Influence of Current Density on the Microstructure of Carbon-Based Cathode Materials during Aluminum Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
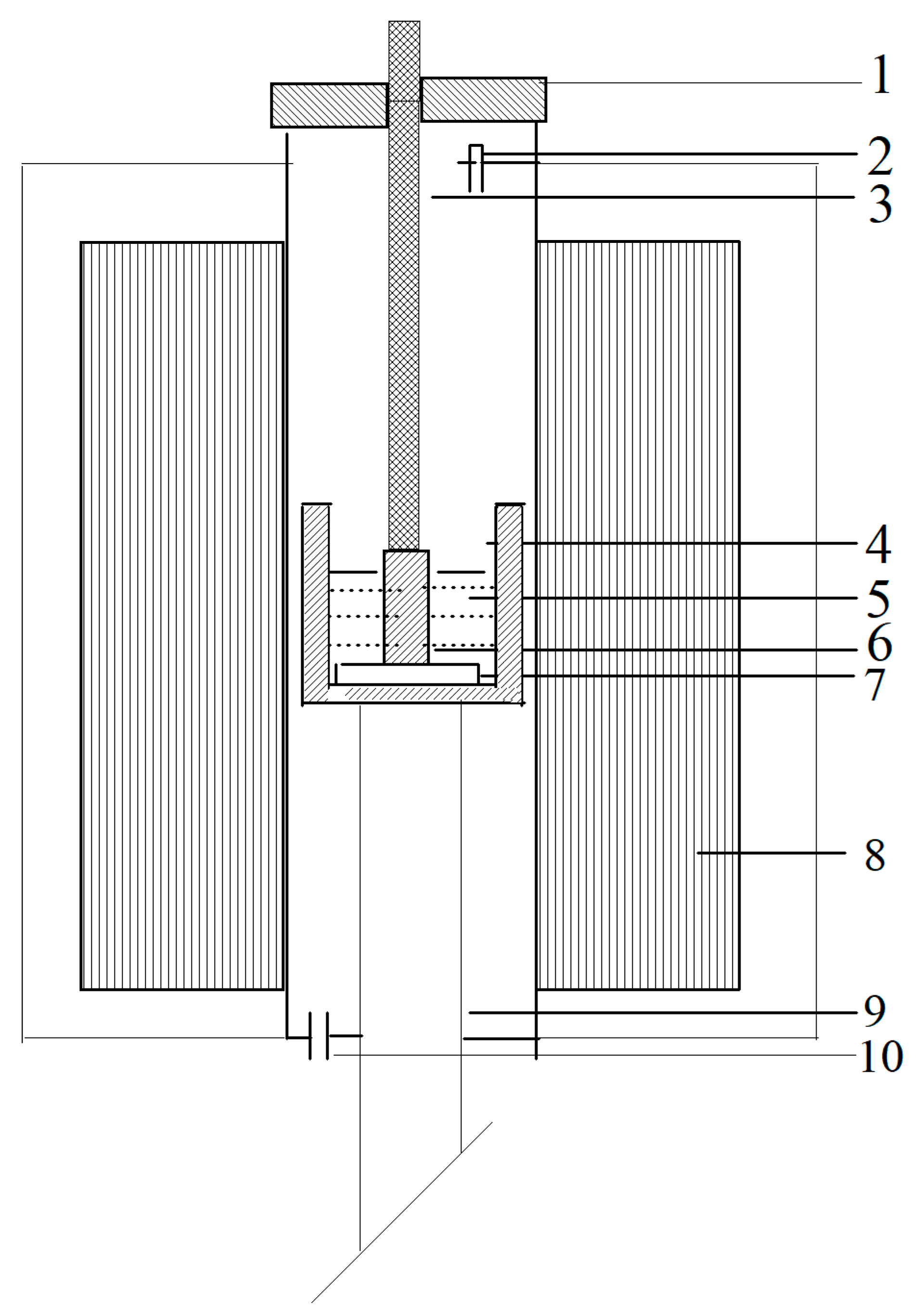
2.2. Cell design and Electrolysis Procedure
2.3. Microstructure Characterization
3. Results and Discussion
3.1. Effect of the Cathode Current Density on Sodium Expansion
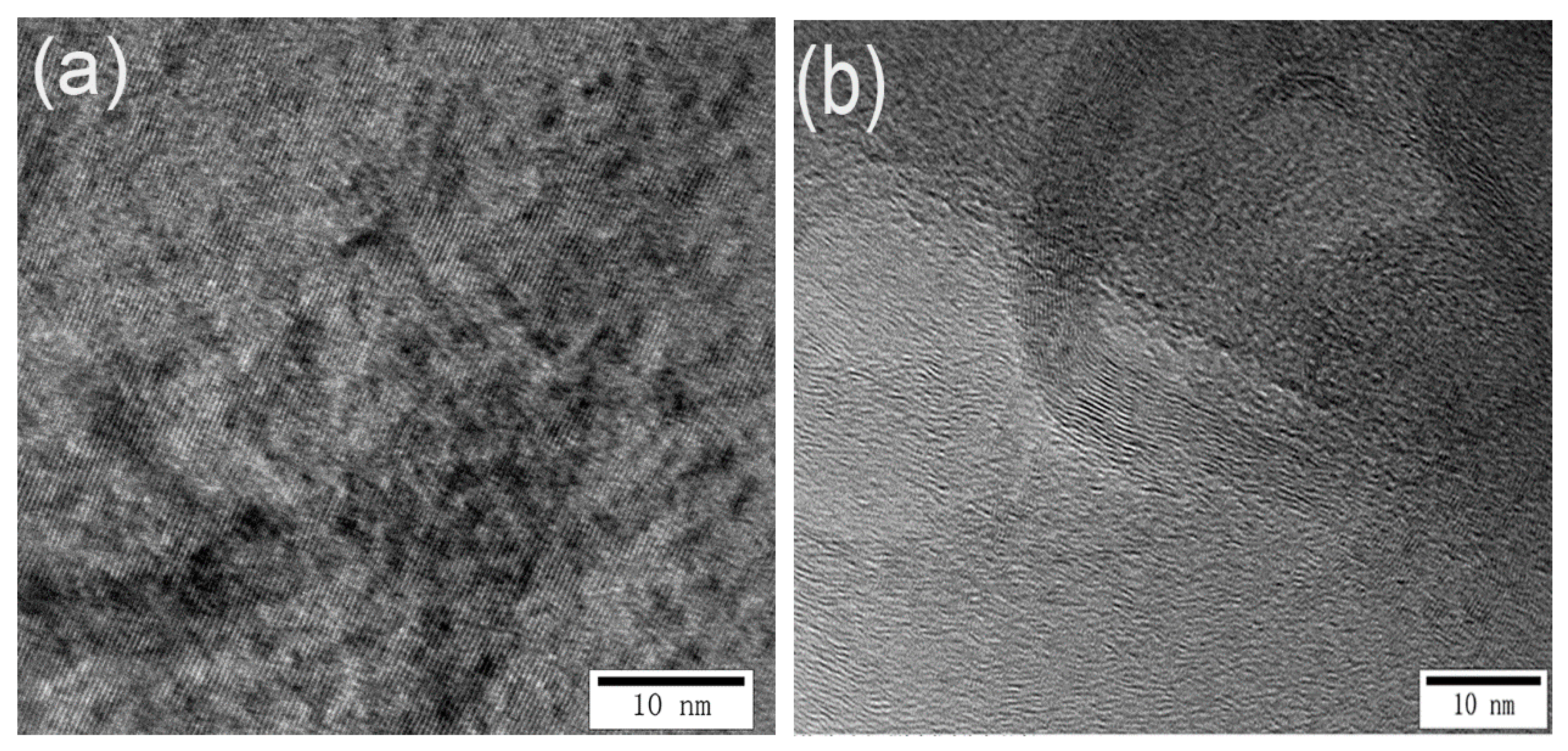
3.2. Effect of the Cathode Current Density on the Microstructure of Carbon Cathodes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, Z.; Lai, Y.; Shu, Y.; Jie, L.; Hwang, J.Y.; Liu, Y. Anodic corrosion behavior of NiFe2O4-based cermet in Na3AlF6-K3AlF6-AlF3 for aluminum electrolysis. Metall. Mater. Trans. B 2015, 46, 1257–1261. [Google Scholar] [CrossRef]
- Wang, W.; Sun, K.; Liu, H. Effects of different aluminum sources on morphologies and properties of ceramic floor tiles from red mud. Constr. Build. Mater. 2020, 241, 118119. [Google Scholar] [CrossRef]
- Wei, S.; Xu, L. Review on research progress of steel and iron wear-resistant material. Acta. Metall. Sinica. [CrossRef]
- Picard, D.; Fafard, M.; Soucy, G.; Bilodeau, J.F. Room temperature long-term creep/relaxation behaviour of carbon cathode material. Mater. Sci. Eng. A 2008, 496, 366–375. [Google Scholar] [CrossRef]
- Chauke, L.; Garbers-Craig, A.M. Reactivity between carbon cathode materials and electrolyte based on industrial and laboratory data. Carbon. 2013, 58, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Chen, W.; Gu, W. High-resolution TEM microscopy study of the creep behaviour of carbon-based cathode materials. Mater. Sci. Eng. A 2017, 687, 107–112. [Google Scholar]
- Zolochevsky, A.; Hop, J.G.; Servant, G.; Foosnæs, T.; Øye, H.A. Rapoport–Samoilenko test for cathode carbon materials: I. Experimental results and constitutive modelling. Carbon 2003, 41, 497–505. [Google Scholar] [CrossRef]
- Hjertenæs, E.; Nguyen, A.Q.; Koch, H. A Reactive ReaxFF Force field for sodium intrusion in graphitic cathodes. Phys. Chem. Chem. Physm. 2016, 18, 31431. [Google Scholar] [CrossRef]
