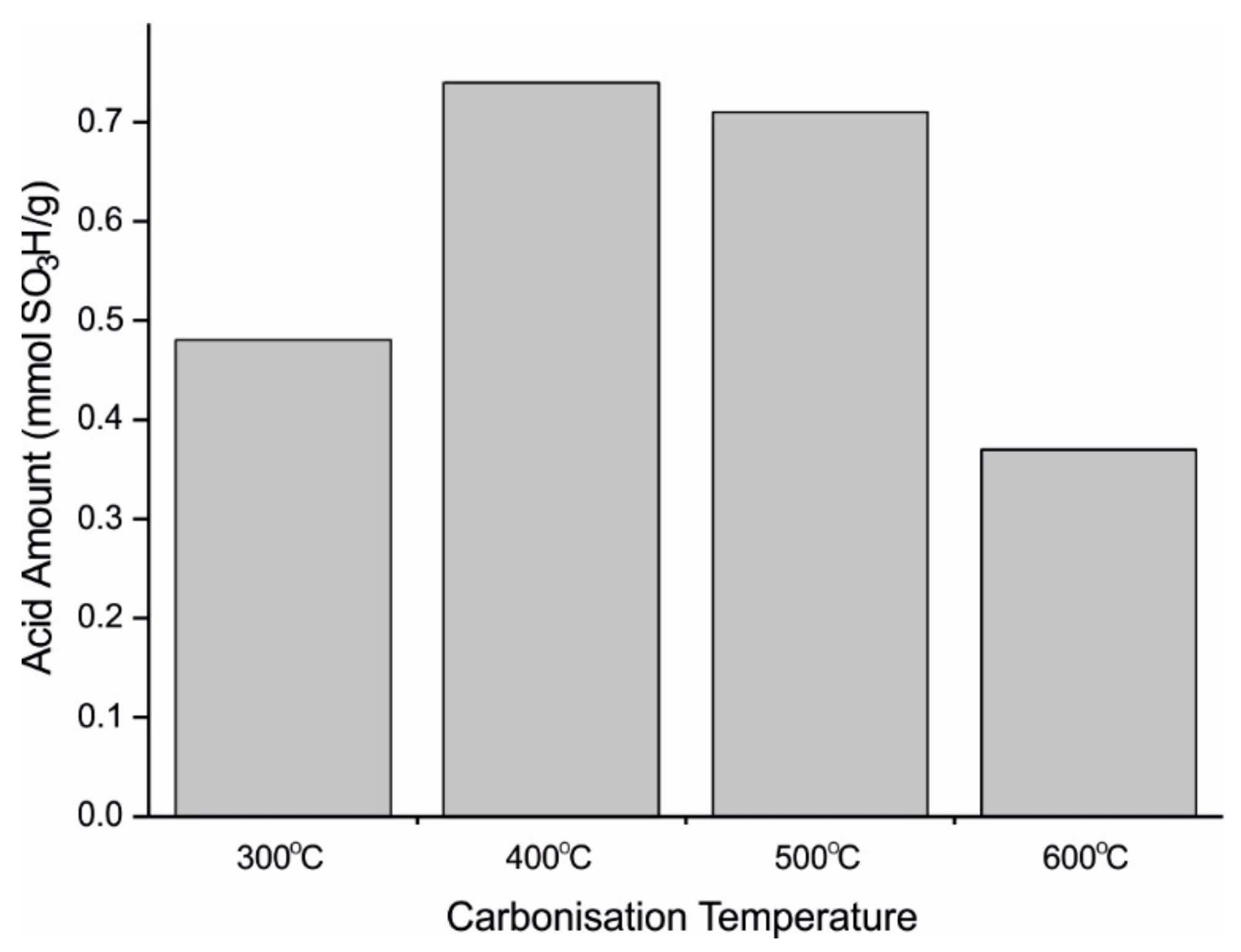
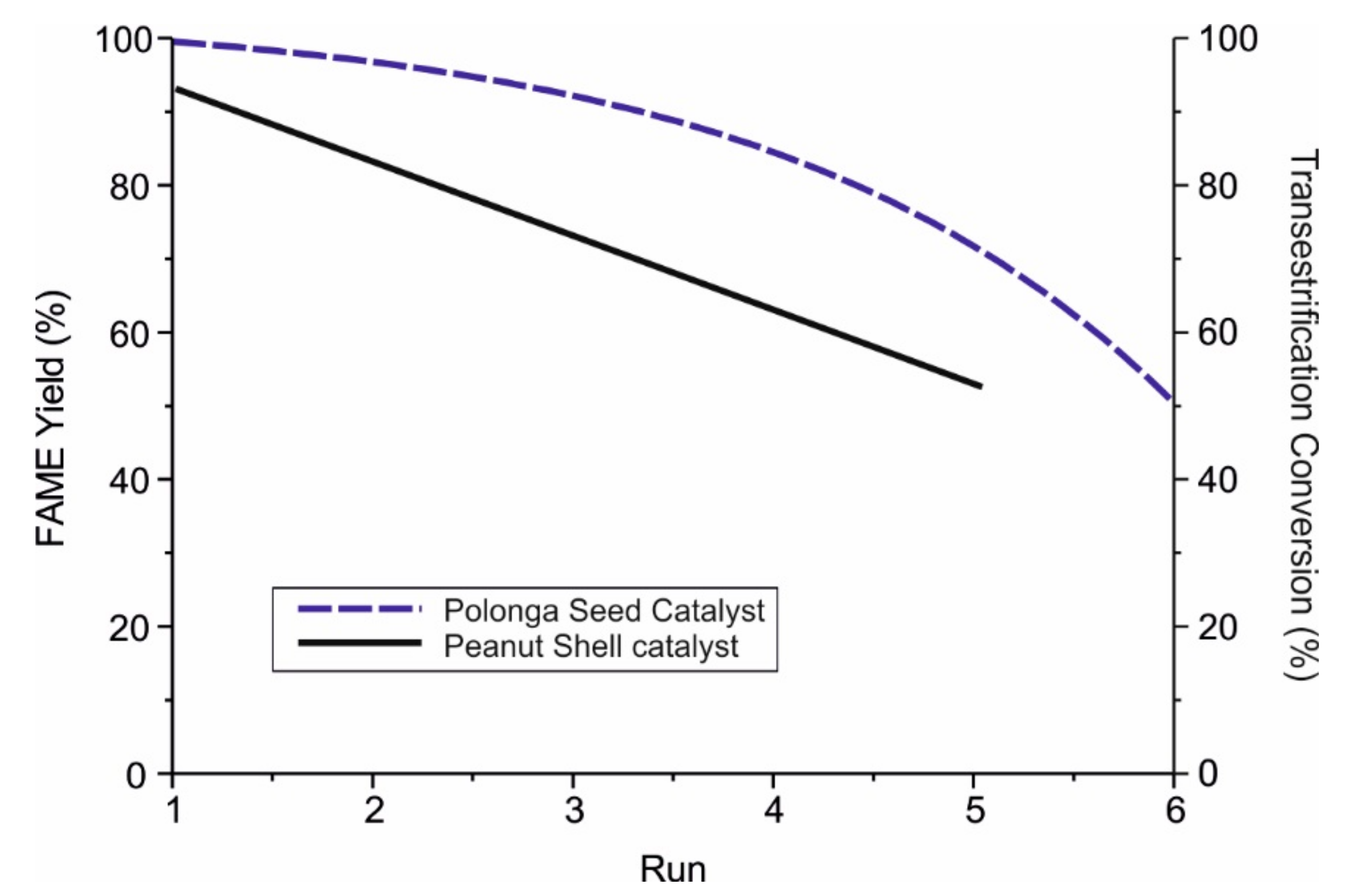

| Catalyst | Catalytic Activity µmol min−1 | Yield of Methyl Oleate wt.% |
|---|---|---|
| Sugar Catalyst | 67 | 95 |
| Sulphated Zirconia | 21 | 85 |
| Amberlyst 15 | 7 | 15 |
| Niobic Acid | 1.5 | 6 |
| Catalyst Derived From | S Content wt.% | Total Acid Site Density mmol/G | SO3H Acid Site Density mmol/G | Surface Area m2/g | Average Pore Volume cm3/g | Average Pore Size nm |
|---|---|---|---|---|---|---|
| D-Glucose | 4.7 | 1.6 | 1.47 | 4.1 | 0.44 | 4.0 |
| Sucrose | 5.1 | 1.71 | 1.59 | 5.0 | 0.52 | 5.1 |
| Cellulose | 5.4 | 1.82 | 1.68 | 5.7 | 0.65 | 6.4 |
| Starch | 5.9 | 1.97 | 1.83 | 7.2 | 0.81 | 8.2 |
| Catalyst | Sulphur wt.% | Average Pore Size nm |
|---|---|---|
| Vegetable oil asphalt | 7.1 | 43.9 |
| Petroleum oil asphalt | 3.6 | 2.2 |
| Catalyst | Surface Area m2/g | Sulphonic Group Density mmol/g | Total Acidity µmol/g |
|---|---|---|---|
| Method A | 14.4 | 0.59 | 83 |
| Method B | 2.6 | 1.04 | 3200 |
| Catalyst | Carbonisation Temperature °C | HCl Treatment | Surface Area (SA) before Sulphonation m2/g | SA after Sulphonation m2/g | Decrease in SA after Sulphonation % | Pore Size before Sulphonation nm | Pore Size after Sulphonation nm | Total Pore Volume after Sulphonation cm3/g | Total Acid Density mmol/g |
