Antifouling Membranes Based on Cellulose Acetate (CA) Blended with Poly(acrylic acid) for Heavy Metal Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
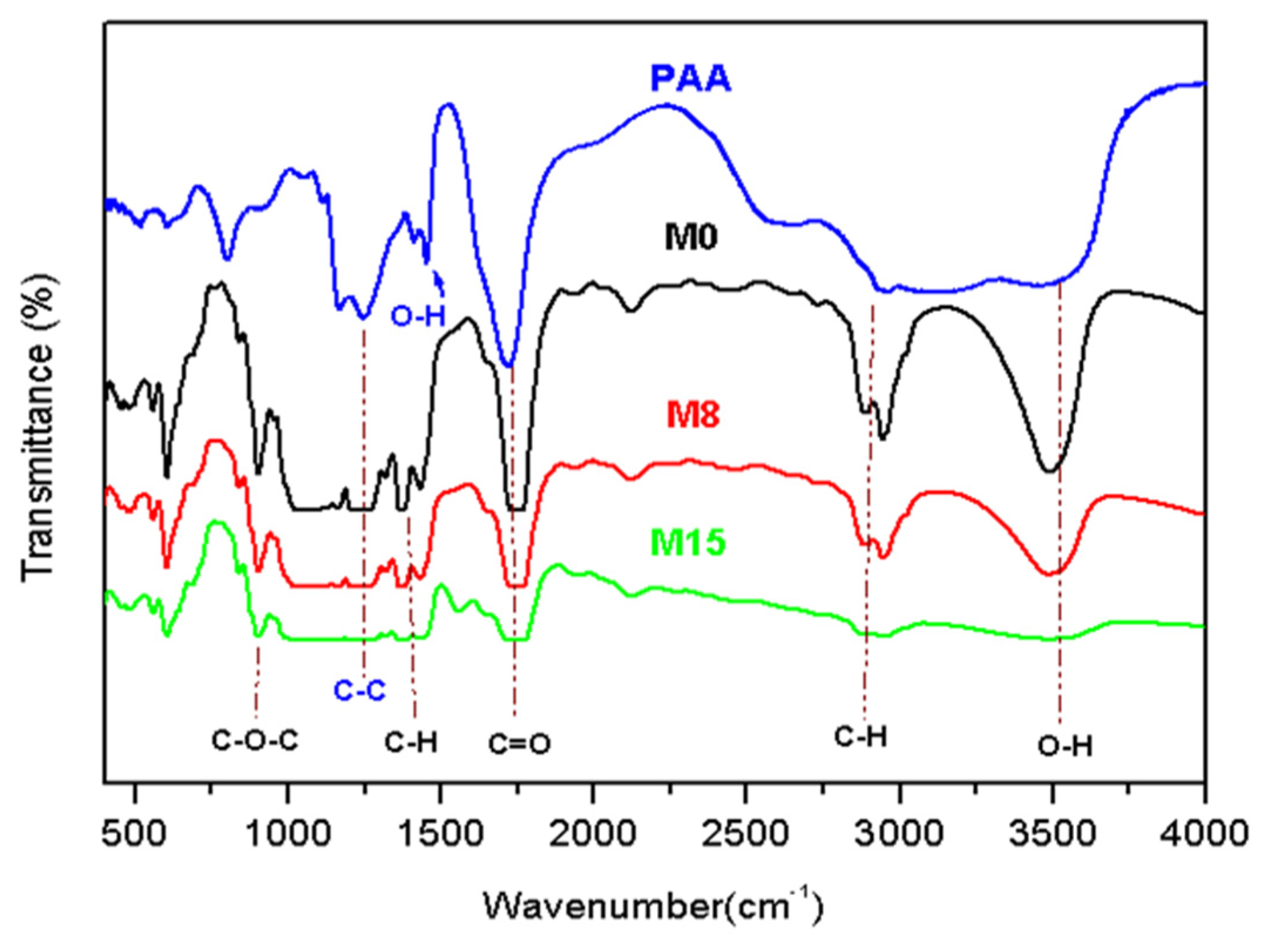
- Fourier Transform infrared spectroscopy (FTIR)
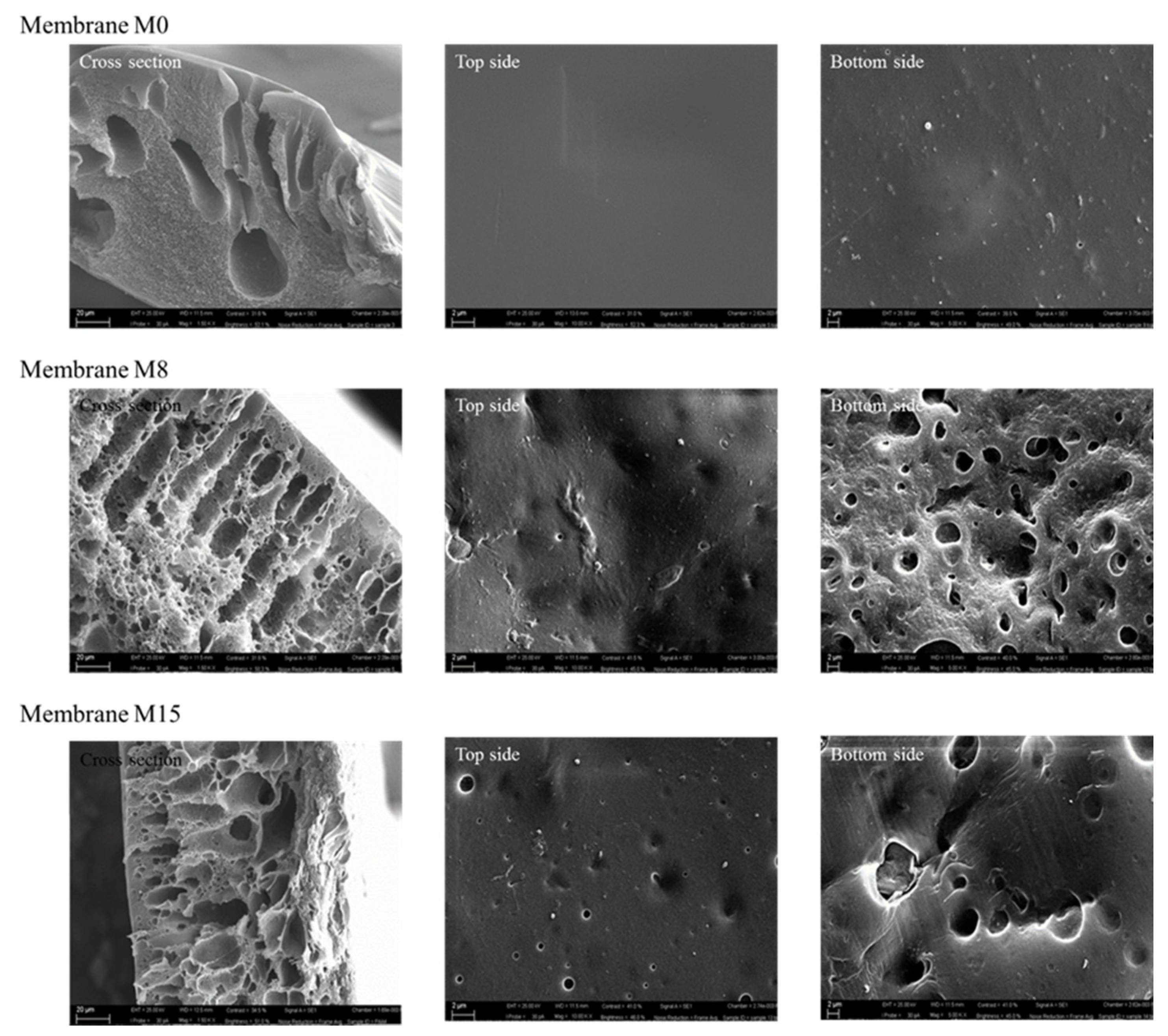
- Scanning Electron Microscopy (SEM)
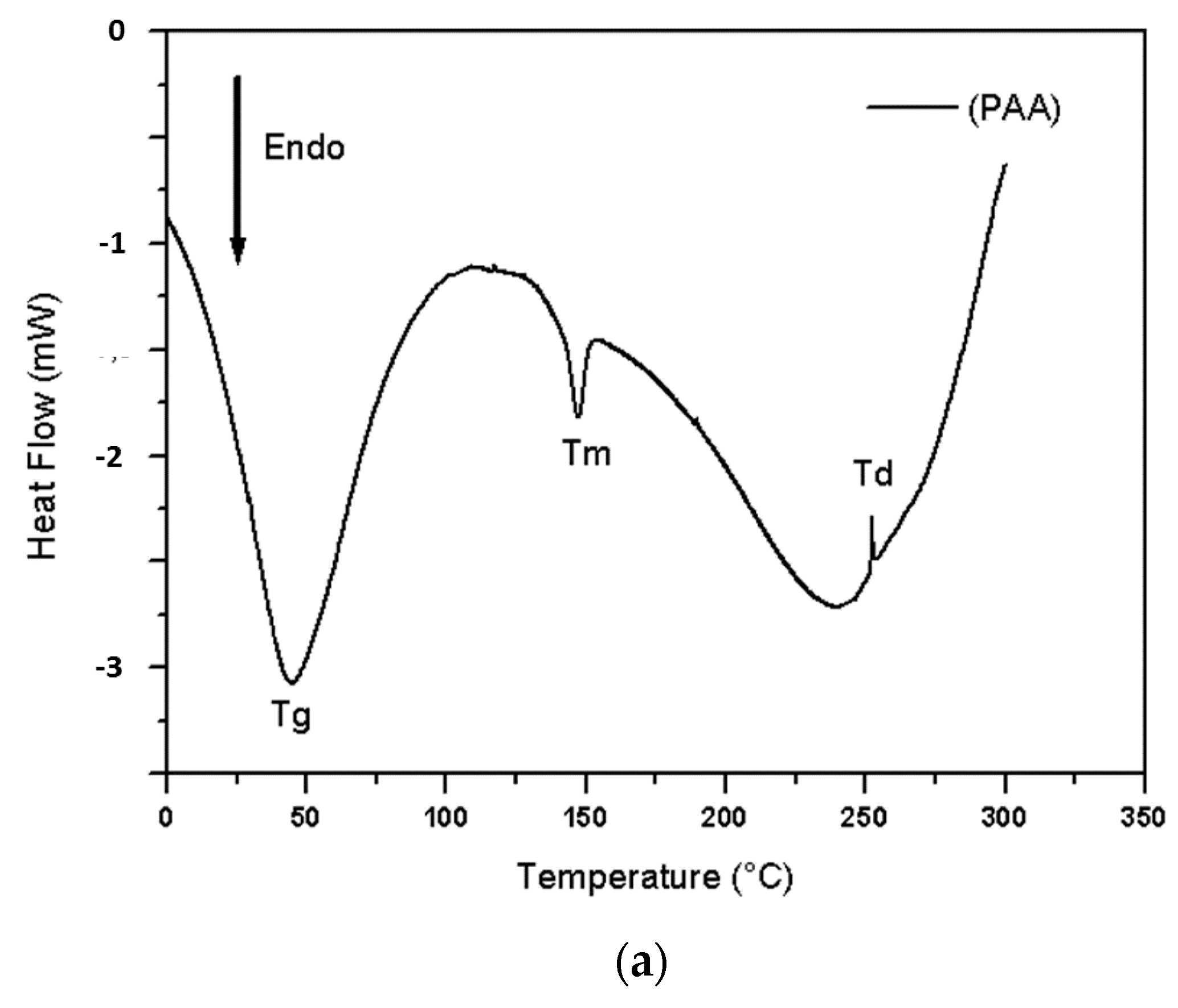
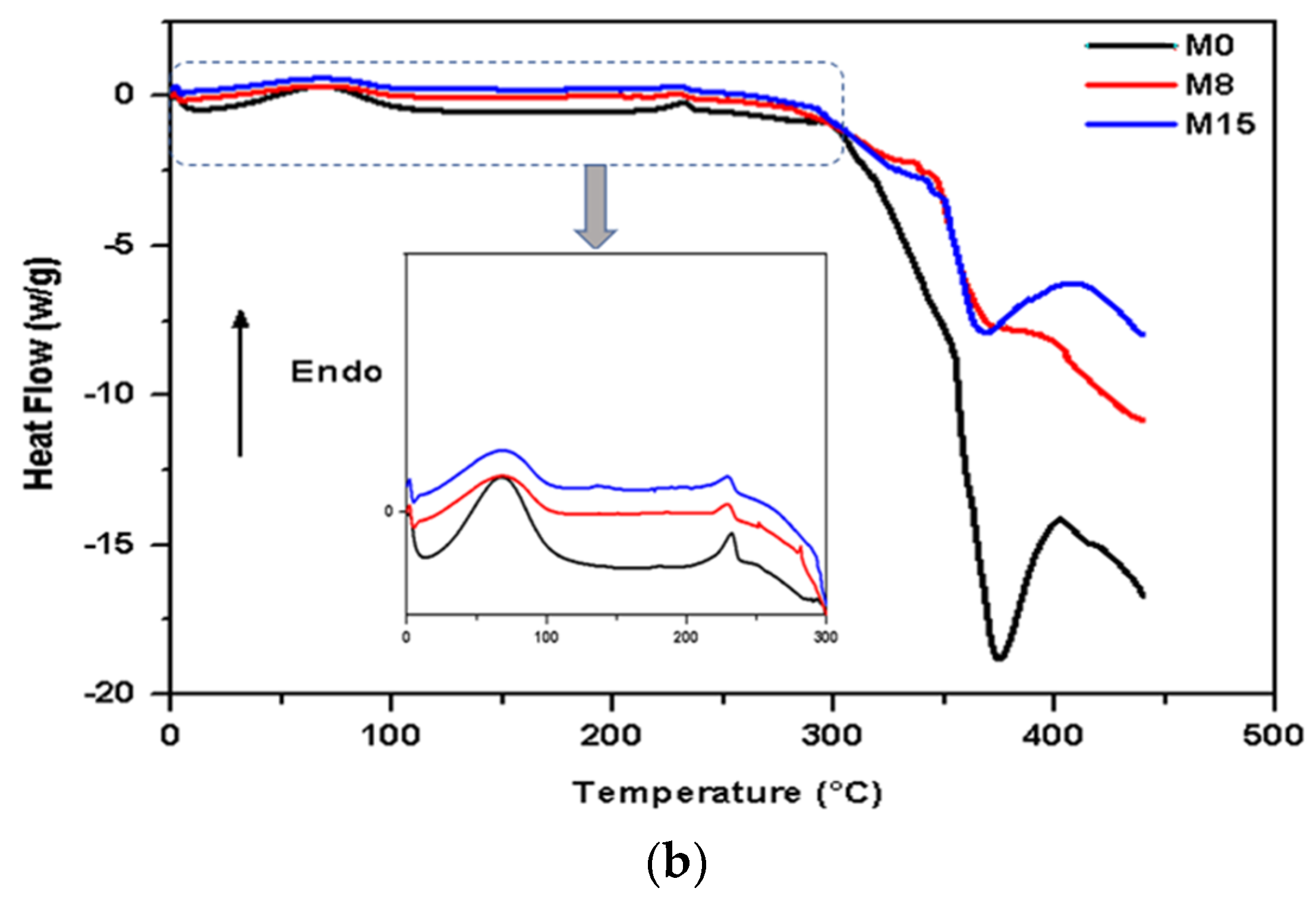
- Differential Scanning Calorimetry (DSC)
- Contact Angle
- Water Content (%)
- Porosity (%) and Pore Size
2.4. Pure Water Permeability and Anti-Fouling Tests
3. Results
3.1. Characterization of the Prepared Membranes
- FTIR and DSC Analysis
- SEM
- Porosity, Pore Size, Contact Angle, Water Content and Pure Water Permeability
3.2. Membrane Performance
- UF Experiments
- Anti-fouling Properties
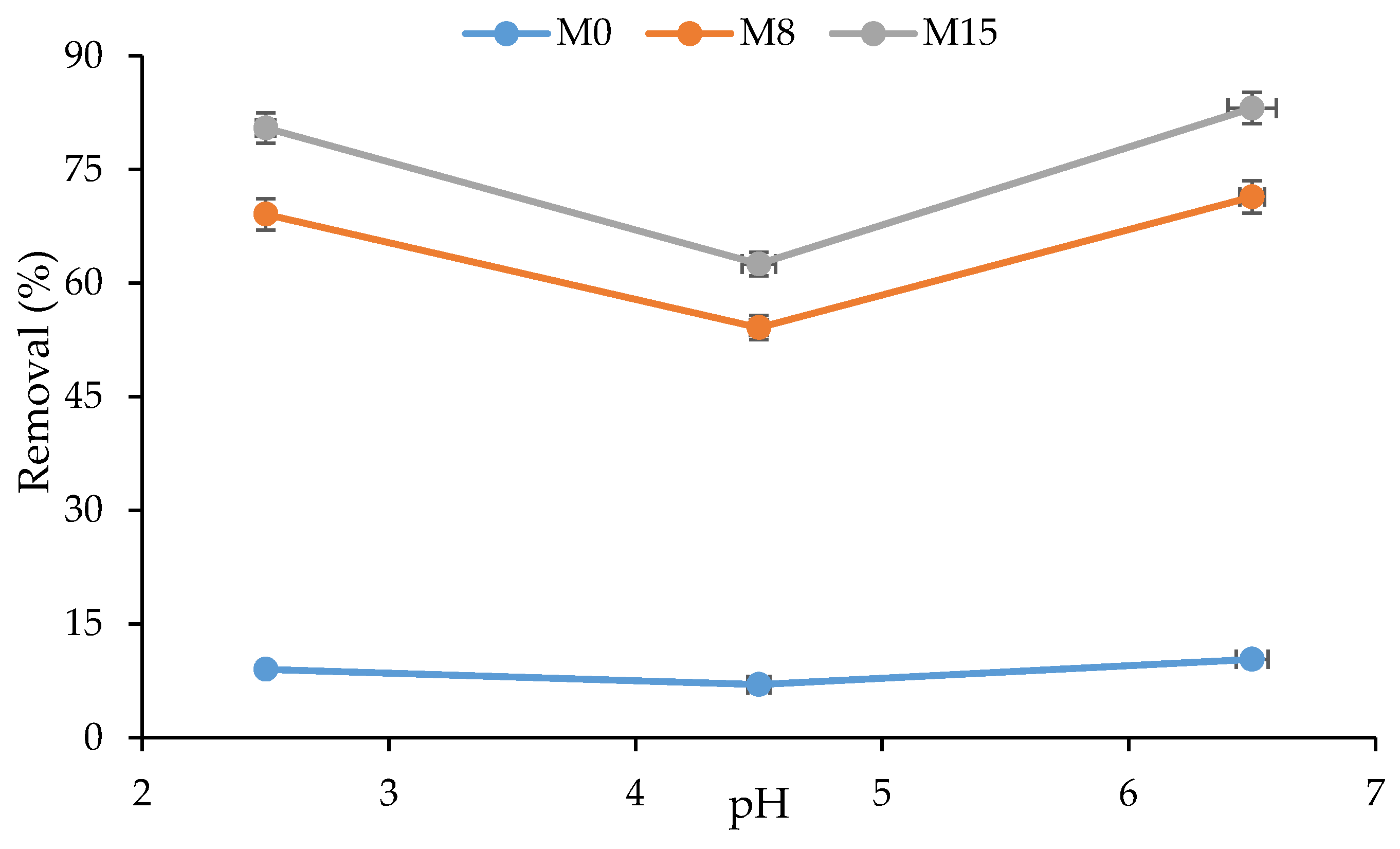
- Cadmium Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Fiksdal, L.; Leiknes, T. The effect of coagulation with MF/UF membrane filtration for the removal of virus in drinking water. J. Membr. Sci. 2006, 279, 364–371. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Apel, Y.P.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Cassano, A.; Drioli, E.; Molinari, R.; Bertolutti, C. Quality improvement of recycled chromium in the tanning operation by membrane processes. Desalination 1997, 108, 193–203. [Google Scholar] [CrossRef]
- Chaufer, B.; Deratani, A. Removal of metal ions by complexation-ultrafiltration using water-soluble macromolecules: Perspective of application to wastewater treatment. Nucl. Chem. Waste Manag. 1988, 8, 175–187. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, X. Polymer-enhanced ultrafiltration: Fundamentals, applications and recent developments. J. Membr. Sci. 2019, 586, 53–83. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Cheng, Y.-H.; Paul, D.R.; Field, R.W.; Freeman, B.D. Fouling mechanisms in constant flux crossflow ultrafiltration. J. Membr. Sci. 2019, 574, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Ontiveros, M.A.; Quintero, Y.; Llanquilef, A.; Morel, M.; Martínez, L.A.; García, A.G.; Garcia, A. Anti-Biofouling and Desalination Properties of Thin Film Composite Reverse Osmosis Membranes Modified with Copper and Iron Nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Zhang, S.; Luan, J.; Mu, Y.; Du, Y.; Wang, G. Fabrication of ultrafiltration membranes with enhanced antifouling capability and stable mechanical properties via the strategies of blending and crosslinking. J. Membr. Sci. 2017, 539, 116–127. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, J.; Xu, X.; Zhang, G.; Yang, Y.; Yang, F. Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J. Colloid Interface Sci. 2017, 505, 341–351. [Google Scholar] [CrossRef]
- Nishigochi, S.; Ishigami, T.; Maruyama, T.; Hao, Y.; Ohmukai, Y.; Iwasaki, Y.; Matsuyama, H. Improvement of Antifouling Properties of Polyvinylidene Fluoride Hollow Fiber Membranes by Simple Dip Coating of Phosphorylcholine Copolymer via Hydrophobic Interactions. Ind. Eng. Chem. Res. 2014, 53, 2491–2497. [Google Scholar] [CrossRef]
- Huner, I.D.; Gulec, H.A. Fouling behavior of poly(ether)sulfone ultrafiltration membrane during concentration of whey proteins: Effect of hydrophilic modification using atmospheric pressure argon jet plasma. Colloids Surf. B Biointerfaces 2017, 160, 510–519. [Google Scholar] [CrossRef]