- Tavassol, H.; Jones, E.M.; Sottos, N.R.; Gewirth, A.A. Electrochemical stiffness in lithium-ion batteries. Nat. Mater. 2016, 15, 1182. [Google Scholar] [CrossRef]
- Xiaoting, J.; Mario, H.; Vincent, M.; Sumpter, B.G.; Jessica, C.D.; Manuel, R.H.J.; Hyungbin, S.; Ya-Ping, H.; Alfonso, R.; Jing, K. Controlled formation of sharp zigzag and armchair edges in graphitic nanoribbons. Science 2009, 323, 1701–1705. [Google Scholar]
- Chon, M.J.; Sethuraman, V.A.; Mccormick, A.; Srinivasan, V.; Guduru, P.R. Real-time measurement of stress and damage evolution during initial lithiation of crystalline silicon. Phys. Rev. Lett. 2011, 107, 045503. [Google Scholar] [CrossRef] [PubMed]
- Tavassol, H.; Chan, M.K.; Catarello, M.G.; Greeley, J.; Cahill, D.G.; Gewirth, A.A. Surface coverage and SEI induced electrochemical surface stress changes during Li deposition in Li-ion battery anodes. J. Electrochem. Soc. 2013, 160, A888. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tokranov, A.; Xiao, X.; Sheldon, B.W. Stress development due to surface processes in graphite electrodes for Li-ion batteries: A first report. Electrochim. Acta 2012, 66, 28–37. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Chon, M.J.; Shimshak, M.; Srinivasan, V.; Guduru, P.R. In situ measurements of stress evolution in silicon thin films during electrochemical lithiation and delithiation. J. Power Sources 2011, 195, 5062–5066. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Xiao, L.; Sushko, M.L.; Han, K.S.; Shao, Y.; Yan, M.; Liang, X.; Mai, L.; Feng, J.; Cao, Y.; et al. Manipulating adsorption–insertion mechanisms in nanostructured carbon materials for high-efficiency sodium ion storage. Adv. Energy Mater. 2017, 7, 1700403. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Liu, H.; Zhang, C. Atomic scale investigations of carbon-based cathode materials deformation mechanism during aluminum electrolysis. Diamond Relat. Mater. 2019, 95, 14–19. [Google Scholar]
