Y2O3:Eu3+ Nanophosphor-Coated Mica or TiO2/Mica as Red-Emitting Pearl Pigment: Coating Factors, Luminescent and Gloss Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Y2O3:Eu3+ Coated on MF or TMF
2.3. Preparation of the Film of Y2O3:Eu@MF and Y2O3:Eu@TMF
2.4. Characterization
3. Results
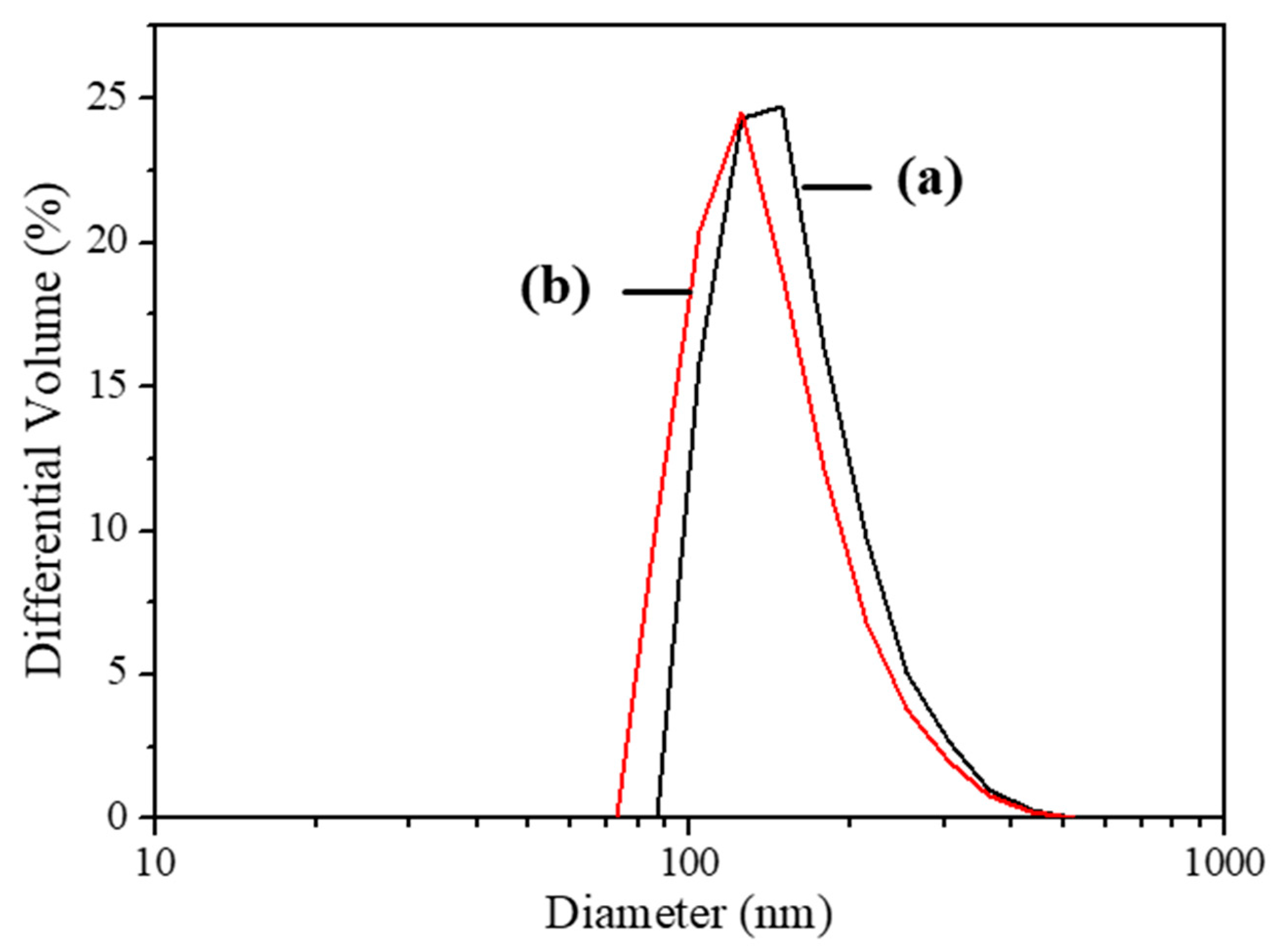
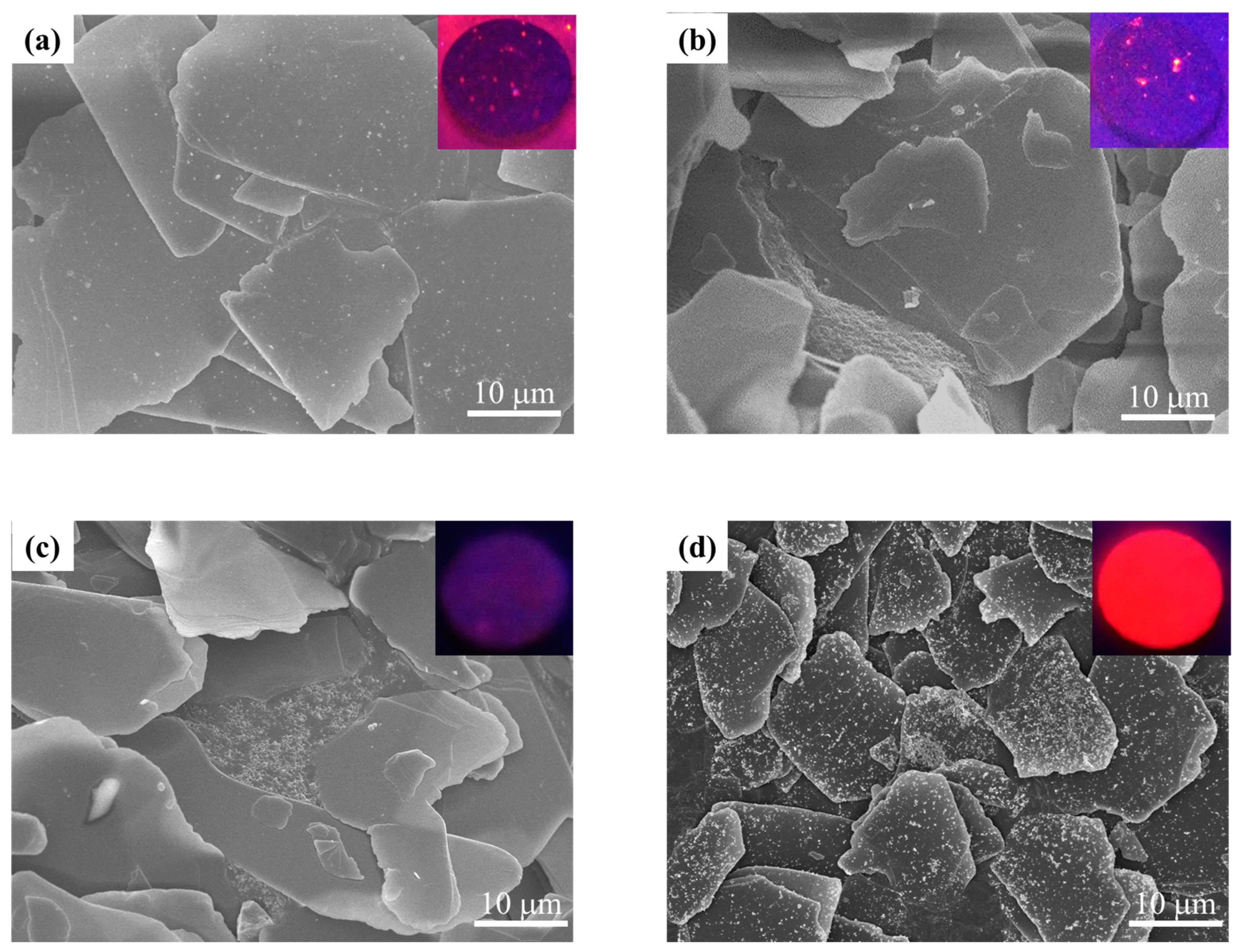
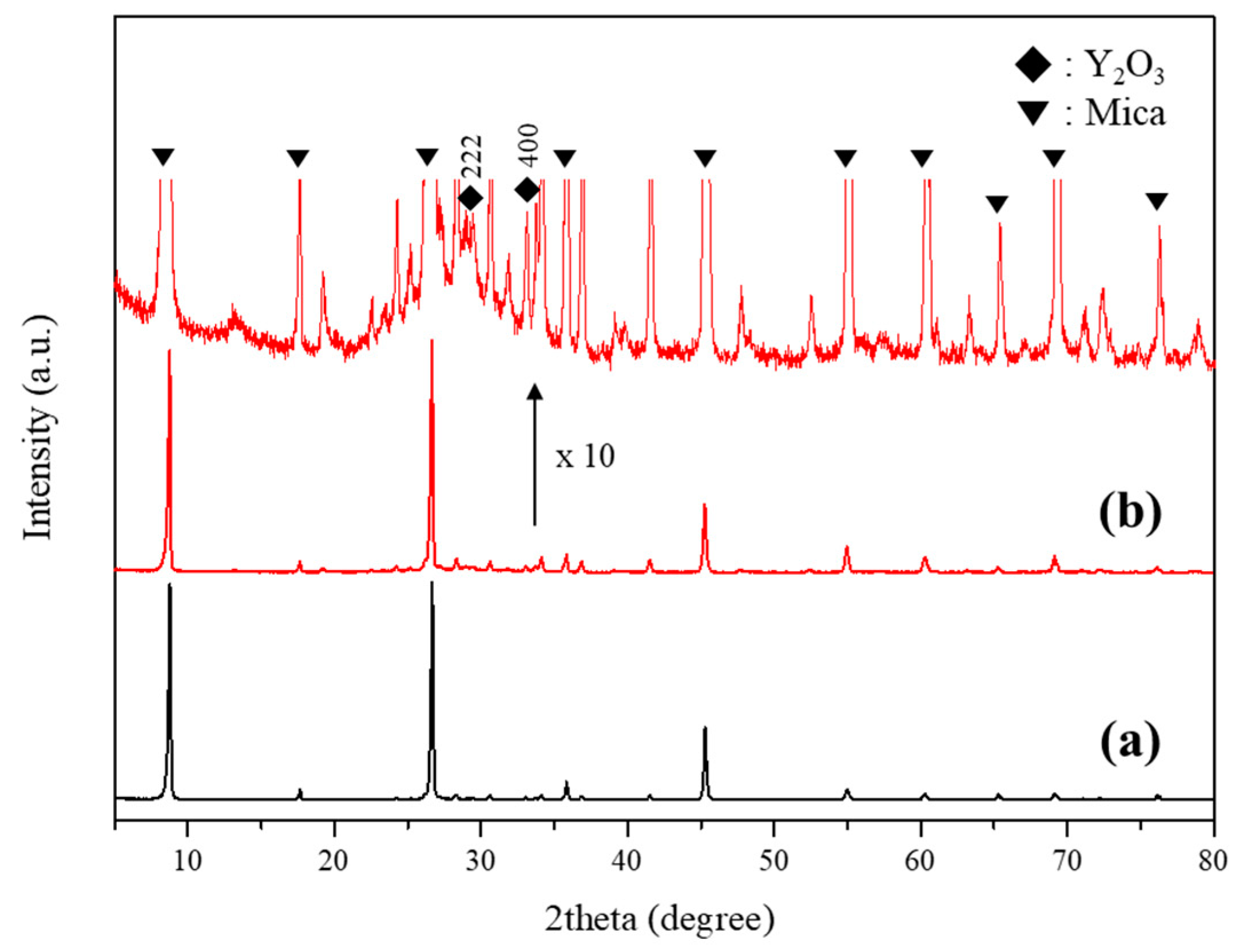
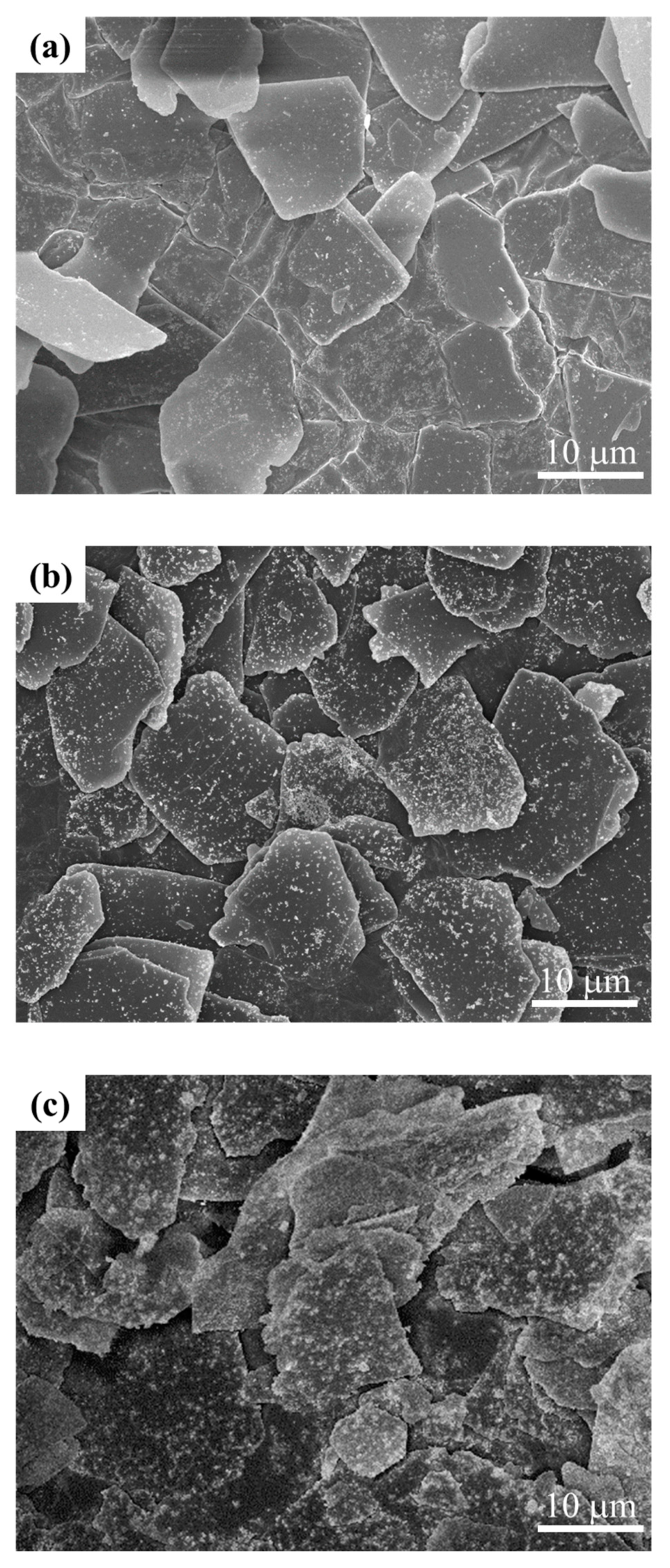
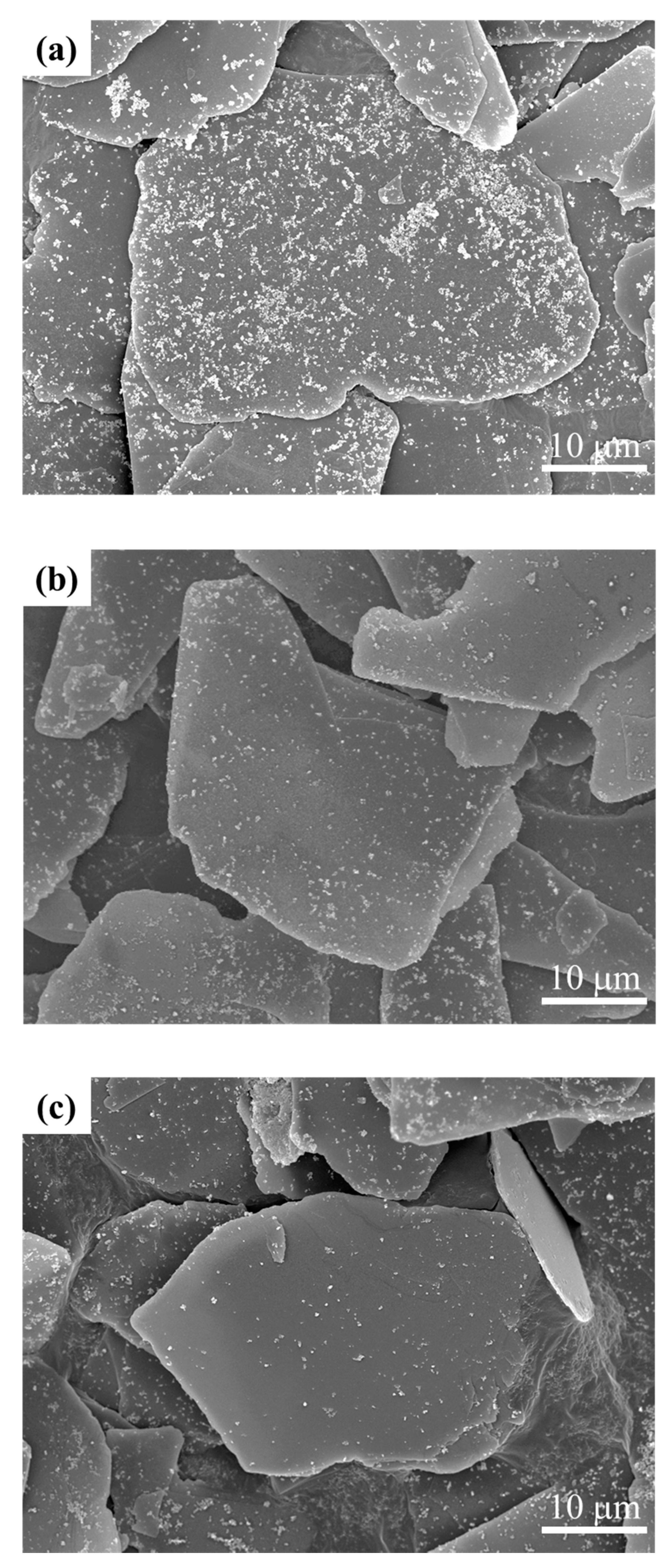
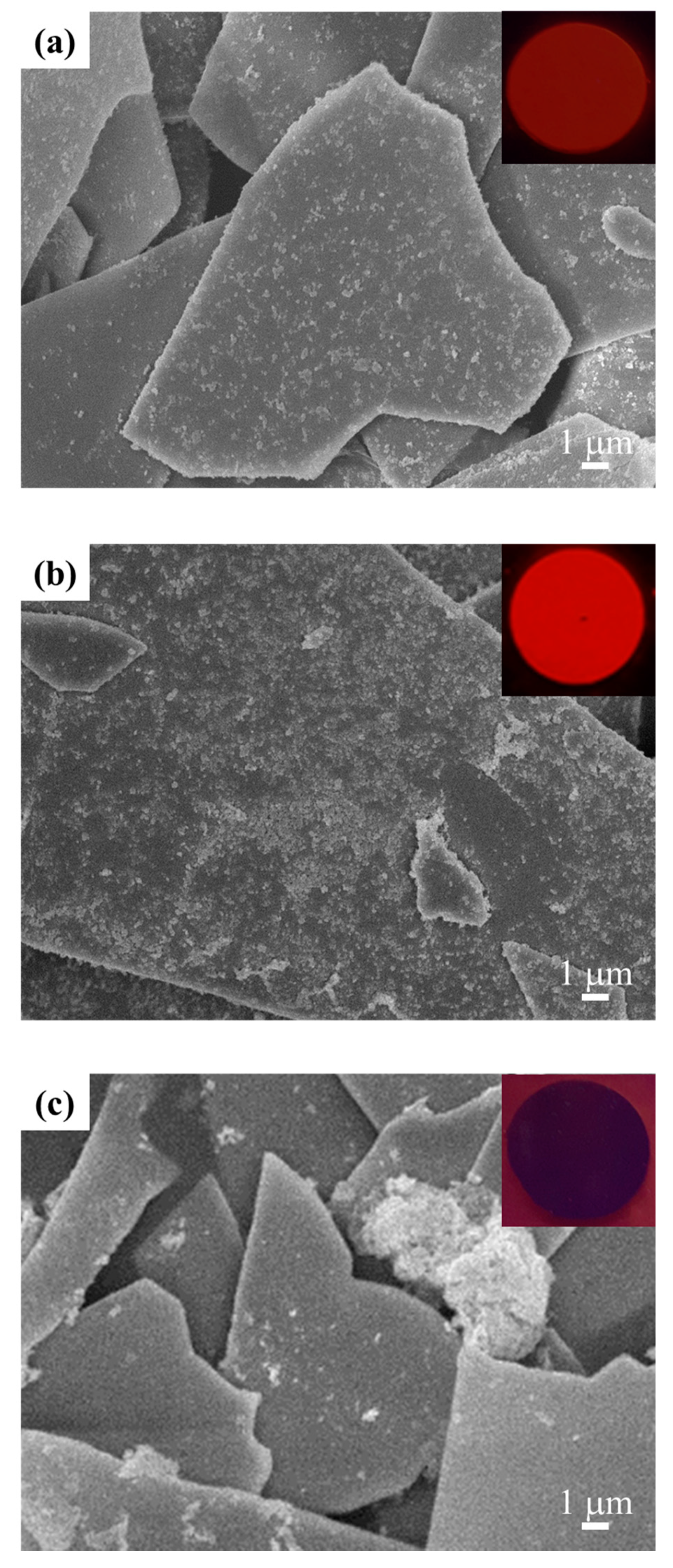
3.1. Y2O3:Eu@MF Prepared by the Variation in pH and Solvents
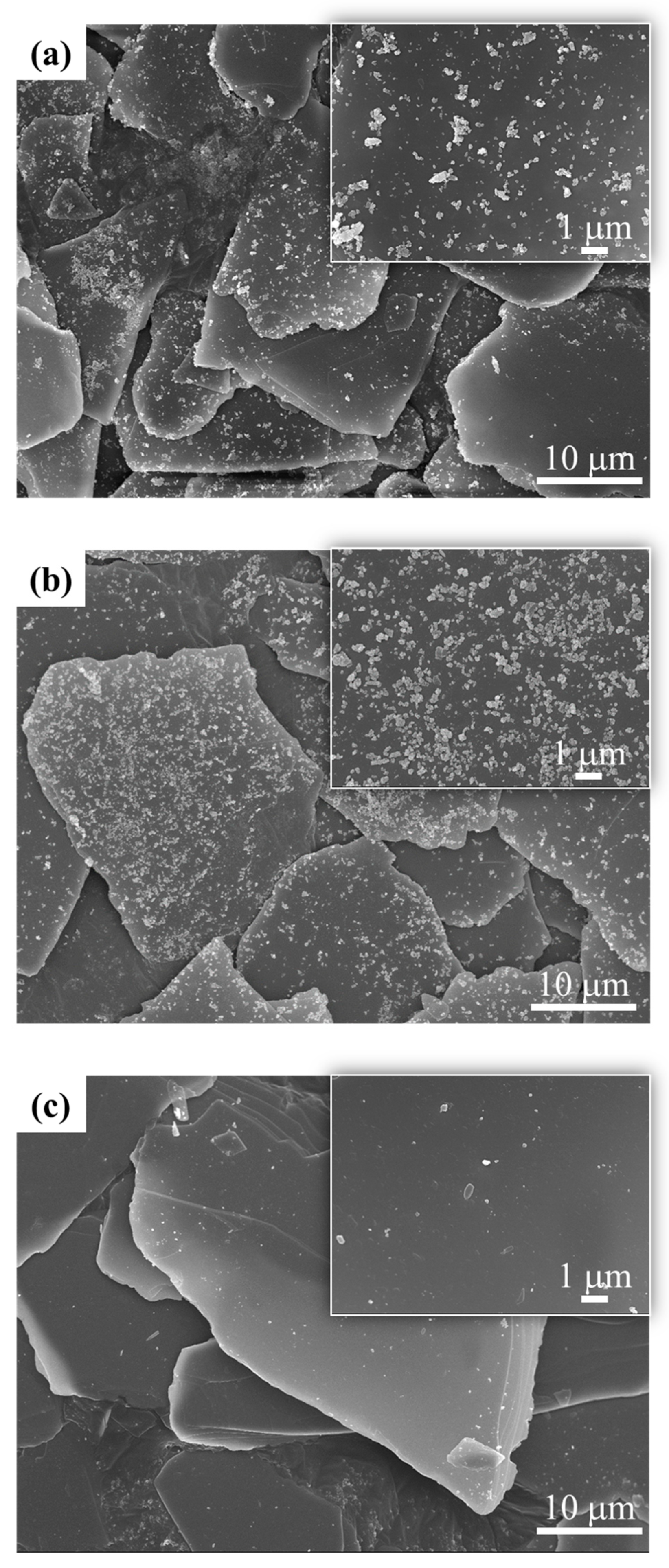
3.2. Y2O3:Eu@MF Prepared by Varying Coating Amount of Y2O3:Eu3+ and Temperatures
3.3. Preparation of Y2O3:Eu@TMF by Varying Loading Amount of Y2O3:Eu3+
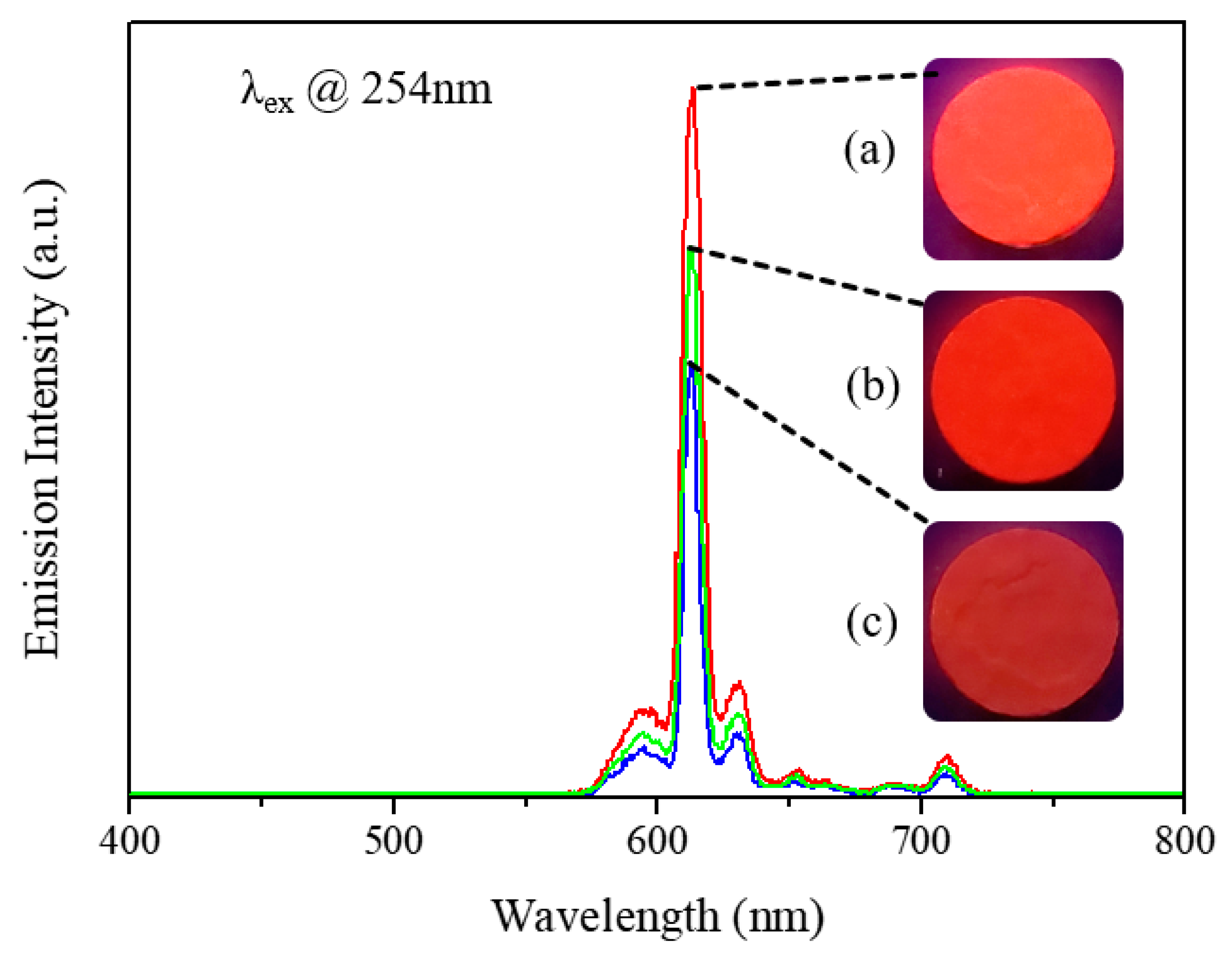
3.4. Gloss and Luminescent Properties of Security Pigment Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.-N.; Li, Y.; Zhang, W.