GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Setups
2.2. Cell Culture
2.3. Viability Assay
2.4. MitoSOX Red Assay
2.5. Surface Calreticulin Detection
2.6. Colorimetric Assays for Chemical Analysis
2.7. Raman Spectroscopy of Glutathione Modifications
2.8. Statistical Analysis
3. Results
3.1. Comparison of Cellular Responses between COST-Jet and nspDBD Treatment of Jurkat T Lymphocytes
3.1.1. COST-Jet and nspDBD Treatment Cause Comparable Reductions in Jurkat T Lymphocyte Viability
- 2-min for the COST-Jet (93.7% ± 3.9%) and 45 Hz for the nspDBD (89.8% ± 10.01%)
- 4-min for the COST-Jet (65.7% ± 3.4%) and 75 Hz for the nspDBD (61.9% ± 9.4%).
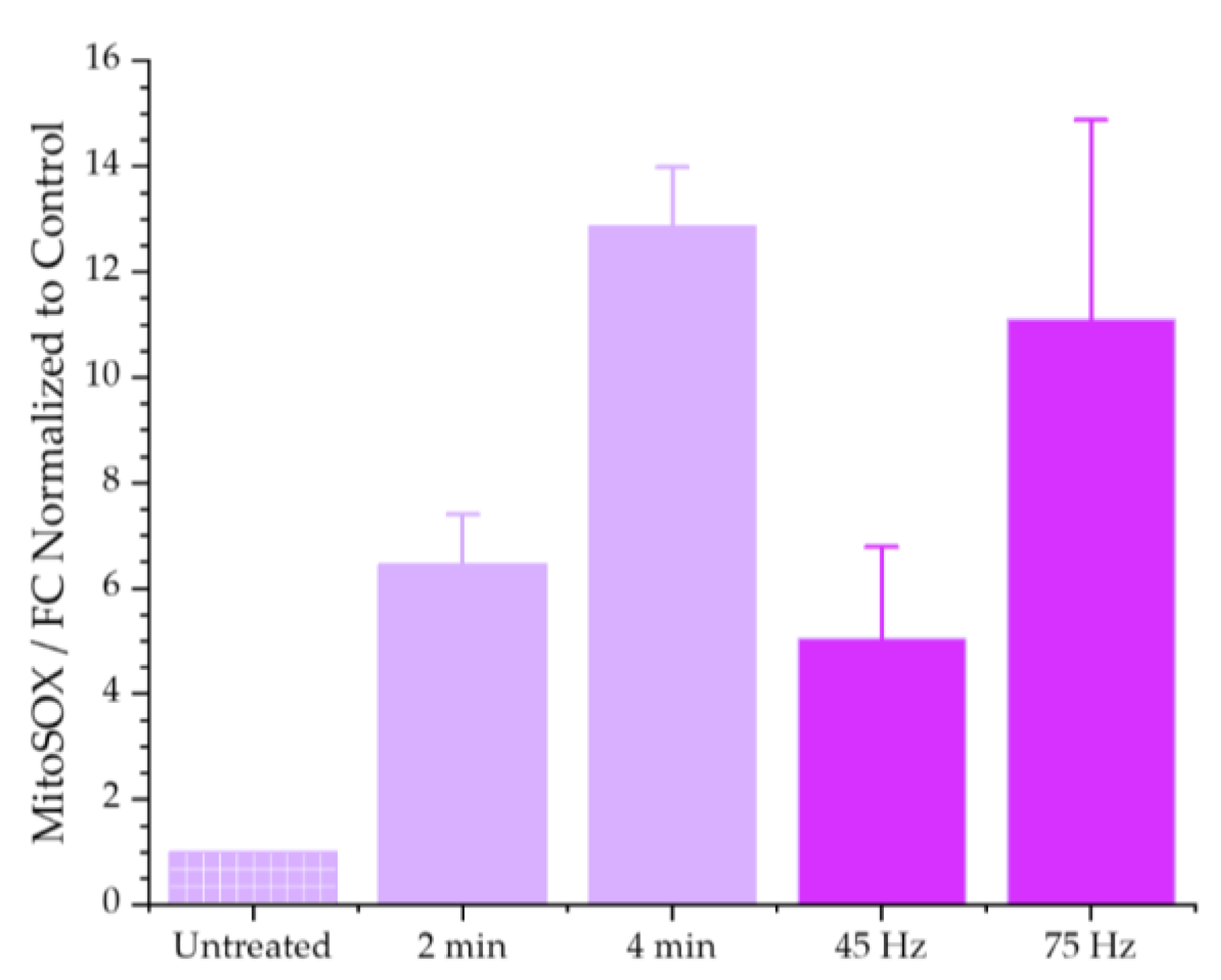
3.1.2. Mitochondrial Superoxide Production Increases in Response to Plasma Treatment
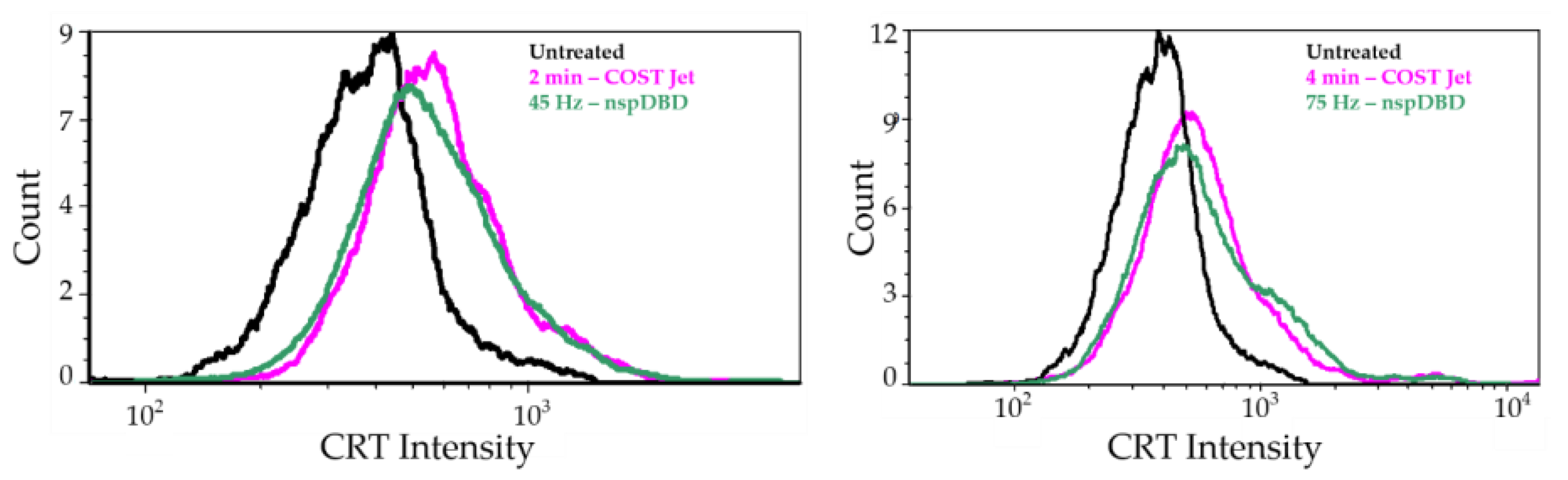
3.1.3. COST-Jet and nspDBD Treatment Increase the Cell Surface Display of Ecto-CRT
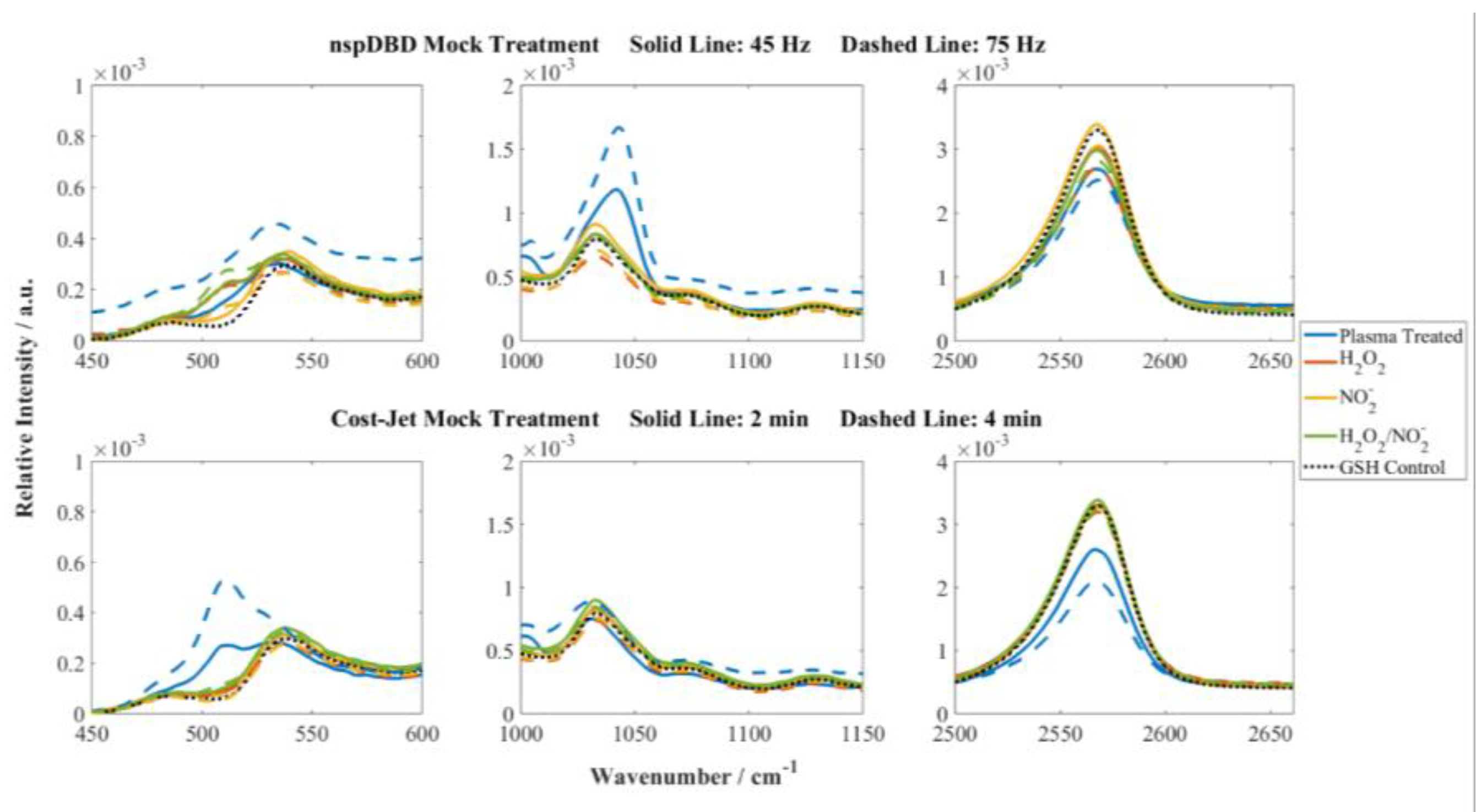
3.2. Chemical Comparison and Mock Treatment Are Inaccurate Markers of Cellular Responses
- For the nspDBD [14], the H2O2 concentration in DI water increased from 109.6 µM to 150.1 µM and NO2− concentrations from 3.9 µM to 9.01 µM when the frequency was increased from 45 to 75 Hz.
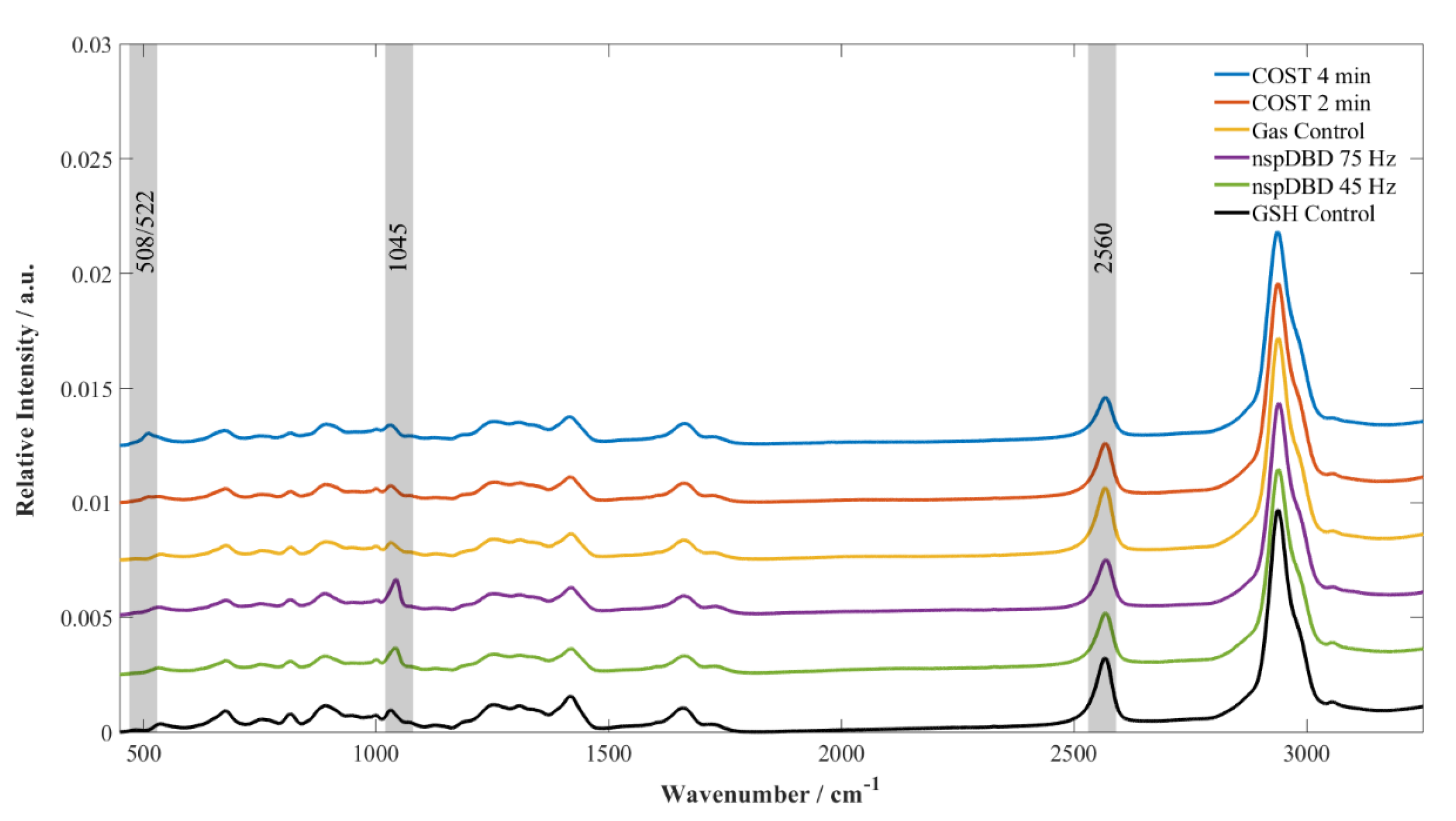
3.3. GSH Modifications Unique to Plasma Source Indicate the Stressors and the Potential for Cellular Response Present During Plasma Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen–A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.-M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [Green Version]
- Korolev, Y.D.; Frants, O.B.; Landl, N.V.; Suslov, A.I. Low-current plasmatron as a source of nitrogen oxide molecules. IEEE Trans. Plasma Sci. 2012, 40, 2837–2842. [Google Scholar] [CrossRef]
- Jiang, Y.; Sokorai, K.; Pyrgiotakis, G.; Demokritou, P.; Li, X.; Mukhopadhyay, S.; Jin, T.; Fan, X. Cold plasma-activated hydrogen peroxide aerosol inactivates Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria innocua and maintains quality of grape tomato, spinach and cantaloupe. Int. J. Food Microbiol. 2017, 249, 53–60. [Google Scholar] [CrossRef]
