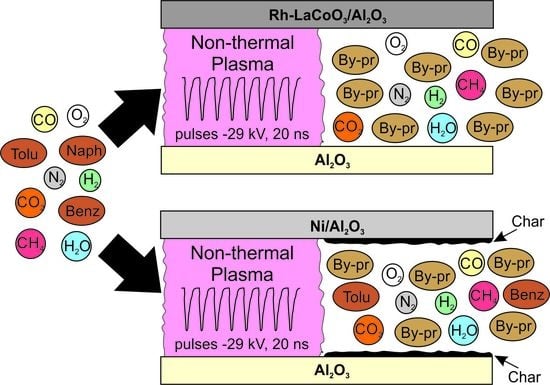
Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge
Abstract
:Featured Application
Abstract
1. Introduction
- Number of tar components—in this work 3 tar representatives were used together while in most cases only 1 or 2 are used [11,15,16,22,23,24,25,26,27,28,29,30,31,32,33,34]. Excluding a few works performed on real biomass producer gas [9,14,21] only Kong et al. [17], Eliott et al. [10], Jamroz et al. [12] and Yu et al. [35] used at least 3 tar components, but in nitrogen, argon and oxygen as a plasma-forming gas;
- DBD plasma with a catalyst—this has been studied by many researchers [18] and is called a one-stage configuration since the catalytic material is in contact with the plasma. The advantage of this configuration is the use of radicals, which are formed in the plasma and which have a very short lifetime;
- Nanosecond high-voltage pulses—investigations have shown that shortening high-voltage pulse leads to increasing the formation of highly energetic electrons, ions, radicals and exited molecules at the same input power [36]. Such short pulses were used for tar removal only by researchers from Eindhoven University of Technology in pulsed corona discharge reactors [4,5,6,7].
2. Materials and Methods
2.1. Non-Thermal Plasma Reactor and Processed Gas
2.2. Catalyst Preparation
2.3. Diagnostics of Products
3. Results and Discussion
3.1. Nanosecond Pulsed Corona Discharge Characteristics
3.2. Tar Removal
3.3. Gaseous Compounds
3.4. Analysis of Liquid and Solid By-products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valderrama Rios, M.L.; González, A.M.; Lora, E.E.S.; Almazán del Olmo, O.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Pemen, A.J.M.; Nair, S.A.; Yan, K.; Van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Pulsed Corona Discharges for Tar Removal from Biomass Derived Fuel Gas. Plasmas Polym. 2003, 8, 209–224. [Google Scholar] [CrossRef]
- Nair, S.A.; Pemen, A.J.M.; Yan, K.; Van Gompel, F.M.; Van Leuken, H.E.M.; Van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Tar removal from biomass-derived fuel gas by pulsed corona discharges. Fuel Process. Technol. 2003, 84, 161–173. [Google Scholar] [CrossRef]
- Nair, S.A.; Yan, K.; Pemen, A.J.M.; Winands, G.J.J.; van Gompel, F.M.; van Leuken, H.E.M.; van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. A high-temperature pulsed corona plasma system for fuel gas cleaning. J. Electrostat. 2004, 61, 117–127. [Google Scholar] [CrossRef]
- Nair, S.A.; Yan, K.; Pemen, A.J.M.; Van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Tar Removal from Biomass-Derived Fuel Gas by Pulsed Corona Discharges. A Chemical Kinetic Study. Ind. Eng. Chem. Res. 2004, 43, 1649–1658. [Google Scholar] [CrossRef]
- Fourcault, A.; Marias, F.; Michon, U. Modelling of thermal removal of tars in a high temperature stage fed by a plasma torch. Biomass Bioenergy 2010, 34, 1363–1374. [Google Scholar] [CrossRef]
- Marias, F.; Demarthon, R.; Bloas, A.; Robert-Arnouil, J.P. Modeling of tar thermal cracking in a plasma reactor. Fuel Process. Technol. 2016, 149, 139–152. [Google Scholar] [CrossRef]
- Eliott, R.M.; Nogueira, M.F.M.; Silva Sobrinho, A.S.; Couto, B.A.P.; MacIel, H.S.; Lacava, P.T. Tar reforming under a microwave plasma torch. Energy Fuels 2013, 27, 1174–1181. [Google Scholar] [CrossRef]
- Wnukowski, M. Decomposition of Tars in Microwave Plasma–Preliminary. J. Ecol. Eng. 2014, 15, 23–28. [Google Scholar]
- Jamróz, P.; Kordylewski, W.; Wnukowski, M. Microwave plasma application in decomposition and steam reforming of model tar compounds. Fuel Process. Technol. 2018, 169, 1–14. [Google Scholar] [CrossRef]
- Wnukowski, M.; Jamróz, P. Microwave plasma treatment of simulated biomass syngas: Interactions between the permanent syngas compounds and their influence on the model tar compound conversion. Fuel Process. Technol. 2018, 173, 229–242. [Google Scholar] [CrossRef]
