Preparation and Properties of Biobased, Cationic, Waterborne Polyurethanes Dispersions from Castor Oil and Poly (Caprolactone) Diol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of WPU
2.3. Characterization
3. Results and Discussion
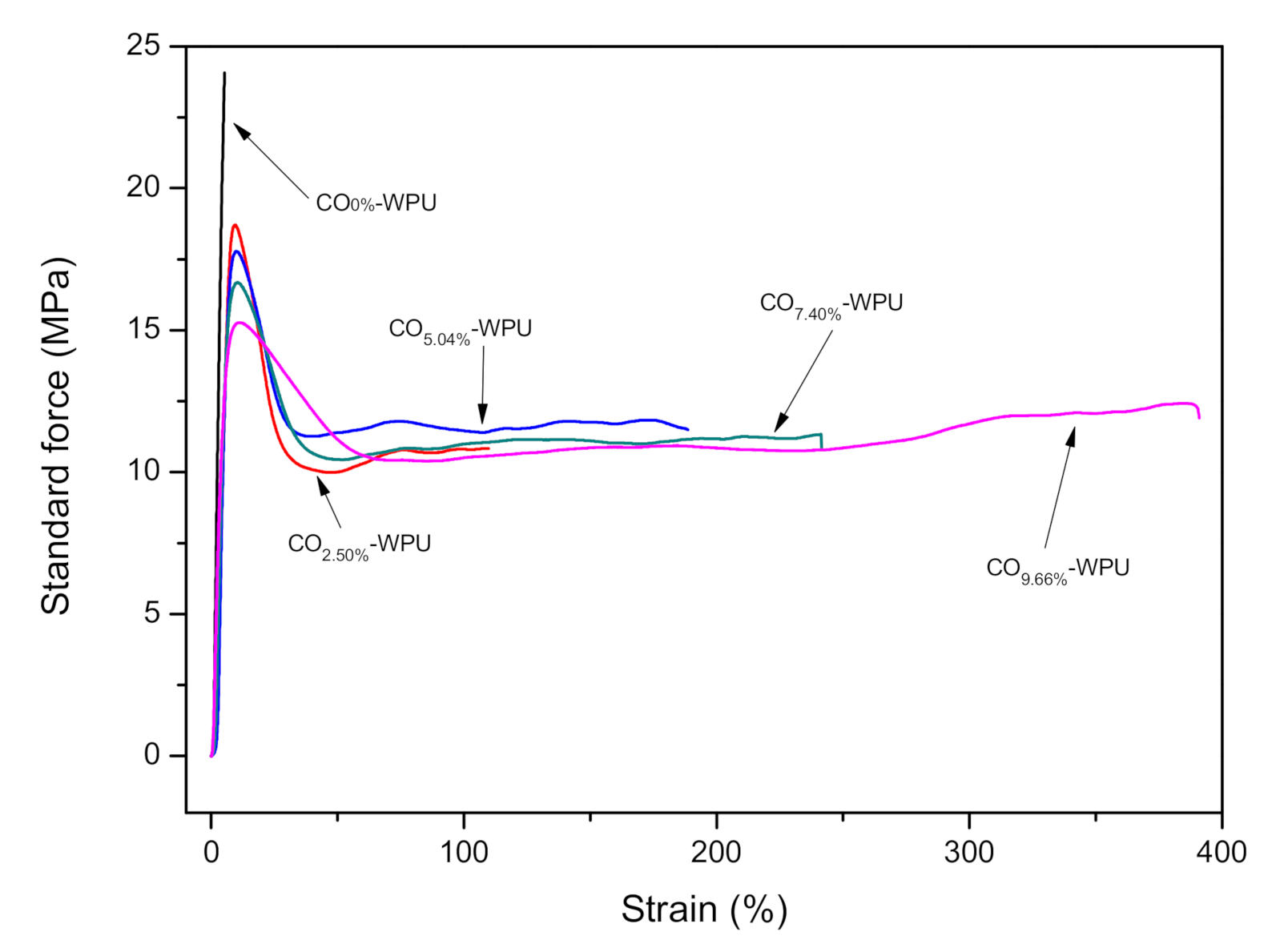
3.1. Mechanical Performance of WPUs
3.2. T-Peel Strength Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.H.; Liu, Y.C.; Chai, T.; Jing, S.M.; Ma, H.; Qin, N.; Zhou, H.; Yan, T.; He, W.M. Synthesis and properties of a nano-silica modified environmentally friendly polyurethane adhesive. RSC Adv. 2015, 5, 44990–44997. [Google Scholar] [CrossRef]
- Kim, E.H.; Myoung, S.W.; Jung, Y.G.; Paik, U. Polyhedral oligomeric silsesquioxane-reinforced polyurethane acrylate. Prog. Org. Coat. 2009, 64, 205–209. [Google Scholar] [CrossRef]
- Valcic, M.D.; Cakic, S.M.; Ristic, I.S.; Cakic, J.D.; Cvetinov, M.J.; Janos, C.J. Polycaprolactone-based biodegradable acrylated polyurethanes: Influence of nanosilica amount on functional properties. Int. J. Adhes. Adhes. 2021, 104. [Google Scholar] [CrossRef]
- Jeon, H.T.; Jang, M.K.; Kim, B.K.; Kim, K.H. Synthesis and characterizations of waterborne polyurethane-silica hybrids using sol-gel process. Colloid Surf. A 2007, 302, 559–567. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, W.J.; Hao, L.F.; Wang, S.; Pei, M.M.; Wang, X.C. Synthesis and characterization of novel fluoroalkyl-terminated hyperbranched polyurethane latex. Appl. Surf. Sci. 2018, 436, 1104–1112. [Google Scholar] [CrossRef]
- Madbouly, S.A. Waterborne Polyurethane Dispersions and Thin Films: Biodegradation and Antimicrobial Behaviors. Molecules 2021, 26, 961. [Google Scholar] [CrossRef]
- Sundar, S.; Vijayalakshmi, N.; Gupta, S.; Rajaram, R.; Radhakrishnan, G. Aqueous dispersions of polyurethane-polyvinyl pyridine cationomers and their application as binder in base coat for leather finishing. Prog. Org. Coat. 2006, 56, 178–184. [Google Scholar] [CrossRef]
- Zhang, M.S.; Hemp, S.T.; Zhang, M.Q.; Allen, M.H.; Carmean, R.N.; Moore, R.B.; Long, T.E. Water-dispersible cationic polyurethanes containing pendant trialkylphosphoniums. Polym. Chem. 2014, 5, 3795–3803. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.H.; Xin, W.; Luo, Y.J. Synthesis and application of a cationic waterborne polyurethane fixative using quaternary ammonium diol as a chain extender. RSC Adv. 2018, 8, 42041–42048. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, F.; Li, Y.; Qiang, X.H. Synthesis of stable cationic waterborne polyurethane with a high solid content: Insight from simulation to experiment. RSC Adv. 2017, 7, 13312–13324. [Google Scholar] [CrossRef] [Green Version]
