Inorganic Waste Generated in Kraft Pulp Mills: The Transition from Landfill to Industrial Applications
Abstract
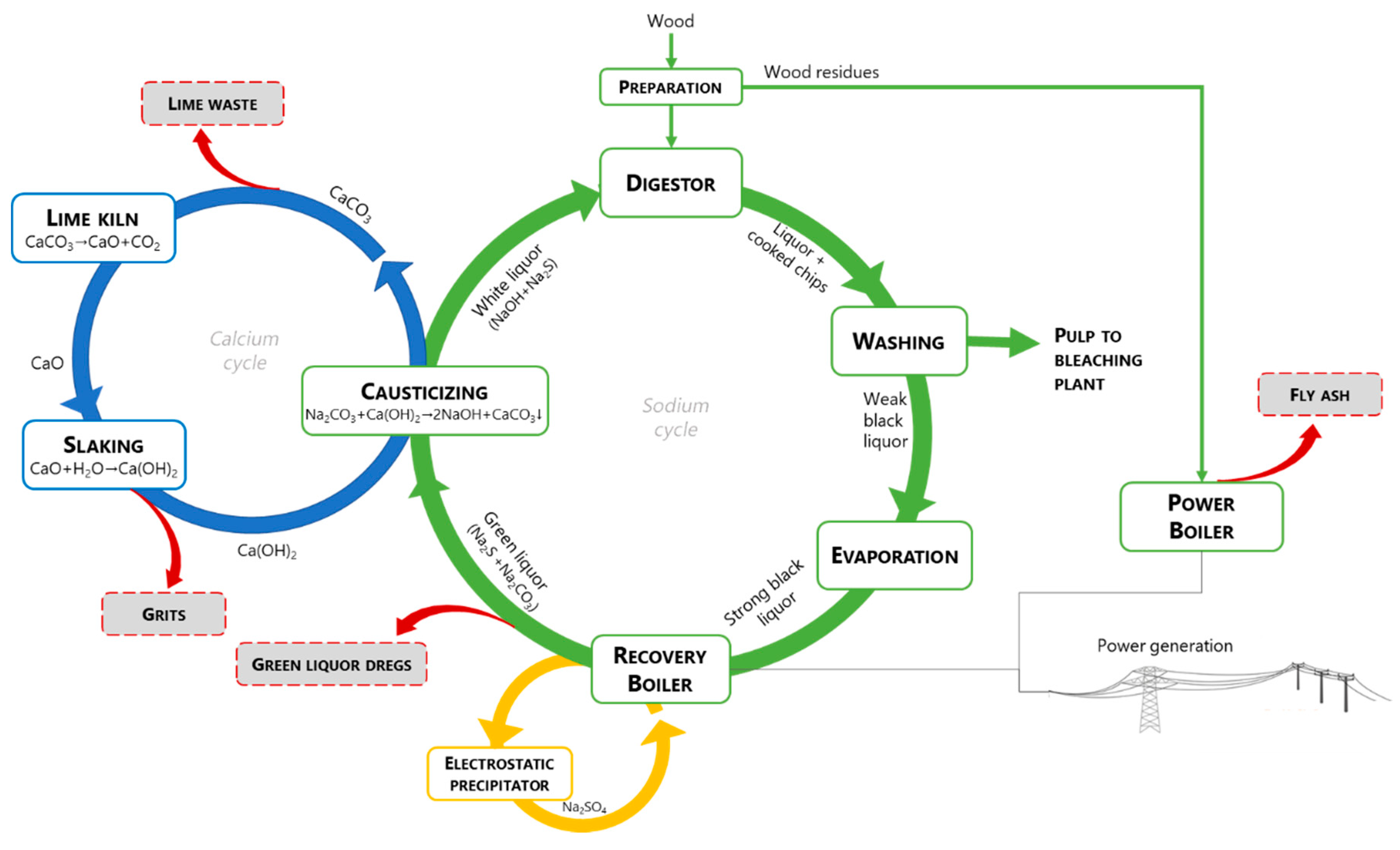
:1. Introduction
2. Kraft Pulp Mill Process and the Recovery of Chemicals
- Higher strength and flexibility of the produced pulps;
- Applicability to various wood species, regardless of their physico-chemical characteristics;
- The wide range of pulp applications;
- The efficient recovery of chemicals used in cooking, off-setting the high capital costs, which makes it economically more viable and competitive.