- Wnukowski, M.; Kordylewski, W.; Łuszkiewicz, D.; Leśniewicz, A.; Ociepa, M.; Michalski, J. Sewage Sludge-Derived Producer Gas Valorization with the Use of Atmospheric Microwave Plasma. Waste Biomass Valorization 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, H.S.; Pilatau, A.; Nozhenko, O.S.; Da Silva Sobrinho, A.S.; Petraconi Filho, G. Microwave Air Plasma Applied to Naphthalene Thermal Conversion. Energy Fuels 2016, 30, 1510–1516. [Google Scholar] [CrossRef]
- Nunnally, T.; Tsangaris, A.; Rabinovich, A.; Nirenberg, G.; Chernets, I.; Fridman, A. Gliding arc plasma oxidative steam reforming of a simulated syngas containing naphthalene and toluene. Int. J. Hydrog. Energy 2014, 39, 11976–11989. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, H.; Li, X.; Xu, R.; Mubeen, I.; Li, L.; Yan, J. Destruction of toluene, naphthalene and phenanthrene as model tar compounds in a modified rotating gliding arc discharge reactor. Catalysts 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, Z.; Das, S.; Kawi, S. Reforming of tar from biomass gasification in a hybrid catalysis-plasma system: A review. Appl. Catal. B Environ. 2019, 250, 250–272. [Google Scholar] [CrossRef]
- Saleem, F.; Harris, J.; Zhang, K.; Harvey, A. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification-A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jahanmiri, A.; Mohamadzadeh Shirazi, M.; Hooshmand, N.; Taghvaei, H. Combination of non-thermal plasma and heterogeneous catalysis for methane and hexadecane co-cracking: Effect of voltage and catalyst configuration. Chem. Eng. J. 2013, 219, 245–253. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Reforming of tars and organic sulphur compounds in a plasma-assisted process for waste gasification. Fuel Process. Technol. 2015, 137, 259–268. [Google Scholar] [CrossRef]
- Chun, Y.N.; Kim, S.C.; Yoshikawa, K. Decomposition of Benzene as a Surrogate Tar in a Gliding Arc Plasma. Environ. Sci. Technol. Technol. 2013, 32, 837–845. [Google Scholar] [CrossRef]
- Liu, S.; Mei, D.; Wang, L.; Tu, X. Steam reforming of toluene as biomass tar model compound in a gliding arc discharge reactor. Chem. Eng. J. 2017, 307, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yakushiji, D.; Kanazawa, S.; Ohkubo, T.; Nomoto, Y. Decomposition of toluene by streamer corona discharge with catalyst. J. Electrostat. 2002, 55, 311–319. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Tu, X.; Wang, Y.; Lu, S.; Yan, J. Decomposition of naphthalene by dc gliding arc gas discharge. J. Phys. Chem. A 2010, 114, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Zhang, H.; Wu, A.; Yan, J.; Ni, M.; Zhang, H.; Buekens, A. Destruction of toluene by rotating gliding arc discharge. Fuel 2016, 176, 78–85. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, F.; Li, X.; Xu, R.; Li, L.; Yan, J.; Tu, X. Steam reforming of toluene and naphthalene as tar surrogate in a gliding arc discharge reactor. J. Hazard. Mater. 2019, 369, 244–253. [Google Scholar] [CrossRef]
- Cimerman, R.; Račková, D.; Hensel, K. Tars removal by non-thermal plasma and plasma catalysis. J. Phys. D Appl. Phys. 2018, 51, 274003. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Lu, N.; Li, J.; Wu, Y. Degradation of benzene by using a silent-packed bed hybrid discharge plasma reactor. Plasma Sci. Technol. 2012, 14, 140–146. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Song, J.; Ahmad, S.; Yang, X.; Sun, Y. Plasma-assisted catalytic reforming of toluene to hydrogen rich syngas. Catal. Sci. Technol. 2017, 7, 4216–4231. [Google Scholar] [CrossRef]
- Liu, S.Y.; Mei, D.H.; Nahil, M.A.; Gadkari, S.; Gu, S.; Williams, P.T.; Tu, X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts. Fuel Process. Technol. 2017, 166, 269–275. [Google Scholar] [CrossRef]
- Liu, S.; Mei, D.; Wang, Y.; Ma, Y.; Tu, X. Plasma reforming of toluene as a model tar compound from biomass gasification: Effect of CO2 and steam. Waste Dispos. Sustain. Energy 2019, 1, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, Y.; Song, J.; Ahmad, S.; Liang, J.; Sun, Y. Plasma-enhanced steam reforming of different model tar compounds over Ni-based fusion catalysts. J. Hazard. Mater. 2019, 377, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Q.; Wang, W.; Wang, K. Plasma catalytic steam reforming of a model tar compound by microwave-metal discharges. Fuel 2018, 234, 1278–1284. [Google Scholar] [CrossRef]