|---|---|---|---|---|---|---|---|---|---|
| Biochar [57] | 450 | No | 204 | 1.9 | 99 | 2.6 | - | 0.01 | 2.6 |
| Biochar [57] | 675 | No | 668 | 640 | 4 | 2.2 | 2.2 | 0.35 | 1.2 |
| Biochar [57] | 875 | No | 1469 | 1411 | 4 | 2.2 | 2.2 | 0.71 | 0.5 |
| Biochar [58] | 675 | Yes | 990 | 949 | 4 | 3.2 | 3.2 | 0.85 | 2.0 |
| Peanut shell [59] | 450 | No | - | 12 | - | - | 39.3 | - | 6.9 |
| Catalyst | Cost Per kg ($) (Taken from Sigma Aldrich) | Carbonisation: Temperature Time | Sulphonation Conditions | Reaction Conditions | Yield: Esterification Transesterification | Reusability |
|---|---|---|---|---|---|---|
| D-glucose [46] | 39.2 | 400 °C 15 h | Conc. H2SO4 150 °C 15 h 10 mL/g | Methanol:WCO weight ratio 1:1 10 wt.% catalyst loading 80 °C | Esterification = 90% | Retains 93% of activity after 50 successive runs |
| D-glucose [39] | 39.2 | 180 °C 4 h | One step hydrothermal carbonisation and sulphonation | Methanol:WCO molar ratio 20:1 10 wt.% catalyst loading 80 °C, 3 h | Esterification = 95% | 5 repeated uses 7% increase in FFAs |