- Krol, P.; Krol, B. Polyurethane cationomers synthesised with 4,4′-methylenebis(phenyl isocyanate), polyoxyethylene glycol and N-methyl diethanolamine. Colloid Polym. Sci. 2008, 286, 1111–1122. [Google Scholar] [CrossRef]
- Xin, H.; Shen, Y.D.; Li, X.R. Novel cationic polyurethane-fluorinated acrylic hybrid latexes: Synthesis, characterization and properties. Colloid Surf. A 2011, 384, 205–211. [Google Scholar] [CrossRef]
- Mohanty, S.; Krishnamurti, N. Synthesis and characterization of aqueous cationomeric polyurethanes and their use as adhesives. J. Appl. Polym. Sci. 1996, 62, 1993–2003. [Google Scholar] [CrossRef]
- Chen, K.L.; Gou, W.W.; Wang, X.M.; Zeng, C.J.; Ge, F.Q.; Dong, Z.J.; Wang, C.X. UV-Cured Fluoride-Free Polyurethane Functionalized Textile with pH-Induced Switchable Superhydrophobicity and Underwater Superoleophobicity for Controllable Oil/Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 16616–16628. [Google Scholar] [CrossRef]
- Wu, D.M.; Qiu, F.X.; Xu, H.P.; Zhang, J.L.; Yang, D.Y. Preparation, Characterization, and Properties of Environmentally Friendly Waterborne Poly(urethane acrylate)/Silica Hybrids. J. Appl. Polym. Sci. 2011, 119, 1683–1695. [Google Scholar] [CrossRef]
- Fang, H.G.; Wang, H.L.; Sun, J.; Wei, H.B.; Ding, Y.S. Tailoring elastomeric properties of waterborne polyurethane by incorporation of polymethyl methacrylate with nanostructural heterogeneity. RSC Adv. 2016, 6, 13589–13599. [Google Scholar] [CrossRef]
- Fei, G.Q.; Zhu, K.; Wang, H.H.; Shen, Y.D.; Zou, J.; Lan, J. Morphology, Dynamic Rheology, and Cohesive Properties of Epoxy-Modified Polyurethane-Acrylate Microemulsions Prepared by In Situ Surfactant-Free Polymerization. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chen, G.D.; Zhou, S.X.; Gu, G.X.; Wu, L.M. Acrylic-based polyurethane/silica hybrids prepared by acid-catalyzed sol-gel process: Structure and mechanical properties. Macromol. Chem. Phys. 2005, 206, 885–892. [Google Scholar] [CrossRef]
- Cakic, S.M.; Ristic, I.S.; M-Cincovic, M.; Stojiljkovic, D.T.; B-Simendic, J. Preparation and characterization of waterborne polyurethane/silica hybrid dispersions from castor oil polyols obtained by glycolysis poly (ethylene terephthalate) waste. Int. J. Adhes. Adhes. 2016, 70, 329–341. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Wang, Z.K.; Ganewatta, M.S.; Tang, C.B. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48. [Google Scholar] [CrossRef]