-P.; Yin, M. Effect of acidity on microstructure and spectroscopic properties of Y2O3:Eu3+ powders and ceramics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Pal, M.; Herrera, M.; Mathew, X. Effect of Eu ion incorporation on the emission behavior of Y2O3 nanophosphors: A detailed study of structural and optical properties. Opt. Mater. 2016, 60, 159–168. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, G.; Yang, H.; Yang, Y.; Zhang, J.; Wang, S. Synthesis and upconversion luminescence of monodispersed, submicron-sized Er3+:Y2O3 spherical phosphors. J. Alloys Compd. 2010, 493, 672–677. [Google Scholar] [CrossRef]
- Liang, H.; Zheng, Y.; Chen, G.; Wu, L.; Zhang, Z.; Cao, W. Enhancement of upconversion luminescence of Y2O3:Er3+ nanocrystals by codoping Li+–Zn2+. J. Alloys Compd. 2011, 509, 409–413. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Nagaraju, G.; Yu, J.S. Versatile properties of CaGd2ZnO5:Eu3+ nanophosphor: Its compatibility for lighting and optical display applications. J. Dalton Trans. 2015, 1790–1799. [Google Scholar] [CrossRef]
- Rakov, N.B.; Lozano, W.; Maciel, G.S.; De Araújo, C.B. Nonlinear luminescence in Eu3+-doped Y2O3 powders pumped at 355 nm. Chem. Phys. Lett. 2006, 428, 134–137. [Google Scholar] [CrossRef]
- Venkatachalaiah, K.N.; Nagabhushana, H.; Darshan, G.P.; Basavaraj, R.B.; Prasad, B.D. Novel and highly efficient red luminescent sensor based SiO2@Y2O3:Eu3+, M+ (M+ = Li, Na, K) composite core–shell fluorescent markers for latent fingerprint recognition, security ink and solid-state lightning applications. J. Sens. Actuator B-Chem. 2017, 310–325. [Google Scholar] [CrossRef]
- Khachatourian, A.M.; Golestani-Fard, F.; Sarpoolaky, H.; Vogt, C.; Vasileva, E.; Mensi, M.; Popov, S.; Toprak, M.S. Microwave synthesis of Y2O3:Eu3+ nanophosphors: A study on the influence of dopant concentration and calcination temperature on structural and photoluminescence properties. J. Lumin. 2016, 169, 1–8. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Y.; Chen, Z.; Jin, X.; Wei, T.-H.; Li, Y.; Qin, Y.; Sun, W. Core–Shell Nanophosphor Architecture: Toward Efficient Energy Transport in Inorganic/Organic Hybrid Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 12798–12807. [Google Scholar] [CrossRef]