- Kruszelnicki, J.; Lietz, A.M.; Parsey, G.; Mohades, S.; Kushner, M.J. Consequences of Environmental Factors in Plasma Treatment of Liquids, Tissues and Materials. Clin. Plasma Med. 2018, 9, 2. [Google Scholar] [CrossRef]
- Lietz, A.M.; Kushner, M.J. Air plasma treatment of liquid covered tissue: Long timescale chemistry. J. Phys. D Appl. Phys. 2016, 49, 425204. [Google Scholar] [CrossRef]
- Norberg, S.A.; Parsey, G.M.; Lietz, A.M.; Johnsen, E.; Kushner, M.J. Atmospheric pressure plasma jets onto a reactive water layer over tissue: Pulse repetition rate as a control mechanism. J. Phys. D Appl. Phys. 2018, 52, 015201. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Naidis, G.; Laroussi, M.; Reuter, S.; Graves, D.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Lackmann, J.-W.; Bruno, G.; Jablonowski, H.; Kogelheide, F.; Offerhaus, B.; Held, J.; Schulz-von der Gathen, V.; Stapelmann, K.; von Woedtke, T.; Wende, K. Nitrosylation vs. oxidation–How to modulate cold physical plasmas for biological applications. PLoS ONE 2019, 14, e0216606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Bekeschus, S.; Lin, A.; Fridman, A.; Wende, K.; Weltmann, K.-D.; Miller, V. A Comparison of Floating-Electrode DBD and kINPen Jet: Plasma parameters to achieve similar growth reduction in colon cancer cells under standardized conditions. Plasma Chem. Plasma Process. 2018, 38, 1–12. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [Green Version]
- Wende, K.; Williams, P.; Dalluge, J.; Gaens, W.V.; Aboubakr, H.; Bischof, J.; Woedtke, T.V.; Goyal, S.M.; Weltmann, K.-D.; Bogaerts, A.; et al. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 2015, 10, 029518. [Google Scholar] [CrossRef] [Green Version]