- Santoro, S.; Drioli, E.; Figoli, A. Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes. Coatings 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Guo, H.; He, H.; Liu, Y.; Li, X.; Zhang, Y.; Yin, H.; Volkov, A.V.; He, T. Unprecedented scaling/fouling resistance of omniphobic polyvinylidene fluoride membrane with silica nanoparticle coated micropillars in direct contact membrane distillation. J. Membr. Sci. 2020, 599, 117819. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Liu, B.; Chen, C.; Wang, S.; Crittenden, J.C. Blended PVC/PVC-g-PEGMA ultrafiltration membranes with enhanced performance and antifouling properties. Appl. Surf. Sci. 2018, 455, 987–996. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Chen, M.; Ma, J.; Chen, S.; Wu, Z. Membrane biofouling control using polyvinylidene fluoride membrane blended with quaternary ammonium compound assembled on carbon material. J. Membr. Sci. 2017, 539, 229–237. [Google Scholar] [CrossRef]
- Bussi, Y.; Golan, S.; Dosoretz, C.G.; Eisen, M.S. Synthesis, characterization and performance of polystyrene/PMMA blend membranes for potential water treatment. Desalination 2018, 431, 35–46. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Z.; Dong, Z.; Ren, P.; Li, Y.; Liu, X. Fabrication and characterization of an ion-imprinted membrane via blending poly(methyl methacrylate-co-2-hydroxyethyl methacrylate) with polyvinylidene fluoride for selective adsorption of Ru(III). React. Funct. Polym. 2017, 115, 1–9. [Google Scholar] [CrossRef]
- Park, S.W.; Bediako, J.K.; Song, M.H.; Choi, J.W.; Lee, H.C.; Yun, Y.S. Facile fabrication of polyacrylic acid-polyvinyl chloride composite adsorbents for the treatment of cadmium-contaminated wastewater. J. Environ. Chem. Eng. 2018, 6, 2401–2408. [Google Scholar] [CrossRef]