- Ye, J.; Hongpeng, Z.; Dingfei, Z. Luminescence Properties of Eu/Tb Activated Y2O3 Phosphors Synthesized by Solid State Process. Rare Met. Mater. Eng. 2016, 45, 2790–2792. [Google Scholar] [CrossRef]
- Zambare, A.P.; Murthy, K.V.R. Photoluminescence studies of Eu doped Yttrium based phosphors. Arch. Phy. Res. 2011, 2, 46–50. [Google Scholar]
- Jung, K.Y.; Lee, J.C.; Kim, D.S.; Choi, B.-K.; Kang, W.-J. Co-doping effect of monovalent alkali metals on optical properties of CeO2:Eu nanophosphor prepared by spray pyrolysis and application for preparing pearlescent pigments with red emission. J. Lumin. 2017, 192, 1313–1321. [Google Scholar] [CrossRef]
- Fisher, M.J.; Wang, W.; Dorhout, P.K.; Fisher, E.R. Synthesis of LaPO4:Eu Nanostructures Using the Sol−Gel Template Method. J. Phys. Chem. C 2008, 112, 1901–1907. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Sato, O.; Taguchi, M.; Einaga, Y.; Murakami, T.; Fujishima, A. Self-Cleaning Particle Coating with Antireflection Properties. Chem. Mater. 2005, 17, 696–700. [Google Scholar] [CrossRef]