| Cellulose [41] | 196.0 | 450 °C 5 h | Fuming sulphuric acid 180 °C 10 h | Esterification:oleic acid 17 wt% catalyst 95 °C, 4 h Transesterification: Triolein 130 °C, 700 kPa, 5 h | Esterification = 99.9% Transesterification = 98.1% | Esterification: No reduction in activity after 10 uses Transesterification: No reduction in activity after four uses |
| D-glucose [10] | 39.2 | 400 °C 15 h | Conc. H2SO4 150 °C 15 h | WCO (27.8% FFA) Methanol:WCO molar ratio 20:1 10 wt% catalyst loading 8 h, 80 °C | Combined Transesterification and Esterification 70% | Not investigated |
| Sucrose [10] | 93.4 | Combined Transesterification and Esterification = 72% | Not investigated | |||
| Cellulose [10] | 196.0 | Combined Transesterification and Esterification = 78% | Not investigated | |||
| Starch [10] | 31.9 | Combined Transesterification and Esterification = 92% | Retained 93% of catalytic ability after 50 uses | |||
| Sulphonated seed cake [3] | By product of oil extraction process. Low-cost inedible oil | 400 °C 10 h | Conc. H2SO4 150 °C 10 h | Methanol:seed oil weight ratio 1:1 0.38 g catalyst (7.5 wt%) 180 °C, 5 h | Combined Transesterification and Esterification = 99% | Begins to deteriorate after three successive uses |