3. Main Properties of the Inorganic Wastes
3.1. Chemical composition
3.2. Mineral phases
3.3. Physico-chemical properties
3.4. Potentially toxic metals
4. Potential Applications
4.1. Green Liquor Dregs
4.2. Slaker Grits
4.3. Lime Mud
4.4. Boiler Fly Ash
5. Forthcoming Developments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Acronyms
| AMD | Acid mine drainage |
| ANC | Acid neutralization capacity |
| BFA | Boiler fly ash |
| CEM | Types of Cement |
| CEPI | Confederation of European Paper Industries |
| CM | Limits in Finnish legislation for ashes use |
| CNP | Calcium hydroxide nanoparticles |
| COD | Chemical oxygen demand |
| dw | Dry weight |
| EC | Electrical conductivity |
| Eh | Redox potential |
| EoW | End-of-waste criteria |
| FF | Finnish legal limit |
| HC | Hydraulic conductivity |
| GLD | Green liquor dregs |
| LCA | Life cycle assessment |
| LM | Lime mud |
| LOI | Loss on ignition |
| MSW | Municipal Solid Wastes |
| NPE | Non-process elements |
| PTM | Potentially toxic metals |
| Sa | Specific area |
| SG | Slaker grits |
| TDS | Total dissolved solids |
| UCS | Unconfined compressive strength |
| VS | Volatile solids |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| WoS | Web of Science |
References
- CEPI Key Statistics. European Pulp & Paper Industry; CEPI: London, UK, 2018. [Google Scholar]
- European Commission. COM Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Maastricht, The Netherlands, 2015. [Google Scholar]
- European Commission. BREF Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper and Board; European Commission: Maastricht, The Netherlands, 2015. [Google Scholar]
- Jia, Y.; Hamberg, R.; Qureshi, A.; Mäkitalo, M.; Maurice, C. Variation of green liquor dregs from different pulp and paper mills for use in mine waste remediation. Environ. Sci. Pollut. Res. 2019, 26, 31284–31300. [Google Scholar] [CrossRef] [PubMed]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; La Scalia, G.; Seabra, M.P.; Labrincha, J.A. Innovative recycling of lime slaker grits from paper-pulp industry reused as aggregate in ambient cured biomass fly ash-based geopolymers for sustainable construction material. Sustainability 2019, 11, 3481. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P. Environmentally Benign Approaches for Pulp Bleaching, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Biermann, C.J. Handbook of Pulping and Papermaking, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Sjöström, E. Wood chemistry: Fundamentals and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Walker, J. Primary Wood Processing: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bajpai, P. Biotechnology for Pulp and Paper Processing, 2nd ed.; Springer: Singapore, 2018. [Google Scholar]
- Golmaei, M.; Kinnarinen, T.; Jernström, E.; Häkkinen, A. Extraction of hazardous metals from green liquor dregs by ethylenediaminetetraacetic acid. J. Environ. Manage. 2018, 212, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tikka, P. Papermaking Science and Technology, Book 6 (Part 2), 2nd ed.; Paper Engineers’ Association/Paperi ja Puu Oy: Helsinki, Finland, 2008. [Google Scholar]
- He, J.; Lange, C.R.; Doughery, M. Laboratory study using paper mill lime mud for agronomic benefit. Process Saf. Environ. Prot. 2009, 87, 401–405. [Google Scholar] [CrossRef]
- Sanchez, D.; Tran, H. Treatment of Lime Slaker Grit and Green Liquor Dregs-Current Practice. In Proceedings of the TAPPI Engineering, Pulping & Environmental Conference, Philadelphia, Pennsylvania, 25–31 August 2005; pp. 1–9. [Google Scholar]
- Poykio, R.; Nurmesniemi, H.; Dahl, O.; Watkins, G.; Manskinen, K. Evaluation of the bio-accessible non-process element concentrations in slaker grits by synthetic sweat and gastric fluids extraction. J. Environ. Occup. Sci. 2014, 3, 65–70. [Google Scholar] [CrossRef]
- Mikkanen, P. Fly Ash Particle Formation in Kraft Recovery Boilers; Helsinki University of Technology: Espoo, Finland, 2000. [Google Scholar]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Wastes from pulp and paper mills—A review of generation and recycling alternatives. Ceramica 2018, 64, 371. [Google Scholar] [CrossRef] [Green Version]
- Sthiannopkao, S.; Sreesai, S. Utilization of pulp and paper industrial wastes to remove heavy metals from metal finishing wastewater. J. Environ. Manage. 2009, 90, 3283–3289. [Google Scholar] [CrossRef]
- Watkins, G.; Pöykiö, R.; Nurmesniemi, H.; Dahl, O. Earth construction and landfill disposal options for slaker grits. Res. J. Appl. Sci. Eng. Technol. 2010, 2, 757–764. [Google Scholar]
- Modolo, R.C.E. Valorization of Solid Wastes from Cellulose and Paper Industry. PhD Thesis, University of Aveiro, Aveiro, Portugal, 2014. [Google Scholar]
- Manskinen, K.; Nurmesniemi, H.; Pöykiö, R. Total and extractable non-process elements in green liquor dregs from the chemical recovery circuit of a semi-chemical pulp mill. Chem. Eng. J. 2011, 166, 954–961. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Pöykiö, R.; Perämäki, P.; Kuokkanen, T. The use of a sequential leaching procedure for heavy metal fractionation in green liquor dregs from a causticizing process at a pulp mill. Chemosphere 2005, 61, 1475–1484. [Google Scholar] [CrossRef]
- Martínez-Lage, I.; Velay-Lizancos, M.; Vázquez-Burgo, P.; Rivas-Fernández, M.; Vázquez-Herrero, C.; Ramírez-Rodríguez, A.; Martín-Cano, M. Concretes and mortars with waste paper industry: Biomass ash and dregs. J. Environ. Manage. 2016, 181, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.R.; Dezena Cabrelon, M.; de Sousa Trichês, E.; Quinteiro, E. Green liquor dregs and slaker grits residues characterization of a pulp and paper mill for future application on ceramic products. J. Clean. Prod. 2019, 240, 118220. [Google Scholar] [CrossRef]
- Cabral, F.; Ribeiro, H.M.; Hilário, L.; Machado, L.; Vasconcelos, E. Use of pulp mill inorganic wastes as alternative liming materials. Bioresour. Technol. 2008, 99, 8294–8298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C.; Yang, S. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Farage, R.M.P.; Silva, C.M.; Passos Rezende, A.A.; Lelis Leal de Souza, J.J.; Teixeira de Matos, A.; Vinha Zanuncio, A.J. Intermediate covering of municipal solid waste landfills with alkaline grits, dregs and lime mud by-products of kraft pulp production. J. Clean. Prod. 2019, 239, 117985. [Google Scholar] [CrossRef]