- Ullah, M.; Ban, S.-M.; Kim, D.-S. Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties. Opt. Mater. 2019, 97, 109366. [Google Scholar] [CrossRef]
- Min, B.H.; Lee, J.C.; Jung, K.Y.; Kim, D.S.; Choi, B.-K.; Kang, W.-J. An aerosol synthesized CeO2:Eu3+/Na+ red nanophosphor with enhanced photoluminescence. RSC Adv. 2016, 6, 81203–81210. [Google Scholar] [CrossRef]
- Jung, K.Y.; Han, J.H.; Kim, D.S.; Choi, B.-K.; Kang, W.-J. Aerosol Synthesis of Gd2O3:Eu/Bi Nanophosphor for Preparation of Photofunctional Pearl Pigment as Security Material. J. Korean Ceram. Soc. 2018, 55, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; You, M.S.; Im, S.H. Formation of uniform TiO2 nanoshell on α-alumina nanoplates for effective metallic luster pigments. Korean J. Chem. Eng. 2016, 33, 2732–2737. [Google Scholar] [CrossRef]
- Tohidifar, M.; Taheri-Nassaj, E.; Alizadeh, P. Precursor content assessment and its influence on the optical interference of a nano-sized mica-hematite pearlescent pigment. Powder Technol. 2010, 204, 194–197. [Google Scholar] [CrossRef]