- Hensel, K.; Kučerová, K.; Tarabová, B.; Janda, M.; Machala, Z.; Sano, K.; Mihai, C.T.; Ciorpac, M.; Gorgan, L.D.; Jijie, R.; et al. Effects of air transient spark discharge and helium plasma jet on water, bacteria, cells, and biomolecules. Biointerphases 2015, 10, 029515. [Google Scholar] [CrossRef]
- Mann, M.S.; Schnabel, U.; Weihe, T.; Weltmann, K.-D.; von Woedtke, T. A reference technique to compare the antimicrobial properties of atmospheric pressure plasma sources. Plasma Med. 2015, 5, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Steuer, A.; Schmidt, A.; Laboha, P.; Babica, P.; Kolb, J.F. Transient Suppression of Gap Junctional Intercellular Communication After Exposure to 100-Nanosecond Pulsed Electric Fields. Bioelectrochemistry 2016, 112, 33–46. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef] [Green Version]
- Lackmann, J.-W.; Wende, K.; Verlackt, C.; Golda, J.; Volzke, J.; Kogelheide, F.; Held, J.; Bekeschus, S.; Bogaerts, A.; Schulz-von der Gathen, V. Chemical fingerprints of cold physical plasmas–an experimental and computational study using cysteine as tracer compound. Sci. Rep. 2018, 8, 7736. [Google Scholar] [CrossRef] [PubMed]
- Kogelheide, F.; Kartaschew, K.; Strack, M.; Baldus, S.; Metzler-Nolte, N.; Havenith, M.; Awakowicz, P.; Stapelmann, K.; Lackmann, J.-W. FTIR spectroscopy of cysteine as a ready-to-use method for the investigation of plasma-induced chemical modifications of macromolecules. J. Phys. D Appl. Phys. 2016, 49, 084004. [Google Scholar] [CrossRef]
- Klinkhammer, C.; Verlackt, C.; Kogelheide, F.; Bogaerts, A.; Metzler-Nolte, N.; Stapelmann, K.; Havenith, M.; Lackmann, J.-W. Elucidation of Plasma-induced Chemical Modifications on Glutathione and Glutathione Disulphide. Sci. Rep. 2017, 7, 13828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Golda, J.; Held, J.; Redeker, B.; Konkowski, M.; Beijer, P.; Sobota, A.; Kroesen, G.; Braithwaite, N.S.J.; Reuter, S.; Turner, M. Concepts and characteristics of the ‘COST Reference Microplasma Jet’. J. Phys. D Appl. Phys. 2016, 49, 084003. [Google Scholar] [CrossRef]