| Petroleum oil asphalt [49] | By-product of petroleum industry | 500–700 °C 4–6 h | Conc. H2SO4 210 °C 10 h 10 mL/g | Cottonseed oil:oleic acid molar ratio 1:1 Methanol:oil molar ratio 20:9 0.3 wt.% catalyst loading 140 °C Esterification 220 °C Transesterification 5 h | Esterification = 54% Transesterification = 87% | Not investigated |
| Vegetable oil asphalt [49] | Low cost can be produced from waste oils and inedible oils | Esterification = 83% Transesterification = 89% | Not investigated | |||
| Biochar A [21] | Waste wood | 475 °C Unknown time | Conc. H2SO4 150 °C 24 h 10 mL/g | Esterification: Waste vegetable oil 28:1 Methanol:oil molar ratio 15:1 5 wt% catalyst 60 °C | Esterification = 89% | Not investigated |
| Biochar B [21] | 475 °C Unknown time | Fuming sulphuric acid 150 °C 5 & 15 h | Transesterification Methanol:oil molar ratio 15:1 5 wt% catalyst loading 24 h, 65 °C | Transesterification = 10% | Not investigated | |
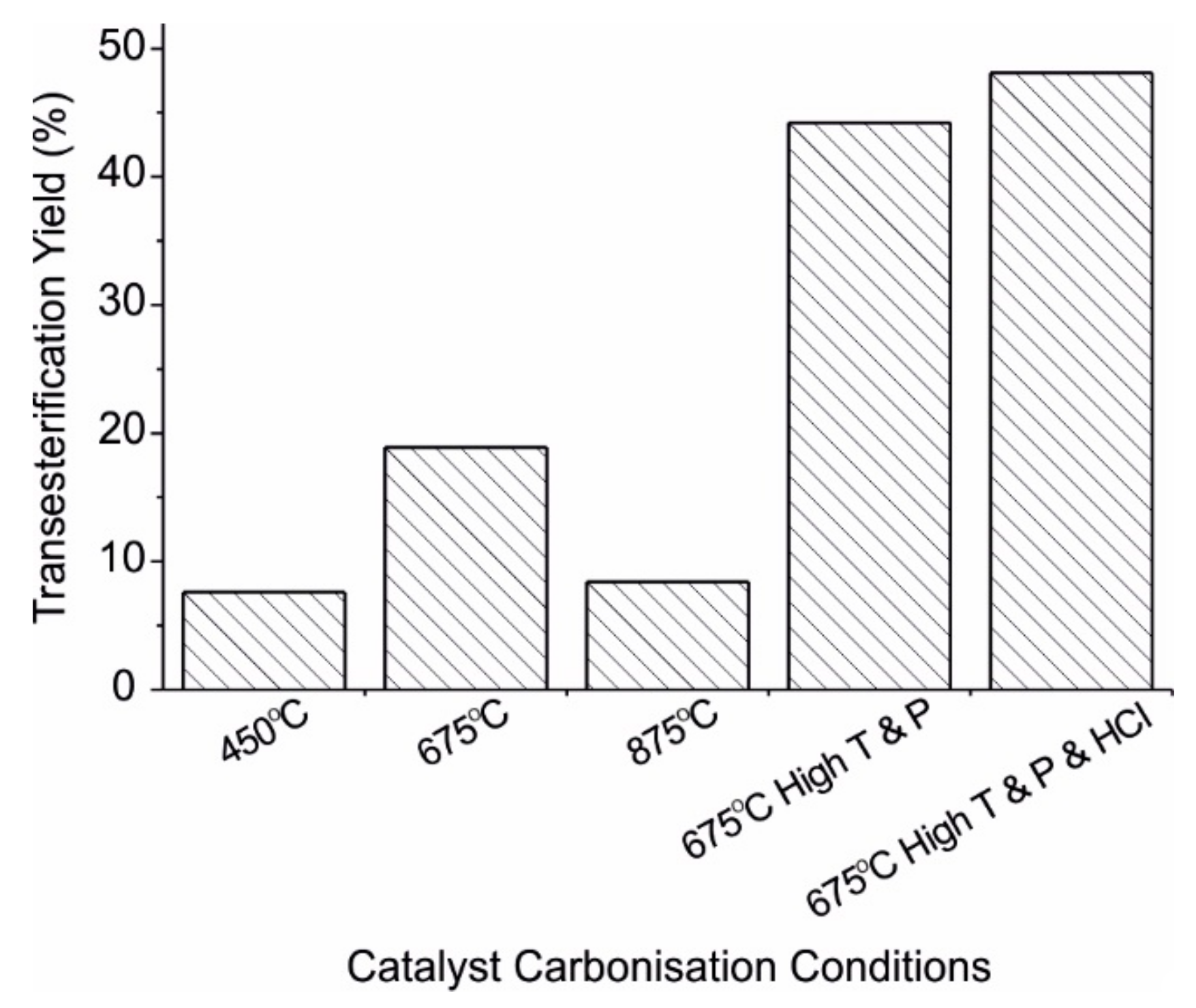
| Biochar [57] | 450 °C 675 °C 875 °C 4.5–7 h | Fuming sulphuric acid 150 °C 15 h 16.5 mL/g | Methanol:canola oil molar ratio 15:1 5 wt% catalyst loading 150 °C, 3 h, 1.52 MPa | Combined Transesterification and Esterification = 44.2 | Loses almost all catalytic ability after one use | |