- Hu, D.; Lian, Z.; Xian, H.; Jiang, R.; Wang, N.; Weng, Y.; Peng, X.; Wang, S.; Ouyang, X. Adsorption of Pb(II) from aqueous solution by polyacrylic acid grafted magnetic chitosan nanocomposite. Int. J. Biol. Macromol. 2020, 154, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Kobayashi, T. Self-assembly functionalized membranes with chitosan microsphere/polyacrylic acid layers and its application for metal ion removal. J. Mater. Sci. 2010, 45, 6694–6700. [Google Scholar] [CrossRef]
- Ounifi, I.; Saidi, N.; Kahloul, M.; Sealeyd, K.S.; Hafiane, A.; Ferjani, E. Synthesis and characterization of ultrafiltration membranes by phase inversion and by uropathogenic Escherichia coli retention performance. Desalin. Water Treat. 2019, 163, 109–117. [Google Scholar] [CrossRef]
- Suthabanditpong, W.; Takai, C.; Razavi-Khosroshahi, H.; Okada, Y.; El-Salmawy, M.S.; Fuji, M. Influence of CaCO3 pore-forming agent on porosity and thermal conductivity of cellulose acetate materials prepared by non-solvent induced phase separation. Adv. Powder Technol. 2019, 30, 207–213. [Google Scholar] [CrossRef]
- Santoro, S.; Sebastian, V.; Moro, A.J.; Portugal, C.A.M.; Lima, J.C.; Coelhoso, I.M.; Crespo, J.G.; Mallada, R. Development of fluorescent thermoresponsive nanoparticles for temperature monitoring on membrane surfaces. J. Colloid Interface Sci. 2017, 486, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Russo, F.; Bulzomì, M.; Di Nicolò, E.; Ursino, C.; Figoli, A. Enhanced Anti-Fouling Behavior and Performance of PES Membrane by UV Treatment. Processes 2021, 9, 246. [Google Scholar] [CrossRef]