- Kamairudin, N.; Hoong, S.S.; Abdullah, L.C.; Ariffin, H.; Biak, D.R.A. Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology. Molecules 2021, 26, 648. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable polymers: Replacing polymers derived from fossil fuels. Polym. Chem. 2014, 5, 3117–3118. [Google Scholar] [CrossRef]
- Coates, G.W.; Hillmyer, M.A. A Virtual Issue of Macromolecules: “Polymers from Renewable Resources”. Macromolecules 2009, 42, 7987–7989. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Lu, Y.S.; Larock, R.C. Aqueous Cationic Polyurethane Dispersions from Vegetable Oils. ChemSuschem 2010, 3, 329–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.B.; Deng, H.H.; Zhang, W.H.; Kang, J.; Zhang, C.Q. Enhanced Mechanical Properties and Functional Performances of Cationic Waterborne Polyurethanes Enabled by Different Natural Phenolic Acids. ACS Sustain. Chem. Eng. 2020, 8, 17447–17457. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Effect of reactive organoclay on physicochemical properties of vegetable oil-based waterborne polyurethane nanocomposites. RSC Adv. 2015, 5, 11524–11533. [Google Scholar] [CrossRef]
- Cakic, S.M.; Valcic, M.D.; Ristic, I.S.; Radusin, T.; Cvetinov, M.J.; Budinski-Simendic, J. Waterborne polyurethane-silica nanocomposite adhesives based on castor oil-recycled polyols: Effects of (3-aminopropyl)triethoxysilane (APTES) content on properties. Int. J. Adhes. Adhes. 2019, 90, 22–31. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Mendieta, J.R.; Ortega-Toro, R. In-depth study from gluten/PCL-based food packaging films obtained under reactive extrusion conditions using chrome octanoate as a potential food grade catalyst. Food Hydrocolloid 2021, 111, 106255. [Google Scholar] [CrossRef]
- Moussaif, N.; Irusta, S.; Yague, C.; Arruebo, M.; Meier, J.G.; Crespo, C.; Jimenez, M.A.; Santamaria, J. Mechanically reinforced biodegradable nanocomposites. A facile synthesis based on PEGylated silica nanoparticles. Polymer 2010, 51, 6132–6139. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Tzetzis, D.; Bikiaris, D. Composite Membranes of Poly(epsilon-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules 2019, 24, 3067. [Google Scholar] [CrossRef] [Green Version]
- Li, J.W.; Cheng, Y.H.; Lee, H.T.; Tsen, W.C.; Chiu, C.W.; Suen, M.C. Properties and degradation of castor oil-based fluoridated biopolyurethanes with different lengths of fluorinated segments. RSC Adv. 2019, 9, 31133–31149. [Google Scholar] [CrossRef] [Green Version]