- Wang, W.; Chen, W.; Liu, H.; Zhang, H. Ripplocations, kink bands and delamination cracks in carbon cathode materials. Carbon Lett. 2019, 29, 377–383. [Google Scholar]
- Martin, E.; Federica, M.; Marco, S.; Vanessa, W. Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science 2013, 342, 716–720. [Google Scholar]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Huang, J.Y.; Chen, S.; Ren, Z.F.; Chen, G.; Dresselhaus, M.S. Real-time observation of tubule formation from amorphous carbon nanowires under high-bias Joule heating. Nano Lett. 2006, 6, 1699–1705. [Google Scholar] [CrossRef]
- Huang, J.Y.; Ding, F.; Yakobson, B.I.; Lu, P.; Qi, L.; Li, J. In situ observation of graphene sublimation and multi-layer edge reconstructions. Proc. Natl. Acad. Sci. USA 2009, 106, 10103–10108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Chen, W. Raman spectroscopy studies of carbon-based cathode materials during aluminum electrolysis. Arch. Metall. Mater. 2019, 64, 1257–1261. [Google Scholar]
- Wang, B.; Wolfe, D.E.; Terrones, M.; Haque, M.A.; Ganguly, S.; Roy, A.K. Electro-graphitization and exfoliation of graphene on carbon nanofibers. Carbon 2017, 117, 201–207. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yang, K.; Yang, Y.; Ma, J.; Pan, K.; Wang, G.; Ren, F.; Pang, H. Enhanced electrochemical performance of Sb2O3 as an anode for Lithium-ion batteries by a stable cross-linked binder. Appl. Sci. Basel 2019, 9, 2677. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Sun, K. Influence of Current Density on the Microstructure of Carbon-Based Cathode Materials during Aluminum Electrolysis. Appl. Sci. 2020, 10, 2228. https://doi.org/10.3390/app10072228
Wang W, Sun K. Influence of Current Density on the Microstructure of Carbon-Based Cathode Materials during Aluminum Electrolysis. Applied Sciences. 2020; 10(7):2228. https://doi.org/10.3390/app10072228
Chicago/Turabian StyleWang, Wei, and Kai Sun. 2020. "Influence of Current Density on the Microstructure of Carbon-Based Cathode Materials during Aluminum Electrolysis" Applied Sciences 10, no. 7: 2228. https://doi.org/10.3390/app10072228