- Ounifi, I.; Ursino, C.; Santoro, S.; Chekir, J.; Hafiane, A.; Figoli, A.; Ferjani, E. Cellulose Acetate Nanofiltration Membranes for Cadmium Remediation. J. Membr. Sci. Res. 2020, 6, 226–234. [Google Scholar]
- Guo, J.; Kim, J. Modifications of polyethersulfone membrane by doping sulfated-TiO2 nanoparticles for improving anti-fouling property in wastewater treatment. RSC Adv. 2017, 7, 33822–33828. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yue, X.; Zhang, S.; Ren, J.; Yang, L.; Wang, Q.; Wang, G. Synthesis of sulfonated polyphenylsulfone as candidates for antifouling ultrafiltration membrane. Sep. Purif. Technol. 2012, 98, 298–307. [Google Scholar] [CrossRef]
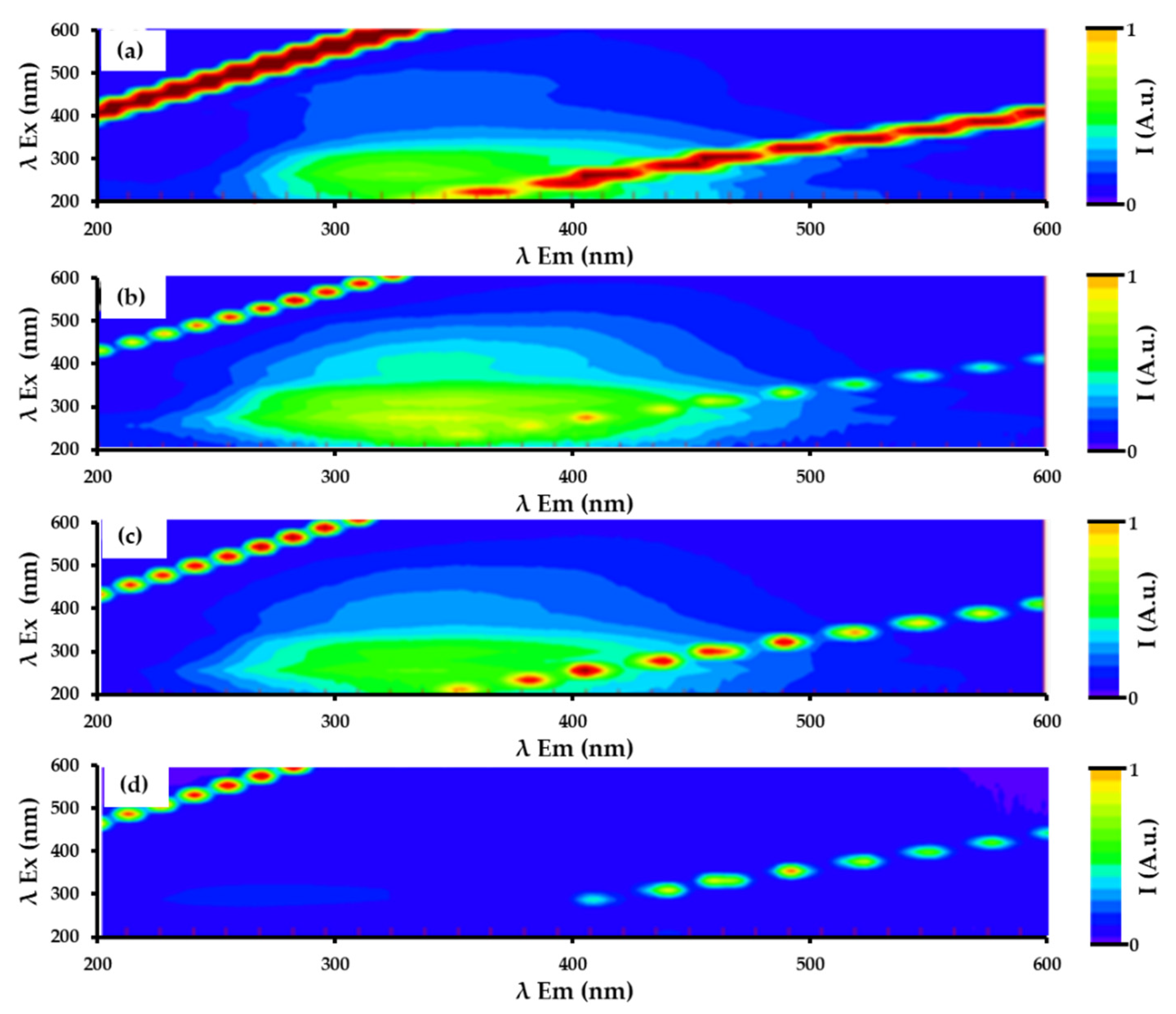
- Ramsay, H.; Simon, D.; Steele, E.; Hebert, A.; Oleschuk, R.D.; Stamplecoskie, K.G. The power of fluorescence excitation–emission matrix (EEM) spectroscopy in the identification and characterization of complex mixtures of fluorescent silver clusters. RSC Adv. 2018, 8, 42080–42086. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Waheed, S.; Khan, S.M.; e-Gul, S.; Shafiq, M.; Farooq, M.; Sanaullah, K.; Jamil, T. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. Desalination 2015, 355, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R. Preparation of chitosan/cellulose acetate blend hollow fibers for adsorptive performance. J. Memb. Sci. 2005, 267, 68–77. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 320631. [Google Scholar] [CrossRef] [Green Version]
- Defieuw, G.; Groeninckx, G.; Reynaers, H. Miscibility and morphology of binary polymer blends of polycaprolactone with solution-chlorinated polyethylenes. Polymer 1989, 30, 595–603. [Google Scholar] [CrossRef]