- Lin, A.; Chernets, N.; Han, J.; Alicea, Y.; Dobrynin, D.; Fridman, G.; Freeman, T.A.; Fridman, A.; Miller, V. Non-Equilibrium Dielectric Barrier Discharge Treatment of Mesenchymal Stem Cells: Charges and Reactive Oxygen Species Play the Major Role in Cell Death. Plasma Process. Polym. 2015, 12, 1117–1127. [Google Scholar] [CrossRef]
- Ellerweg, D.; Benedikt, J.; von Keudell, A.; Knake, N.; Schulz-von der Gathen, V. Characterization of the effluent of a He/O2 microscale atmospheric pressure plasma jet by quantitative molecular beam mass spectrometry. New J. Phys. 2010, 12, 013021. [Google Scholar] [CrossRef]
- Sousa, J.S.; Niemi, K.; Cox, L.; Algwari, Q.T.; Gans, T.; O’connell, D. Cold atmospheric pressure plasma jets as sources of singlet delta oxygen for biomedical applications. J. Appl. Phys. 2011, 109, 123302. [Google Scholar] [CrossRef] [Green Version]
- Gorbanev, Y.; Verlackt, C.; Tinck, S.; Tuenter, E.; Foubert, K.; Cos, P.; Bogaerts, A. Combining experimental and modelling approaches to study the sources of reactive species induced in water by the COST RF plasma jet. Phys. Chem. Chem. Phys. 2018, 20, 2797–2808. [Google Scholar] [CrossRef]
- Kelly, S.; Golda, J.; Turner, M.M.; Schulz-von der Gathen, V. Gas and heat dynamics of a micro-scaled atmospheric pressure plasma reference jet. J. Phys. D Appl. Phys. 2015, 48, 444002. [Google Scholar] [CrossRef]
- Kruszelnicki, J.; Lietz, A.M.; Kushner, M.J. Interactions between water droplets and atmospheric pressure plasmas. Bull. Am. Phys. Soc. 2017, 62. [Google Scholar]
- Wartewig, S. IR and Raman Spectroscopy: Fundamental Processing; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lin, A.; Truong, B.; Pappas, A.; Kirifides, L.; Oubarri, A.; Chen, S.; Lin, S.; Dobrynin, D.; Fridman, G.; Fridman, A. Uniform Nanosecond Pulsed Dielectric Barrier Discharge Plasma Enhances Anti-Tumor Effects by Induction of Immunogenic Cell Death in Tumors and Stimulation of Macrophages. Plasma Process. Polym. 2015, 12, 1392–1399. [Google Scholar] [CrossRef]