- Mahmoudkhani, M.; Richards, T.; Theliander, H. Recycling of solid residues to the forest: Experimental and theoretical study of the release of sodium from lime mud and green liquor dregs aggregates. Process Saf. Environ. Prot. 2004, 82, 230–237. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Labrincha, J.A. Upcycling unexplored dregs and biomass fly ash from the paper and pulp industry in the production of eco-friendly geopolymer mortars: A preliminary assessment. Constr. Build. Mater. 2018, 184, 464–472. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Golmaei, M.; Jernström, E.; Häkkinen, A. Separation, treatment and utilization of inorganic residues of chemical pulp mills. J. Clean. Prod. 2016, 133, 953–964. [Google Scholar] [CrossRef]
- Martins, F.M.; Martins, J.M.; Ferracin, L.C.; da Cunha, C.J. Mineral phases of green liquor dregs, slaker grits, lime mud and wood ash of a Kraft pulp and paper mill. J. Hazard. Mater. 2007, 147, 610–617. [Google Scholar] [CrossRef]
- Cherian, C.; Siddiqua, S. Pulp and Paper Mill Fly Ash: A Review. Sustainability 2019, 11, 4394. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, F.B.; Holanda, J.N.F. Reuse of grits waste for the production of soil-cement bricks. J. Environ. Manage. 2013, 131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Vilarinho, C.; Trancoso, D.; Ferreira, P.; Nunes, F.; Miragaia, A. Utilisation of pulp and paper industry wastes as raw materials in cement clinker production. Int. J. Mater. Eng. Innov. 2009, 1, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.A.; Santos, A.F.; Góis, J.C.; Quina, M.J. Thermal dehydration of urban biosolids with green liquor dregs from pulp and paper mill, Journal of Environmental Management. J. Environ. Manage. 2020, 261, 109944. [Google Scholar] [CrossRef]
- Modolo, R.; Benta, A.; Ferreira, V.M.; Machado, L.M. Pulp and paper plant wastes valorisation in bituminous mixes. Waste Manag. 2010, 30, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Mäkitalo, M.; Maurice, C.; Jia, Y.; Öhlander, B. Characterization of green liquor dregs, potentially useful for prevention of the formation of acid rock drainage. Minerals 2014, 4, 330–344. [Google Scholar] [CrossRef] [Green Version]
- Royer-Tardif, S.; Whalen, J.; Rivest, D. Can alkaline residuals from the pulp and paper industry neutralize acidity in forest soils without increasing greenhouse gas emissions? Sci. Total Environ. 2019, 663, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Eroǧlu, H.; Acar, H.H.; Üçüncü, O.; Imamoǧlu, S. Soil stabilization of forest roads sub-base using lime mud waste from the chemical recovery process in alkaline pulp mill. J. Appl. Sci. 2006, 6, 1199–1203. [Google Scholar]
- Etiegni, L.; Campbell, A.G.; Mahler, R.L. Evaluation of wood ash disposal on agricultural land. i. potential as a soil additive and liming agent. Commun. Soil Sci. Plant Anal. 1991, 22, 243–256. [Google Scholar] [CrossRef]
- Pöykiö, R.; Nurmesniemi, H. Calcium carbonate waste from an integrated pulp and paper mill as a potential liming agent. Environ. Chem. Lett. 2008, 6, 47–51. [Google Scholar] [CrossRef]
- Pöykiö, R.; Mäkelä, M.; Watkins, G.; Nurmesniemi, H.; Dahl, O. Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment. Trans. Nonferrous Met. Soc. China 2016, 26, 256–264. [Google Scholar] [CrossRef]
- Alvarenga, P.; Rodrigues, D.; Mourinha, C.; Palma, P.; de Varennes, A.; Cruz, N.; Tarelho, L.A.C.; Rodrigues, S. Use of wastes from the pulp and paper industry for the remediation of soils degraded by mining activities: Chemical, biochemical and ecotoxicological effects. Sci. Total Environ. 2019, 686, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Modolo, R.C.E.; Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Tarelho, L.A.C. Lime mud from cellulose industry as raw material in cement mortars. Mater. Constr. 2014, 64, 316. [Google Scholar] [CrossRef] [Green Version]
- Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Preparation of clinker from paper pulp industry wastes. J. Hazard. Mater. 2015, 286, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Simão, L.; Jiusti, J.; Lóh, N.J.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Waste-containing clinkers: Valorization of alternative mineral sources from pulp and paper mills. Process Saf. Environ. Prot. 2017, 109, 106–116. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Mäkelä, M.; Pöykiö, R.; Manskinen, K.; Dahl, O. Comparison of the forest fertilizer properties of ash fractions from two power plants of pulp and paper mills incinerating biomass-based fuels. Fuel Process. Technol. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Ribeiro, A.S.M.; Monteiro, R.C.C.; Davim, E.J.R.; Fernandes, M.H.V. Ash from a pulp mill boiler-Characterisation and vitrification. J. Hazard. Mater. 2010, 179, 303–308. [Google Scholar] [CrossRef]
- Torres, C.M.; Silva, C.M.; Pedroti, L.G.; Fernandes, W.; Ballotin, F.C.; Zanuncio, J.C. Cement Portland production with dregs and grits from kraft pulp mills incorporated to clinker. In Proceedings of the 6th International Workshop Advances in Cleaner Production, São Paulo, Brazil, 24–26 May 2017; pp. 1–12. [Google Scholar]
- Mäkitalo, M. Green Liquor Dregs as Sealing Layer Material to Cover Sulphidic Mine Waste Deposits. PhD Thesis, Luleå tekniska universitet, Luleå, Sweden, 2012. [Google Scholar]
- Pasandín, A.R.; Pérez, I.; Ramírez, A.; Cano, M.M. Moisture damage resistance of hot-mix asphalt made with paper industry wastes as filler. J. Clean. Prod. 2016, 112, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Nurmesniemi, H.; Pöykiö, R.; Keiski, R.L. A case study of waste management at the Northern Finnish pulp and paper mill complex of Stora Enso Veitsiluoto Mills. Waste Manag. 2007, 27, 1939–1948. [Google Scholar] [CrossRef]
- Pöykö, R.; Nurmesniemi, H.; Kuokkanen, T.; Perämäki, P. Green liquor dregs as an alternative neutralizing agent at a pulp mill. Environ. Chem. Lett. 2006, 4, 37–40. [Google Scholar] [CrossRef]
- Sebogodi, K.R.; Johakimu, J.K.; Sithole, B.B. Beneficiation of pulp mill waste green liquor dregs: Applications in treatment of acid mine drainage as new disposal solution in South Africa. J. Clean. Prod. 2019, 246, 118979. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Sądej, W.; Suski, M.S.; Wyrwas, A.; Skrocki, D. Impact of Paper Mill Waste on Physicochemical Properties of Soil, Crop Yield, and Chemical Composition of Plants. CLEAN Soil Air Water 2019, 47, 1900080. [Google Scholar] [CrossRef]
- Pértile, P.; Albuquerque, J.A.; Gatiboni, L.C.; da Costa, A.; Luciano, R.V. Corrective Potential of Alkaline Residue (Dregs) from Cellulose Industry in an Acid Soil Cultivated Under No-tillage. Commun. Soil Sci. Plant Anal. 2017, 48, 1868–1880. [Google Scholar] [CrossRef]
- Zambrano, M.; Pichún, C.; Alvear, M.; Villarroel, M.; Velásquez, I.; Baeza, J.; Vidal, G. Green liquor dregs effect on Kraft mill secondary sludge composting. Bioresour. Technol. 2010, 101, 1028–1035. [Google Scholar] [CrossRef]
- PaperChain Circular Case 1. Available online: https://www.paperchain.eu/circular-cases/circular-case-1/ (accessed on 24 January 2020).