- Bragança, F.C.; Rosa, D.S. Thermal, mechanical and morphological analysis of poly(ε-caprolactone), cellulose acetate and their blends. Polym. Adv. Technol. 2003, 14, 669–675. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [Green Version]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 43, 212–220. [Google Scholar] [CrossRef]
- Wei, Q.; Li, J.; Qian, B.; Fang, B.; Zhao, C. Preparation, characterization and application of functional polyethersulfone membranes blended with poly(acrylic acid) gels. J. Membr. Sci. 2009, 337, 266–273. [Google Scholar] [CrossRef]
- Sierra, M.M.D.; Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J. Fluorescence fingerprint of fulvic and humic acids from varied origins as viewed by single-scan and excitation/emission matrix techniques. Chemosphere 2005, 58, 715–733. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic acid fouling during microfiltration. J. Membr. Sci. 1999, 157, 1–12. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.G.; Karabelas, A.J. A study of ultrafiltration membrane fouling by humic acids and flux recovery by backwashing: Experiments and modeling. J. Membr. Sci. 2005, 266, 40–50. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Huang, T.; Dong, C.; Li, J.; Zhao, B.; Zhang, S. Chemical Cleaning of Ultrafiltration Membrane Fouled by Humic Substances: Comparison between Hydrogen Peroxide and Sodium Hypochlorite. Int. J. Environ. Res. Public Health 2019, 16, 2568. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.R.; Mukherjee, M.; De, S. Preparation, characterization and humic acid removal capacity of chitosan coated iron-oxide- polyacrylonitrile mixed matrix membrane. J. Water Process Eng. 2015, 6, 93–104. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Pang, W.Y.; Shafie, Z.M.H.M.; Zaulkiflee, N.D. PES/PVP/TiO2 mixed matrix hollow fiber membrane with antifouling properties for humic acid removal. J. Water Process Eng. 2019, 31, 100827–100835. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Sogbaike, C.E.; Ebhoaye, J.E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep. Purif. Technol. 2005, 44, 85–89. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Oztürk, D.; Rosales, M.; Diaz, D.I.; Mautner, A. Incorporation of CuO nanoparticles into thin-film composite reverse osmosis membranes (TFC-RO) for antibiofouling properties. Polym. Bull. 2018, 75, 2053–2069. [Google Scholar] [CrossRef]
- Lam, B.; Déon, S.; Morin-Crini, N.; Crini, G.; Fievet, P. Polymer-enhanced ultrafiltration for heavy metal removal: Influence of chitosan and carboxymethyl cellulose on filtration performances. J. Clean. Prod. 2018, 171, 927–933. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Childress, A.E. Zeta potential of commercial RO membranes: Influence of source water type and chemistry. Desalination 2001, 140, 87–95. [Google Scholar] [CrossRef]


| Membrane Name | Blend Composition | |
|---|---|---|
| CA wt. % | PAA wt. % | |
| M0 | 100 | 0 |
| M8 | 92 | 8 |
| M15 | 85 | 15 |
| Concentration [mg/L] | pH | Zeta Potential ζ [mV] | Particle Size in Suspension [nm] |
|---|---|---|---|
| 100 | 6 | −27.1 ± 0.01 | 532.7 ± 4.5 |
| Samples | Tg [°C] | Tm [°C] | Td [°C] |
|---|---|---|---|
| PAA | 44.5 | 147.23 | 252.78 |
| M0 | 63.71 | 231.80 | 369.40 |
| M8 | 65.30 | 227.47 | 362.68 |
| M15 | 64.12 | 226.55 | 361.01 |
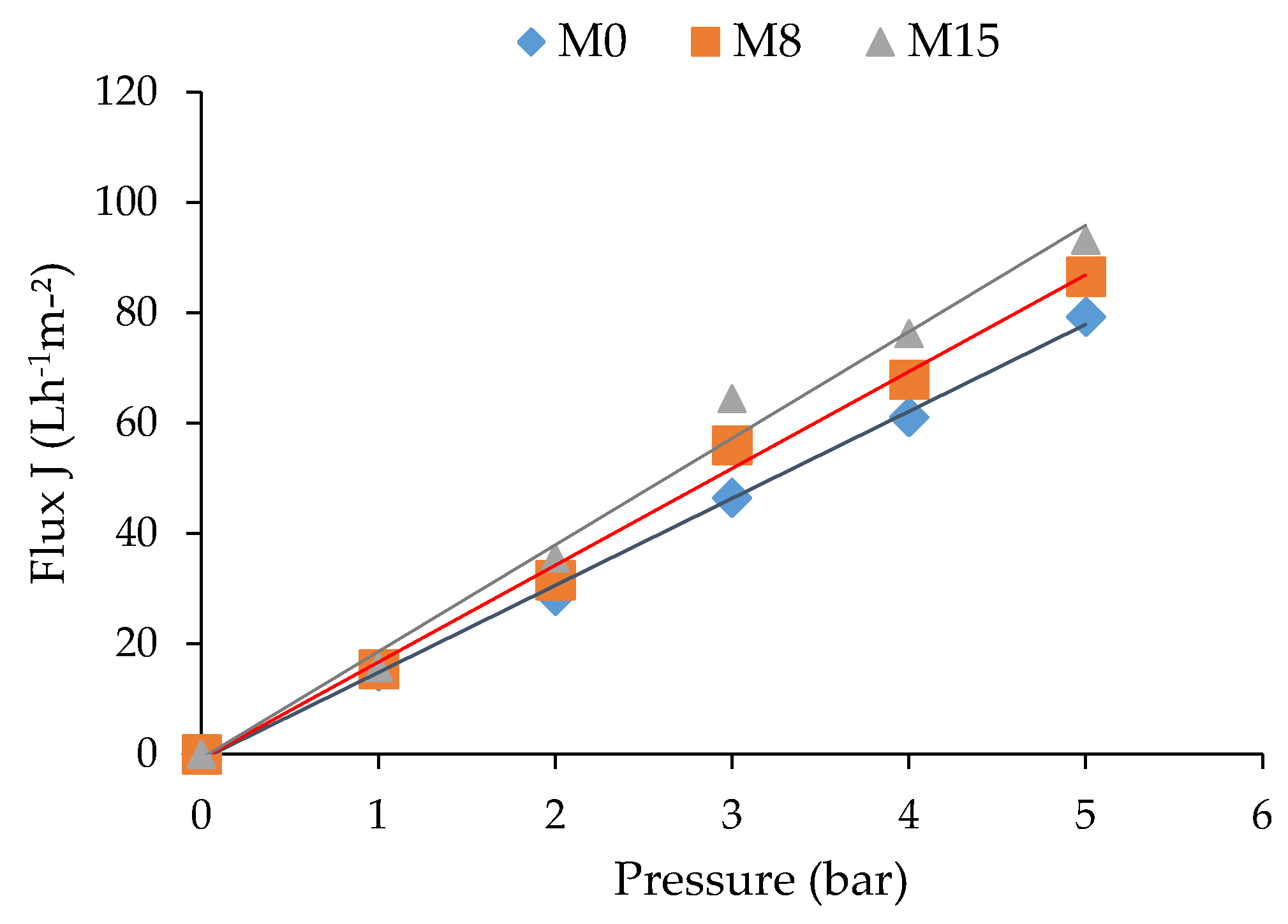