- Vermeylen, S.; De Waele, J.; Vanuytsel, S.; De Backer, J.; Van der Paal, J.; Ramakers, M.; Leyssens, K.; Marcq, E.; Van Audenaerde, J.; Smits, E.L.J. Cold atmospheric plasma treatment of melanoma and glioblastoma cancer cells. Plasma Process. Polym. 2016, 13, 1195–1205. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Human Glioblastoma Spheroids Using Cold Atmospheric Plasma: The Combined Effect of Short- and Long-Lived Reactive Species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Obeid, M.; Tesniere, A.; Panaretakis, T.; Tufi, R.; Joza, N.; Van Endert, P.; Ghiringhelli, F.; Apetoh, L.; Chaput, N.; Flament, C.; et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol. Rev. 2007, 220, 22–34. [Google Scholar] [CrossRef]
- Beebe, S.J.; White, J.; Blackmore, P.F.; Deng, Y.; Somers, K.; Schoenbach, K.H. Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol. 2003, 22, 785–796. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Baty, J.W.; Hampton, M.B.; Winterbourn, C.C. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem. J. 2005, 389, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Girard, P.-M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rödder, K.; Jablonowski, H.; Schmidt, A.; von Woedtke, T.; Weltmann, K.-D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlackt, C.; Van Boxem, W.; Bogaerts, A. Transport and accumulation of plasma generated species in aqueous solution. Phys. Chem. Chem. Phys. 2018, 20, 6845–6859. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Gorbanev, Y.; O’Connell, D.; Vann, R.; Chechik, V.; Woude, M.W. Nontarget Biomolecules Alter Macromolecular Changes Induced by Bactericidal Low–Temperature Plasma. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Hefny, M.M.; Pattyn, C.; Lukes, P.; Benedikt, J. Atmospheric plasma generates oxygen atoms as oxidizing species in aqueous solutions. J. Phys. D Appl. Phys. 2016, 49, 404002. [Google Scholar] [CrossRef]