- Siqueira, F.B.; Holanda, J.N.F. Application of grits waste as a renewable carbonate material in manufacturing wall tiles. Ceram. Int. 2018, 44, 19576–19582. [Google Scholar] [CrossRef]
- Paiva, H.; Simões, F.; Morais, M.; Ferreira, V.M. Pilot test involving pulp and paper industry wastes in road pavements. In Wastes: Solutions, Treatments and Opportunities III; Vilarinho, C., Castro, F., Gonçalves, M., Fernando, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 20–26. [Google Scholar]
- Pérez-López, R.; Quispe, D.; Castillo, J.; Nieto, J.M. Acid neutralization by dissolution of alkaline paper mill wastes and implications for treatment of sulfide-mine drainage. Am. Mineral. 2011, 96, 781–791. [Google Scholar] [CrossRef]
- Farage, R.; Quina, M.J.; Gando-Ferreira, L.; Silva, C.M.; Souza, J.L.; Torres, C.M. Kraft pulp mill dregs and grits as permeable reactive barrier for removal of copper and sulfate in acid mine drainage. Sci. Rep. 2020, 10, 4083. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Dahl, O.; Watkins, G.; Pöykiö, R. Slaker grits from the causticising process of a pulp mill—A potential fertiliser and liming agent material for use in agriculture and forestry. Int. J. Mater. Eng. Innov. 2010, 1, 312–324. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Pöykiö, R.; Watkins, G.; Dahl, O. Total and extractable heavy metal, phosphorous and sulfur concentrations in slaker grits from the causticizing process of a pulp mill for use as a soil amendment. Chem. Speciat. Bioavailab. 2010, 22, 87–97. [Google Scholar] [CrossRef]
- Sarkar, R.; Kurar, R.; Gupta, A.K.; Mudgal, A.; Gupta, V. Use of paper mill waste for brick making. Cogent Eng. 2017, 4, 1405768. [Google Scholar] [CrossRef]
- Vu, H.; Khan, M.; Chilakala, R.; Lai, T.; Thenepalli, T.; Ahn, J.; Park, D.; Kim, J. Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal. Sustainability 2019, 11, 1524. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, R.; Zhao, J.; Han, K.; Lu, C. Sulfation behavior of white mud from paper manufacture as SO2 sorbent at fluidized bed combustion temperatures. J. Therm. Anal. Calorim. 2012, 107, 241–248. [Google Scholar] [CrossRef]
- Mäkelä, M.; Harju-Oksanen, M.L.; Watkins, G.; Ekroos, A.; Dahl, O. Feasibility assessment of inter-industry solid residue utilization for soil amendment—Trace element availability and legislative issues. Resour. Conserv. Recycl. 2012, 67, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Niu, S.; Lu, C.; Liu, M.; Huo, M. Transesterification catalyzed by industrial waste - Lime mud doped with potassium fluoride and the kinetic calculation. Energy Convers. Manag. 2014, 86, 1110–1117. [Google Scholar] [CrossRef]
- Shen, J.; Fatehi, P.; Soleimani, P.; Ni, Y. Recovery of lignocelluloses from pre-hydrolysis liquor in the lime kiln of kraft-based dissolving pulp production process by adsorption to lime mud. Bioresour. Technol. 2011, 102, 10035–10039. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Han, P.; Lu, X.; Wang, Y. A review on the dewaterability of bio-sludge and ultrasound pretreatment. Ultrason. Sonochem 2004, 11, 337–348. [Google Scholar]
- Hannam, K.D.; Venier, L.; Hope, E.; McKenney, D.; Allen, D.; Hazlett, P.W. AshNet: Facilitating the use of wood ash as a forest soil amendment in Canada. For. Chron. 2017, 93, 17–20. [Google Scholar] [CrossRef] [Green Version]
- IEA Bioenergy. Options for Increased Use of Ash From Biomass Combustion and Co-Firing; IEA: Paris, France, 2018. [Google Scholar]
- Elliot, A.; Mahmood, T. Beneficial uses of pulp and paper power boiler ash residues. Tappi J. 2006, 5, 9–16. [Google Scholar]
- Rangan, V. Fly ash-based geopolymer concrete. In Proceedings of the International Workshop on Geopolymer Cement and Concrete, Mumbai, India, 28 June 2010; Allied Publishers Private Limited: Mumbai, India, 2010; pp. 68–106. [Google Scholar]
- Lessard, J.M.; Omran, A.; Tagnit-Hamou, A.; Gagné, R. Production of RCC using biomass fly and bottom ashes: From laboratory to fieldwork. J. Mater. Civ. Eng. 2017, 29, 04017225. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Miranda, T.; Oliveira, D.; Silva, R. Soil stabilisation using alkaline activation of fly ash for self compacting rammed earth construction. Constr. Build. Mater. 2012, 36, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Vu, D.H.; Wang, K.S.; Chen, J.H.; Nam, B.X.; Bac, B.H. Glass-ceramic from mixtures of bottom ash and fly ash. Waste Manag. 2012, 32, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Arm, M.; Vestin, J.; Lind, B.B.; Lagerkvist, A.; Nordmark, D.; Hallgren, P. Pulp mill fly ash for stabilization of low-volume unpaved forest roads—field performance. Can. J. Civ. Eng. 2014, 41, 955–963. [Google Scholar] [CrossRef]