- Lee, H.J.; Ban, S.M.; Jung, K.-Y.; Choi, B.-K.; Kang, K.-J.; Kim, D.S. Nano Dispersion of Aggregated Y2O3:Eu Red Phosphor and Photoluminescent Properties of Its Nanosol. Korean J. Mater. Res. 2017, 27, 100–106. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterization of instability of concentrated dispersions by a new optical analyser: The TURBISCAN MA 1000. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 111–123. [Google Scholar] [CrossRef]
- Zuend, A.; Marcolli, C.; Booth, A.M.; Lienhard, D.M.; Soonsin, V.; Krieger, U.K.; Topping, D.O.; McFiggans, G.; Peter, T.; Seinfeld, J.H. New and extended parameterization of the thermodynamic model AIOMFAC: Calculation of activity coefficients for organic-inorganic mixtures containing carboxyl, hydroxyl, carbonyl, ether, ester, alkenyl, alkyl, and aromatic functional groups. Atmos. Chem. Phys. Discuss. 2011, 11, 9155–9206. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.K.; Haranath, D.; Saini, S.; Singh, V.N.; Shanker, V. Synthesis and characterization of ultra-fine Y2O3:Eu3+ nanophosphors for luminescent security ink applications. Nanotechnology 2010, 21, 055607. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Tang, H.Z.; Zhu, G.D.; Li, C.C.; Lin, C.K. The optical properties and sunscreen application of spherical h-BN-TiO2/mica composite powder. Ceram. Int. 2014, 4691–4696. [Google Scholar] [CrossRef]





| Sample | Coating Condition | ||||
|---|---|---|---|---|---|
| Solvent | Y2O3:Eu3+ Loaded Amount Based on MF or TMF (wt%) | pH | Stirring Temperature (°C) | Calcination Temperature (°C) | |
| Y2O3:Eu@MF | Ethanol | 10 | 7 | 65 | 650 |
| 8 | |||||
| H2O | 7 | 85 | |||
| 8 | |||||
| 5 | |||||
| 20 | |||||
| 10 | 75 | ||||
| 95 | |||||
| 85 | 750 | ||||
| 850 | |||||
| Y2O3:Eu@TMF | 650 | ||||
| 20 | |||||
| 30 | |||||
| Gross of Angle | 20° | 60° | 85° | |
|---|---|---|---|---|
| Sample * | ||||
| MF | 4.8 | 17.1 | 19.3 | |
| Y2O3:Eu@MF | 10 wt% ** | 3.9 | 14.8 | 15.6 |
| 20 wt% | 4.5 | 16.3 | 18.2 | |
| TMF | 17.8 | 58.3 | 62.9 | |