- Ellerweg, D.; von Keudell, A.; Benedikt, J. Unexpected O and O3 production in the effluent of He/O2 microplasma jets emanating into ambient air. Plasma Sources Sci. Technol. 2012, 21, 034019. [Google Scholar] [CrossRef]
- Mastanaiah, N.; Banerjee, P.; Johnson, J.A.; Roy, S. Examining the role of ozone in surface plasma sterilization using dielectric barrier discharge (DBD) plasma. Plasma Process. Polym. 2013, 10, 1120–1133. [Google Scholar] [CrossRef]
- Fujii, Y.; Tomita, K.; Sano, H.; Yamasaki, A.; Hitsuda, Y.; Adcock, I.M.; Shimizu, E. Dissociation of DNA damage and mitochondrial injury caused by hydrogen peroxide in SV-40 transformed lung epithelial cells. Cancer Cell Int. 2002, 2, 16. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef] [Green Version]
- Bruno, G.; Heusler, T.; Lackmann, J.-W.; von Woedtke, T.; Weltmann, K.-D.; Wende, K. Cold physical plasma-induced oxidation of cysteine yields reactive sulfur species (RSS). Clin. Plasma Med. 2019, 14, 100083. [Google Scholar] [CrossRef]
- Uchida, G.; Mino, Y.; Suzuki, T.; Ikeda, J.-I.; Suzuki, T.; Takenaka, K.; Setsuhara, Y. Decomposition and oxidation of methionine and tryptophan following irradiation with a nonequilibrium plasma jet and applications for killing cancer cells. Sci. Rep. 2019, 9, 6625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Kanzaki, M.; Kaneko, T. Calcium influx through TRP channels induced by short-lived reactive species in plasma-irradiated solution. Sci. Rep. 2016, 6, 25728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Et Biophys. Acta (Bba) Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, S.; Shaban, M.; Montazersadgh, F.; Alkayal, A.; Liu, D.; Kong, M.G.; Buckley, B.R.; Iza, F. trans-Stilbene epoxidation by He+O2 atmospheric pressure plasma: Epoxidation without oxidant waste stream. Plasma Process. Polym. 2020, 17, 1900162. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Van der Paal, J.; Van Boxem, W.; Dewilde, S.; Bogaerts, A. Reaction of chloride anion with atomic oxygen in aqueous solutions: Can cold plasma help in chemistry research? Phys. Chem. Chem. Phys. 2019, 21, 4117–4121. [Google Scholar] [CrossRef]