- Šķēls, P.; Bondars, K.; Plonis, R.; Haritonovs, V.; Paeglītis, A. Usage of Wood Fly Ash in Stabilization of Unbound Pavement Layers and Soils. In Proceedings of the Historical Experience and Challenges of Proceedings of 13th Baltic Sea Geotechnical Conference, Vilnius, Lithuaniam, 22–24 September 2016; pp. 1–4. [Google Scholar]
- Rios, S.; Cristelo, N.; Miranda, T.; Araújo, N.; Oliveira, J.; Lucas, E. Increasing the reaction kinetics of alkali-activated fly ash binders for stabilisation of a silty sand pavement sub-base. Road Mater. Pavement Des. 2018, 19, 201–222. [Google Scholar] [CrossRef] [Green Version]
- Malakootian, M.; Almasi, A.; Hossaini, H. Pb and Co removal from paint industries effluent using wood ash. Int. J. Environ. Sci. Technol. 2008, 5, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Laohaprapanon, S.; Marques, M.; Hogland, W. Removal of Organic Pollutants from Wastewater Using Wood Fly Ash as a Low-Cost Sorbent. CLEAN Soil Air Water 2010, 38, 1055–1061. [Google Scholar] [CrossRef]
- Das, S.K. Yudhbir Geotechnical properties of low calcium and high calcium fly ash. Geotech. Geol. Eng. 2006, 24, 249–263. [Google Scholar] [CrossRef]
- Rissanen, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Partial Replacement of Portland-Composite Cement by Fluidized Bed Combustion Fly Ash. J. Mater. Civ. Eng. 2017, 29, 04017061. [Google Scholar] [CrossRef] [Green Version]
- Ohenoja, K.; Tanskanen, P.; Wigren, V.; Kinnunen, P.; Körkkö, M.; Peltosaari, O.; Österbacka, J.; Illikainen, M. Self-hardening of fly ashes from a bubbling fluidized bed combustion of peat, forest industry residuals, and wastes. Fuel 2016, 165, 440–446. [Google Scholar] [CrossRef]
- Huotari, N.; Tillman-Sutela, E.; Moilanen, M.; Laiho, R. Recycling of ash—For the good of the environment? For. Ecol. Manage. 2015, 348, 226–240. [Google Scholar] [CrossRef]
- Fu, K.; Ren, X.Y.; Lin, J.Q.; Yue, P. Comparative analysis of environmental impacts between dregs disposal and conventional cement production by life cycle assessment (LCA). Proc. Adv. Mater. Res. 2013, 777, 461–466. [Google Scholar] [CrossRef]
- Sartz, L.; Hamilton, I.; Mácsik, J.; Maurice, C.; Sädbom, S.; Westin, G.; Bäckström, M. Green liquor dregs from pulp and paper industry used in mining waste management: A symbiosis project (GLAD) between two Swedish base industries. In Proceedings of the 13th International mine water association congress, Rauha-Lappeenranta, Finland, 31 January 2017; Wolkersdorfer, C., Sartz, L., Sillanpää, M., Häkkinen, A., Eds.; Mine Water & Circular Economy, Lappeenranta University of Technology: Lappeenranta, Finland, 2017; pp. 862–868. [Google Scholar]


| Wastes | Industrial Information (a) | [3] | [20] | [21] | [22] | [23] |
|---|---|---|---|---|---|---|
| GLD | 12 | 10–20 (b) | 12 | 15 | 4–20 | 12.8 |
| SG | 10 | 7 | 16 | |||
| LM | 25 | 10–20 | 15 | 13 | ||
| BFA | 30 | 9 (c) | 20 | 5 |
| GLD | SG | LM | BFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [4] | [24] | [25] | [5] | [24] | [26] | [27] | [28] | [5] | [23] | [26] | [29] | |
| CaO | 34.3 | 33.0 | 34.9 | 49.45 | 55.8 | 44.4–52.0 | 57.12 | 54.1 | 16.7 | 34.9 | 0.8–10.4 | 16.5 |
| MgO | 10.9 | 4.65 | 5.94 | 0.45 | 0.47 | 0.6–3.4 | 0.91 | 0.86 | 3.44 | 4.4 | 0.7–1.9 | 3.07 |
| SiO2 | 0.23 | 2.35 | ni | 0.47 | 1.31 | 3.4–11.0 | 3.58 | 0.34 | 38.5 | 11.6 | 33.9–59.7 | 34.0 |
| Al2O3 | 1.94 | 0.69 | 0.47 | 0.29 | 0.42 | 0.5–1.4 | 0.07 | 0.07 | 14.8 | 4.4 | 16.5–35.4 | 13.5 |
| Fe2O3 | 0.61 | 0.65 | 0.59 | 0.05 | <0.1 | 0.2–1.2 | 0.20 | 0.15 | 5.94 | 2.6 | 1.5–19.7 | 4.95 |
| Na2O | 2.10 | 11.7 | 9.49 | 4.52 | 0.60 | ni | 2.32 | 0.91 | 1.53 | 1.4 | ni | 1.52 |
| K2O | <0.1 | 1.03 | 0.37 | 0.27 | <0.1 | ni | 0.26 | 0.06 | 5.97 | 6.5 | ni | 5.49 |
| P2O5 | ni | 0.33 | 0.37 | 0.38 | 0.65 | ni | 0.03 | 0.96 | 1.12 | 1.6 | ni | 1.11 |
| TiO2 | ni | <0.1 | ni | ni | <0.1 | ni | ni | ni | 0.76 | 0.25 | ni | 0.65 |
| MnO | 4.21 | 0.37 | 0.06 | ni | <0.1 | ni | ni | 0.09 | 0.50 | 1.4 | ni | 0.45 |
| SO3 | 3.6 | 2.82 | ni | 1.86 | 0.11 | ni | 0.4 | ni | 2.66 | 11.4 | ni | 2.77 |
| LOI | ni | 42.10 * | ni | 41.1 | 40.10 | 31.5–43.5 | ni | ni | 6.38 | 15.8 | 1.2–33.6 | 14.3 |
| GLD | SG | LM | BFA |
|---|---|---|---|
| Calcite (CaCO3) [4,24,31] | Calcite (CaCO3) [5,24,31] | Calcite (CaCO3) [31] | Calcite (CaCO3) [5,23] |
| Dolomite (CaMg(CO3)2) [23] | Dolomite (CaMg(CO3)2) [23] | Ca(1−x)MgxCO3[31] | Dolomite (CaMg(CO3)2) [23] |
| Cesanite (Ca2Na3(SO4)3(OH)) [23] | Quartz (SiO2) [24] | Halite (NaCl) [23] | |
| Natrite (Na2CO3) [24,23,33,34] | Pirssonite (Na2Ca(CO3)2.2H2O) [31] | Quartz (SiO2) [5,23,31] | |