| Y2O3:Eu@TMF | 10 wt% | 14.2 | 42.6 | 51.1 |
| 20 wt% | 10.1 | 35.7 | 42.4 | |
| 30 wt% | 15.3 | 55.1 | 59.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, S.-M.; Ullah, M.; Jung, K.Y.; Choi, B.-K.; Kang, K.-J.; Kang, M.C.; Kim, D.-S. Y2O3:Eu3+ Nanophosphor-Coated Mica or TiO2/Mica as Red-Emitting Pearl Pigment: Coating Factors, Luminescent and Gloss Properties. Appl. Sci. 2021, 11, 4365. https://doi.org/10.3390/app11104365
Ban S-M, Ullah M, Jung KY, Choi B-K, Kang K-J, Kang MC, Kim D-S. Y2O3:Eu3+ Nanophosphor-Coated Mica or TiO2/Mica as Red-Emitting Pearl Pigment: Coating Factors, Luminescent and Gloss Properties. Applied Sciences. 2021; 11(10):4365. https://doi.org/10.3390/app11104365
Chicago/Turabian StyleBan, Se-Min, Mahboob Ullah, Kyeong Youl Jung, Byung-Ki Choi, Kwang-Jung Kang, Myung Chang Kang, and Dae-Sung Kim. 2021. "Y2O3:Eu3+ Nanophosphor-Coated Mica or TiO2/Mica as Red-Emitting Pearl Pigment: Coating Factors, Luminescent and Gloss Properties" Applied Sciences 11, no. 10: 4365. https://doi.org/10.3390/app11104365