| Biochar [21] | Waste product | 675 °C Unknown time | Fuming sulphuric acid 150 °C 15 h 16.5 mL/g | Methanol:oil (70% canola 30% oleic acid) molar ratio 15:1 5wt% catalyst loading 150 °C, 1.52 MPa, 3 h | Combined Transesterification and Esterification = 48.1 | Reaction yield decreases by 8% upon reuse of catalyst |
| Peanut shell biochar [22] | Waste product from peanut industry | 450 °C 15 h | Conc. H2SO4 200 °C 10 h 100 mL/g | Refined cottonseed oil Methanol:oil molar ratio 9:1 2 wt% catalyst loading 85 °C, 2 h | Combined Transesterification and Esterification = 90.2% | Retains 50% of catalytic activity after 5 runs. Easily regenerated with 1 M H2SO4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clohessy, J.; Kwapinski, W. Carbon-Based Catalysts for Biodiesel Production—A Review. Appl. Sci. 2020, 10, 918. https://doi.org/10.3390/app10030918
Clohessy J, Kwapinski W. Carbon-Based Catalysts for Biodiesel Production—A Review. Applied Sciences. 2020; 10(3):918. https://doi.org/10.3390/app10030918
Chicago/Turabian StyleClohessy, Jack, and Witold Kwapinski. 2020. "Carbon-Based Catalysts for Biodiesel Production—A Review" Applied Sciences 10, no. 3: 918. https://doi.org/10.3390/app10030918