| Pirssonite (Na2Ca(CO3)2.2H2O) [4, 21,30] | Portlandite (Ca(OH)2) [31] | Sylvite (KCl) [23] | |
| Manganite (Mn4O8H4) [4] | Wustite (FeO) [31] | Anhydrite (CaSO4) [23] | |
| Sodium sesquicarbonate (Na3H(CO3)2) [35] | Larnite (Ca2 SiO4) [31] | Portlandite (Ca(OH)2) [23] | |
| Brucite (Mg(OH)2) [35] | Brucite (Mg(OH)2) [31] | Periclase (MgO) [23] |
| Property | GLD | SG | LM | BFA |
|---|---|---|---|---|
| Moisture (%) | 50.8 [36]; 48.0–57.0 [17], 54.0 [37] | 15.7 [36]; 28.4 [19], 7.0–16.0 [17], | 41.1 [38], 1.1–45.6 [17], 28.0 [39], 39–60 [26] | 0.30–0.80 [40] |
| pH | 12.8 [36]; 12.2 [25], 12.9 [4] | 13.1 [36]; 12.6 [25], 13.1 [19] | 12.6 [41] | 11 [32]; 12.8 [42]; 13.3 [43] |
| EC (mS/cm) | 26.2 [36]; 9.76 [4] | 20,8 [36]; 94.3 [19] | 7.3 [41] | 13.6 [42]; 11.63 [43] |
| VS (% TS) | 8.3 [36] | 2.4 [19] | ||
| ANC (% CaCO3) | 64.4–95.6 [25], 8.3 mmol H+/g [4] | 69.4–100 [25] | 106 [41] | 54.3–77.7 [25] |
| D50 (µm) | 11.6 [24], 8.97 [4]; 6 [29] | 24.1 [24] | 49.3 [5]; 150–250 [32] | |
| Density (g/cm3) | 2.498 [24], 2.47–2.60 [37] | 2.703 [24] | 2.83 [44], 2.43 [39] | 2.4–2.8 [32], 2.615 [23] |
| Bulk density (g/cm3) | 1.2–1.64 [4], 0.44–0.67 [37] | 0.15–1.3 [32] | ||
| Sa (m2/g) | 72.08 [24]; 12–21 [37] | 2.901 [24] | 5.17 [44] | 3.03 [5]; 4.2–101 [32], 3.25 [23] |
| HC (m/s) | 8.8 × 10−9 − 1 × 10−8 [4] | |||
| Kjeldahl N (%) | 0.07 [25] | 0.05 [25] | 0.17 [25] | |
| Chlorides (%) | 0.30 [29]; 0.8 [36] | 0.1 [12]; 0.02 [19]; 0.1 [36] | 0.08 [44]; <LQ [45]; 0.06 [46] | 1.5 [29]; 2.7 [23]; 1.2 [45]; 1.2 [43]; 0.10 [46] |
| GLD | SG | LM | BFA | Limit FF | Limit CM | Crust * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [4] | [25] | [30] | [19] | [25] | [28] | [41] | [25] | [42] | [47] | [47] | [19] | [48] | |
| Pb | 6.12 | 46.8 | 13 | <3 | 34.1 | 6.79 | <3 | 44.3 | 28.7 | 31 | 150 | 300 | 12.5 |
| Cd | 3.81 | 5.19 | 9.4 | 0.3 | 4.75 | 0.91 | <0.3 | 4.7 | 2.9 | 3.3 | 25 | 15 | 0.2 |
| Cu | 229 | 80.9 | 102 | <10 | 4.6 | 0.73 | 4.1 | 25.8 | 63.6 | 72 | 700 | 400 | 55 |
| Cr | 295 | 56.0 | 118 | 12.6 | 12.4 | 16.7 | 7.0 | 24.1 | 66.9 | 74 | 300 | 400 | 100 |
| Ni | 233 | 189 | 84 | 23.9 | 25.2 | ni | 4.0 | 97.4 | 32.4 | 33 | 150 | ni | 75 |
| Zn | 3197 | 160 | 1000 | 9.9 | 15.0 | ni | 36 | 68.9 | 295.3 | 320 | 4500 | 2000 | 70 |
| Hg | <0.05 | ni | ni | <0.03 | ni | <0.04 | <0.03 | ni | 0.03 | 0.1 | 1.0 | ni | ni |
| V | ni | ni | 1.9 | 39.0 | ni | ni | ni | ni | 92.7 | ni | ni | 400 | 135 |
| Mo | 0.29 | ni | 1.7 | <1 | ni | ni | 2 | ni | 3.8 | ni | ni | 50 | 1.5 |
| As | <0.1 | ni | 0.3 | <3 | ni | 0.38 | 2.7 | ni | 13.0 | 14 | 40 | 50 | 1.8 |
| Application | Highlights | Scale |
|---|---|---|
| Construction materials | ||
| Concrete | GLD as a replacement of part of the cement in concrete is not suitable since the loss in mechanical properties is significant [23]. | Laboratory |
| Cement | Substitution of clinker up to 10% is feasible to obtain Portland cement (CP I-S and CP II-F) [50]. | Laboratory |
| Clinker production | The mixture of GLD (0.13 wt %) with standard materials is technically viable and does not present noticeable environmental effects [34]. | Industrial |
| Geopolymer mortars | GLD can be used as a fine filler up to 25 wt % incorporation. The obtained mortars exhibited enhanced tensile and compressive strength [29]. | Laboratory |
| Geotechnical | ||
| Landfill cover | Alternating layers of 0.15 m of GLD (70 wt %) and SG (30 wt %) covered by 1 m of MSW have the potential for replacing soil as intermediate covering in landfills [27]. | Industrial |
| Road pavement construction | GLD require washing before incorporation as aggregates in bituminous mixtures to guarantee stability in terms of water sensitivity [36]. | Laboratory |
| Sealing layer in mines | A mixture with the proportions 7:2:1 of tailings: GLD: fly ash was found to be geotechnically satisfactory to be used as a sealing layer in dry covers on mine [37]. GLD showed high water retention capacity and low hydraulic conductivity, which prevents water percolation and oxygen transport [51]. | Laboratory |
| Hot-mix asphalt | GLD used as filler in hot-mix asphalt leads to poor water resistance, despite displaying adequate mechanical properties (stiffness and permanent deformation) [52]. | Laboratory |
| Environmental | ||
| Neutralize acidic wastewaters | The liming effect of GLD (39.6% Ca eq) is similar to commercial limestone (38%). The pH of 10.7 indicates a strong liming effect [53,54]. | Industrial |
| Acid mine drainage remediation | GLD exhibited high buffering capacity [4] at low dosages of 1 g/L [55] for remediation of acid mine drainage. | Laboratory |