| Membranes | Porosity (%) | rm [nm] | Water Uptake (%) | Lp (L m−2 h−1 bar−1) |
|---|---|---|---|---|
| M0 | 44.58 ± 0.2 | 12.92 ± 0.02 | 50.64 ± 0.12 | 15.51 ± 1.2 |
| M8 | 64.50 ± 0.4 | 10.13 ± 0.02 | 69.87 ± 0.24 | 17.30 ± 1.4 |
| M15 | 75.65 ± 0.7 | 9.65 ± 0.03 | 78.23 ± 0.1 | 19.98 ± 0.5 |
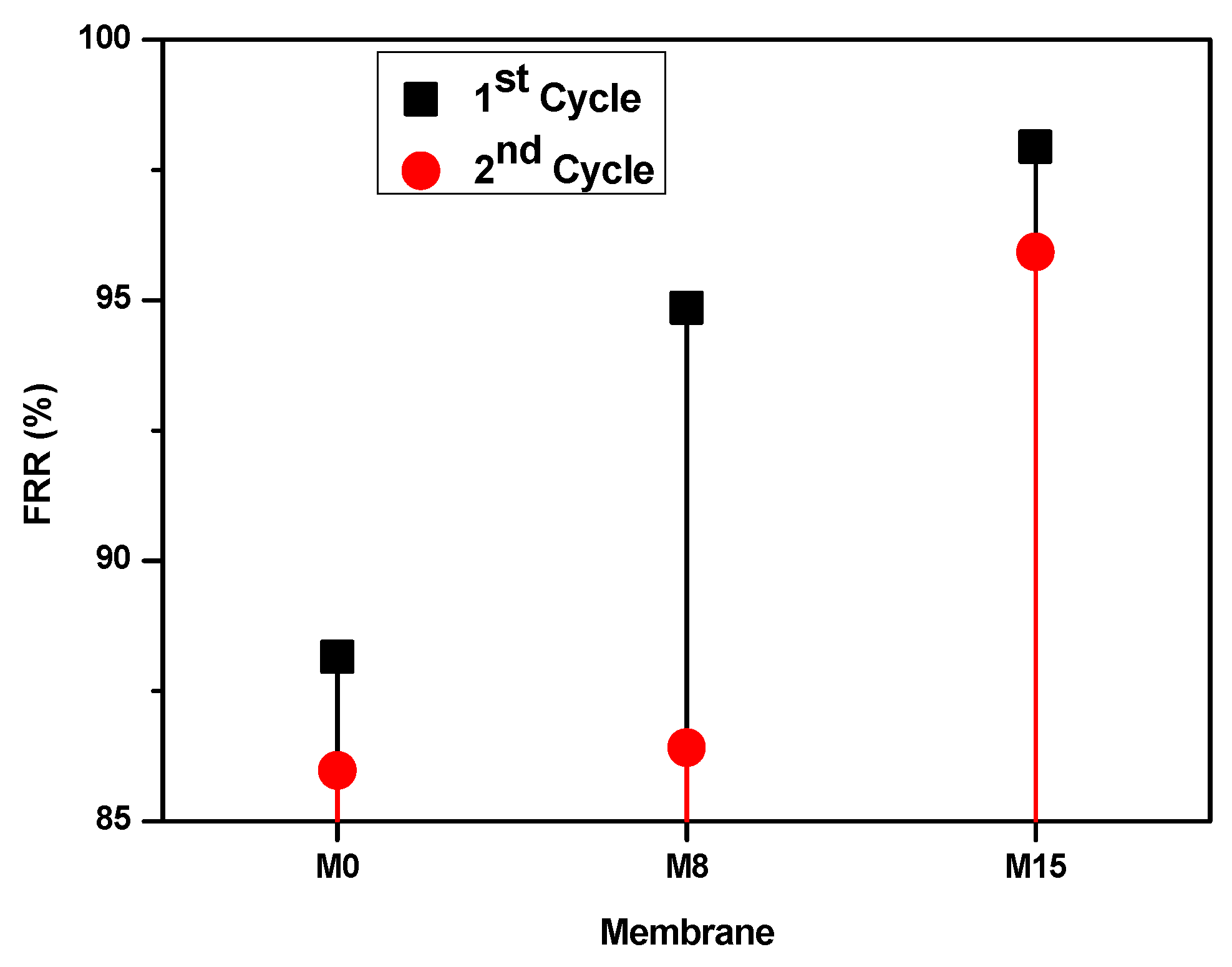
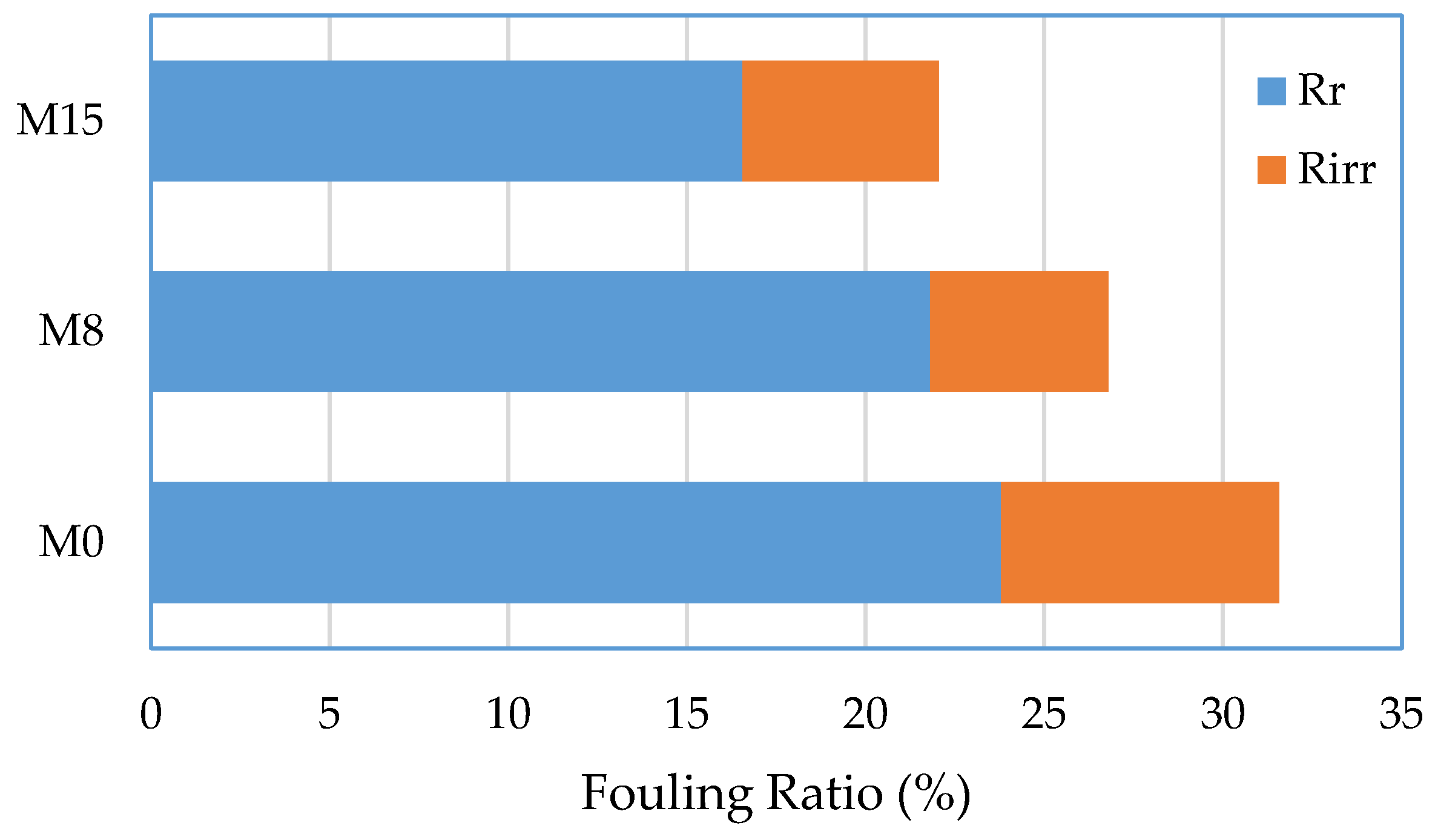
| Membrane | Pressure (bar) | Permeate Flux (L/m2·h) | HA Rejection (%) | FRR (%) | Reference |
|---|---|---|---|---|---|
| PAN/CS/Fe3O4 | 5.5 | 25.5 | 96.5 | - | [49] |
| PES/PVP/TiO2 | 1 | 21.927 | 92.96 | 99.03 | [50] |
| CA/OMTT | 3 | 160 | 95.4 | - | [51] |
| RC/SA | 1 | - | 91 | 67 | [52] |
| M0 | 4 | 61.12 | 95.7 | 88.16 | This work |
| M15 | 4 | 76.29 | 99.9 | 97.65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ounifi, I.; Guesmi, Y.; Ursino, C.; Santoro, S.; Mahfoudhi, S.; Figoli, A.; Ferjanie, E.; Hafiane, A. Antifouling Membranes Based on Cellulose Acetate (CA) Blended with Poly(acrylic acid) for Heavy Metal Remediation. Appl. Sci. 2021, 11, 4354. https://doi.org/10.3390/app11104354
Ounifi I, Guesmi Y, Ursino C, Santoro S, Mahfoudhi S, Figoli A, Ferjanie E, Hafiane A. Antifouling Membranes Based on Cellulose Acetate (CA) Blended with Poly(acrylic acid) for Heavy Metal Remediation. Applied Sciences. 2021; 11(10):4354. https://doi.org/10.3390/app11104354
Chicago/Turabian StyleOunifi, Ibtissem, Youssef Guesmi, Claudia Ursino, Sergio Santoro, Selim Mahfoudhi, Alberto Figoli, Ezzedin Ferjanie, and Amor Hafiane. 2021. "Antifouling Membranes Based on Cellulose Acetate (CA) Blended with Poly(acrylic acid) for Heavy Metal Remediation" Applied Sciences 11, no. 10: 4354. https://doi.org/10.3390/app11104354