- Yu, L.; Tu, X.; Li, X.; Wang, Y.; Chi, Y.; Yan, J. Destruction of acenaphthene, fluorene, anthracene and pyrene by a dc gliding arc plasma reactor. J. Hazard. Mater. 2010, 180, 449–455. [Google Scholar] [CrossRef]
- Smulders, E.H.W.M.; Van Heesch, B.E.J.M.; Van Paasen, S.S.V.B. Pulsed power corona discharges for air pollution control. IEEE Trans. Plasma Sci. 1998, 26, 1476–1484. [Google Scholar] [CrossRef] [Green Version]
- Ammendola, P.; Piriou, B.; Lisi, L.; Ruoppolo, G.; Chirone, R.; Russo, G. Dual bed reactor for the study of catalytic biomass tars conversion. Exp. Therm. Fluid Sci. 2010, 34, 269–274. [Google Scholar] [CrossRef]
- Van de Kamp, W.; De Wild, P.; Zielke, U.; Suomalainen, M.; Knoef, H.; Good, J.; Liliedahl, T.; Unger, C.; Whitehouse, M.; Neeft, J.; et al. Tar measurement standard for sampling and analysis of tars and particles in biomass gasification product gas. Proc. 14th Eur. Biomass Conf. Exhib. 2005, 2005, 791–794. [Google Scholar]
- CEN/BT/TF 143. Biomass Gasification–Tar and Particles in Product Gases–Sampling and Analysis; Draft Document; Document of CEN: TC BT/TF 143 WICSC 030022.4, 10/2004; British Standards Institution, European Committee for Standardization: London, UK, 2004.
- Bityurin, V.A.; Filimonova, E.A.; Naidis, G.V. Simulation of naphthalene conversion in biogas initiated by pulsed corona discharges. IEEE Trans. Plasma Sci. 2009, 37, 911–919. [Google Scholar] [CrossRef]
- NIST Chemical Kinetics Database on the Web. Available online: http://kinetics.nist.gov (accessed on 15 December 2019).
- Xu, B.; Xie, J.; Yin, X.; Liu, H.; Sun, C.; Wu, C. Mechanisms of Toluene Removal in Relation to the Main Components of Biosyngas in a Catalytic Nonthermal Plasma Process. Energy Fuels 2019, 33, 4287–4301. [Google Scholar] [CrossRef]
- Mei, D.; Wang, Y.; Liu, S.; Alliati, M.; Yang, H.; Tu, X. Plasma reforming of biomass gasification tars using mixed naphthalene and toluene as model compounds. Energy Convers. Manag. 2019, 195, 409–419. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.M.; Batiot-Dupeyrat, C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. J. CO2 Util. 2015, 12, 54–61. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2015, 24, 015011. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Mei, D.; Tu, X.; Bogaerts, A. Gliding arc plasma for CO2 conversion: Better insights by a combined experimental and modelling approach. Chem. Eng. J. 2017, 330, 11–25. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Li, X.; Wang, W.; Yan, J.; Tu, X. Warm plasma activation of CO2 in a rotating gliding arc discharge reactor. J. CO2 Util. 2018, 27, 472–479. [Google Scholar] [CrossRef]
- Den Harder, N.; van den Bekerom, D.C.M.; Al, R.S.; Graswinckel, M.F.; Palomares, J.M.; Peeters, F.J.J.; Ponduri, S.; Minea, T.; Bongers, W.A.; van de Sanden, M.C.M.; et al. Homogeneous CO2 conversion by microwave plasma: Wave propagation and diagnostics. Plasma Process. Polym. 2017, 14, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Mohsenian, S.; Sheth, S.; Bhatta, S.; Nagassou, D.; Sullivan, D.; Trelles, J.P. Design and characterization of an electromagnetic-resonant cavity microwave plasma reactor for atmospheric pressure carbon dioxide decomposition. Plasma Process. Polym. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; ISBN 9781139471732. [Google Scholar]
- Liu, Y.; Li, X.S.; Liu, J.L.; Wu, J.; Ye, D.; Zhu, A.M. Cycled storage-discharge (CSD) plasma catalytic removal of benzene over AgMn/HZSM-5 using air as discharge gas. Catal. Sci. Technol. 2016, 6, 3788–3796. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dors, M.; Kurzyńska, D. Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge. Appl. Sci. 2020, 10, 991. https://doi.org/10.3390/app10030991
Dors M, Kurzyńska D. Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge. Applied Sciences. 2020; 10(3):991. https://doi.org/10.3390/app10030991
Chicago/Turabian StyleDors, Mirosław, and Daria Kurzyńska. 2020. "Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge" Applied Sciences 10, no. 3: 991. https://doi.org/10.3390/app10030991