| Soil amendment | Doses up to 20 t/ha achieved neutralization of acidic soil, not causing deterioration of soil properties or depressing crop yields [56]. After 5.5 years since application, positive effects were observed on the soil chemical attributes [57]. | Laboratory |
| Drying adjuvant of sewage sludge | A dose of 0.15 g GLD per g of sewage sludge reduced by 8% the energy required for the evaporation of humidity at 130 °C. A reduction in phytotoxicity was also observed in tests with garden cress, revealing a good potential for agricultural applications [34]. | Laboratory |
| Agricultural | ||
| Liming material | Valid option to substitute commercial agricultural limestone [25,41]. | Industrial |
| Co-composting | The addition of a moderate amount of GLD (5–8 wt %) with kraft mill sludge did not show a negative effect on the biological activities during the composting process [58]. | Laboratory |
| Application | Highlights | Scale |
|---|---|---|
| Construction materials | ||
| Cement | The incorporation of SG up to 10% presents good results of compressive strength and elasticity modulus to produce Portland cement (CP I-S and CP II-F) [50]. | Laboratory |
| Clinker production | The incorporation of 0.25 wt % of SG shows the potential to be directly incorporated in clinker manufacture [34]. | Industrial |
| Ceramic wall tiles | The total replacement of traditional calcareous material by SG revealed a positive influence on the properties and microstructure of wall tiles [60]. | Laboratory |
| Ceramic building materials (soil-cement bricks) | SG can replace up to 20 wt % Portland cement in soil-cement bricks [33]. | Laboratory |
| Geotechnical | ||
| Road pavement construction | Incorporation as aggregates in bituminous mixtures revealed a good performance and it might be directly tested industrially [36]. Mixtures of GLD and SG as a natural filler and fine aggregates in bituminous mixtures have the possibility to be scaled up with success [61]. | Laboratory |
| Environmental | ||
| Acid mine drainage | Metal removal and neutralization of acid mine drainage (AMD) in treatment systems can be controlled by the addition of alkaline SG [62,63]. | Laboratory |
| Landfill cover | A mixture of GLD (70%) and SG (30%) was used as covering material. COD removal in the leachates (91%) after 15 months suggest no impact on the microorganisms responsible for the stabilization of the organic fraction of the MSW [27]. | Industrial |
| Agricultural | ||
| Soil amendment and fertilizer | A mixture of SG and green liquor sludge (7 t/ha) buffered soil acidity [38]. 0.96 tons of SG would be required to replace 1 ton of a commercial ground limestone product [63]. | Laboratory |
| Application | Highlights | Scale |
|---|---|---|
| Construction materials | ||
| Geopolymer mortars | Efficient reuse as aggregates with the best mechanical resistance achieved using a binder-aggregate ratio of 1 to 5, belonging to at least class M10 (UCS ≥10 MPa) [5]. Mortars prepared with belite-based cement (CEM II A-L) using 16 wt % of LM showed properties that fulfill the requirements of indoor and outdoor plasters [45]. | Laboratory |
| Cement | A belite-based cement (CEM II A-L) was prepared with 80 wt % of F4 clinker, 16 wt % of LM and 4 wt % gypsum [45]. | Laboratory |
| Clinker | Belitic and Portland clinkers were obtained using only mixtures of biomass sludge, LM and BFA from the pulp and paper process [45]. | Laboratory |
| Anorthite ceramics | Anorthite was the major phase in formulations containing 36 wt % LM and 64 wt % BFA synthesized at 1100 °C [26]. | Laboratory |
| Bricks | A mixture of soil:LM (80:20wt %) achieved a compressive strength that satisfies the requirements of International standard codes for the production of burnt bricks [66]. | Laboratory |
| Geotechnical | ||
| Landfill cover | LM showed strong potential for replacing soils as intermediate covering in municipal solid waste landfill [27]. | Industrial |
| Environmental | ||
| Phosphorous removal | LM was used to synthesize Ca(OH)2 nanoparticles (CNP). P removal from aqueous solutions after 10 min was 53% for a CNP/P mass ratio of 2.2 [67]. | Laboratory |
| Desulfurization of flue gas | LM performed better than commercial limestone in the same conditions, due to higher specific surface area and near-optimal pore size [68]. | Laboratory |
| SO2 sorbent | The CaO derived from LM achieves the maximum sulfation conversion of 83% at about 940 °C which is 1.7 times higher than that derived from limestone at about 880 °C [68]. | Laboratory |
| Agricultural | ||