- Jirásek, V.; Lukeš, P. Formation of reactive chlorine species in saline solution treated by non-equilibrium atmospheric pressure He/O2 plasma jet. Plasma Sources Sci. Technol. 2019, 28, 035015. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303. [Google Scholar] [CrossRef] [Green Version]




| Parameter | COST-Jet 1 | nspDBD 2 |
|---|---|---|
| Treatment Distance | 4 mm | 1 mm |
| Treatment Time | 0, 1, 2, 3, 4, 5 min | 10 s |
| Frequency | 13.56 MHz | 30, 45, 60, 75 Hz |
| Treated Volume | 500 µL | 100 µL |
| Gas Flow Rate | He + 0.6% O2 (1 slm + 6 sccm) | - |
| Pulse Width | - | 20 ns |
| Rise Time | - | 2 ns |
| Energy/Pulse | - | 0.9 mJ/pulse |
| Treatment Condition | H2O2 (µM) | NO2− (µM) |
|---|---|---|
| COST-Jet: 2 min | 6.50 | 1.15 |
| COST-Jet: 4 min | 17.35 | 2.01 |
| nspDBD: 45 Hz | 108.78 | 3.92 |
| nspDBD: 75 Hz | 149.93 | 9.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranieri, P.; Mohamed, H.; Myers, B.; Dobossy, L.; Beyries, K.; Trosan, D.; Krebs, F.C.; Miller, V.; Stapelmann, K. GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment. Appl. Sci. 2020, 10, 2025. https://doi.org/10.3390/app10062025
Ranieri P, Mohamed H, Myers B, Dobossy L, Beyries K, Trosan D, Krebs FC, Miller V, Stapelmann K. GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment. Applied Sciences. 2020; 10(6):2025. https://doi.org/10.3390/app10062025
Chicago/Turabian StyleRanieri, Pietro, Hager Mohamed, Brayden Myers, Leah Dobossy, Keely Beyries, Duncan Trosan, Fred C. Krebs, Vandana Miller, and Katharina Stapelmann. 2020. "GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment" Applied Sciences 10, no. 6: 2025. https://doi.org/10.3390/app10062025