| Fertilizer and soil amendment | LM (7.5 t/ha) buffered soil acidity, increased pH, doubled the base saturation, and reduced exchangeable acidity [28,38]. | Laboratory |
| Liming agent | The soil amendment shows promising results in the replacement of commercial liming materials (neutralizing value 38.3% Ca eq, d.w.) [69]. | Laboratory |
| Others | ||
| Catalyst | LM doped with potassium fluoride was used as a transesterification catalyst. Oil conversion of 99.09% was achieved with 5 wt % catalyst, methanol/oil molar ratio = 12, t = 2 h and T = 64 °C [70]. | Laboratory |
| Sorbent | LM with the addition of a cationic polymer adsorbed 134.1 mg/g of lignocelluloses from pre-hydrolysis wood chip liquor [71]. | Laboratory |
| Application | Highlights | Scale |
|---|---|---|
| Construction materials | ||
| Geopolymer concrete | Replacement up to 10% of the cement in concrete is feasible without virtually affecting the mechanical properties [23]. BFA has been successfully used with a silicon and aluminum oxides constitution of 80 wt %, and the Si-to-Al ratio of 2 [76]. | Laboratory/Industrial |
| Geopolymer mortars | BFA was used as the main source of aluminosilicate in the binder precursor (70 wt % substitution to metakaolin), comprising a binder-aggregate ratio of 1 to 5. All tested formulations belong at least to class M10 (UCS ≥10 MPa) [5]. BFA up to 75 wt % were used as aluminosilicate source [29]. | Laboratory |
| Roller-compacted concrete | Mixtures containing 10–20% BFA + 50% BBA (boiler bottom ash) with a water-to-binder ratio of 0.35–0.37 were used for the construction of a storage slab (area = 792 m2, thickness = 0.3 m) [77]. | Industrial |
| Rammed-earth construction | BFA activated with a sodium-based solution was used to enhance residual granitic soils properties for earth wall molds construction [78]. | Laboratory |
| Clinker production | Belitic and Portland clinkers were obtained. The chlorides present in the FA do not affect the durability of the clinker since most are eliminated during the thermal treatment [45]. | Laboratory |
| Anorthite ceramics | Formulations of 36 wt % LM and 64 wt % BFA produced anorthite with lightweight, high water absorption and good chemical stability [26]. | Laboratory |
| Alkali-activated bricks | Alkali-activated bricks can be produced using BFA with similar costs as the clay fired brick but with reduced environmental impact [79]. | Laboratory |
| Geotechnical | ||
| Filling mine cavities | The fly ash originated from Stora Enso mills in Finland has been used as a hardener in filling mine cavities since 2003 [53]. | Industrial |
| Stabilization of roads | Improved road performance can be achieved by mixing existing unbound road bases with BFA without any other additives [80]. | Industrial |
| Pavement construction | A mixture of soil and wood FA (10 wt %) showed to be a valuable material for hydraulically bound mixtures [81]. Alkali-activated low-calcium FA was successfully used as a binder for soil stabilization in road platforms [82]. | Laboratory |
| Environmental | ||
| Remediation of soils degraded by mining activities | Granules of 90 wt % BFA and 10 wt % biological sludge improved soil quality (pH correction and extractable P and K), but were not able to support permanent plant cover [43]. | Laboratory |
| Adsorbent | Pb and Co removal from paint industries effluent reached 96.1% and 99% at pH 2 with a contact time of 3 h and 100 g/L wood ash [83]. BFA dosage of 160 g/L reduced COD by 37% from real industrial wastewater [84]. | Laboratory |
| Agricultural | ||
| Liming material | The soil extractable K and P increased, indicating that besides the liming effect BFA can also contribute to improving soil fertility [25]. | Laboratory |
| Soil amendment and fertilizer | Ash levels up to 2 wt % (40 t/ha), heat biomass increased more than with the control soil [40]. FA can be a potential soil fertilizer regarding some nutrient deficiencies [32,38]. | Laboratory |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quina, M.J.; Pinheiro, C.T. Inorganic Waste Generated in Kraft Pulp Mills: The Transition from Landfill to Industrial Applications. Appl. Sci. 2020, 10, 2317. https://doi.org/10.3390/app10072317
Quina MJ, Pinheiro CT. Inorganic Waste Generated in Kraft Pulp Mills: The Transition from Landfill to Industrial Applications. Applied Sciences. 2020; 10(7):2317. https://doi.org/10.3390/app10072317
Chicago/Turabian StyleQuina, Margarida J., and Carolina T. Pinheiro. 2020. "Inorganic Waste Generated in Kraft Pulp Mills: The Transition from Landfill to Industrial Applications" Applied Sciences 10, no. 7: 2317. https://doi.org/10.3390/app10072317