- Cernadas, T.; Morgado, S.; Alves, P.; Goncalves, F.A.M.M.; Correia, T.R.; Correia, I.J.; Ferreira, P. Preparation of functionalized poly(caprolactone diol)/castor oils blends to be applied as photocrosslinkable tissue adhesives. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Field, J.; Haycock, J.W.; Boissonade, F.M.; Claeyssens, F. A Tuneable, Photocurable, Poly(Caprolactone)-Based Resin for Tissue Engineering-Synthesis, Characterisation and Use in Stereolithography. Molecules 2021, 26, 1199. [Google Scholar] [CrossRef]
- Hailemeskel, B.Z.; Hsu, W.H.; Addisu, K.D.; Andrgie, A.T.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C. Diselenide linkage containing triblock copolymer nanoparticles based on Bi(methoxyl poly(ethylene glycol))-poly(epsilon-carprolactone): Selective intracellular drug delivery in cancer cells. Mater. Sci. Eng. C Mater. 2019, 103. [Google Scholar] [CrossRef]
- Kwak, Y.S.; Kim, H.D. Preparation and properties of waterborne polyurethanes based on triblock glycol (CL)(4.5)-PTMG-(CL)(4.5) for water vapor permeable coatings: Effect of soft segment content. Fiber Polym. 2002, 3, 153–158. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.Q.; Li, S.J.; Cheng, Y.L.; Lei, W.Q.; Meng, X.J. Recent Advances in Synthesis of Waterborne Polyurethane and Their Application in Water-based Ink: A Review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Wang, G.; Ma, G.Z.; Hou, C.Y.; Guan, T.T.; Ling, L.X.; Wang, B.J. Preparation and Properties of Waterborne Polyurethane/Nanosilica Composites: A Diol as Extender with Triethoxysilane Group. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, J.; Ye, L. Structure and properties of polyvinyl alcohol/polyurethane blends. Compos. Part B Eng. 2015, 69, 389–396. [Google Scholar] [CrossRef]
- Barni, A.; Levi, M. Aqueous polyurethane dispersions: A comparative study of polymerization processes. J. Appl. Polym. Sci. 2003, 88, 716–723. [Google Scholar] [CrossRef]
- Wu, H.; Wan, Y.; Cao, X.Y.; Wu, Q. Interlocked chitosan/poly(DL-lactide) blends. Mater. Lett. 2008, 62, 330–334. [Google Scholar] [CrossRef]
- Feng, F.; Ye, L. Morphologies and Mechanical Properties of Polylactide/Thermoplastic Polyurethane Elastomer Blends. J. Appl. Polym. Sci. 2011, 119, 2778–2783. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, H.; Tan, H.F.; Du, X.W. Modified Shape Memory Epoxy Resin Composites by Blending Activity Polyurethane. J. Appl. Polym. Sci. 2013, 127, 3152–3158. [Google Scholar] [CrossRef]




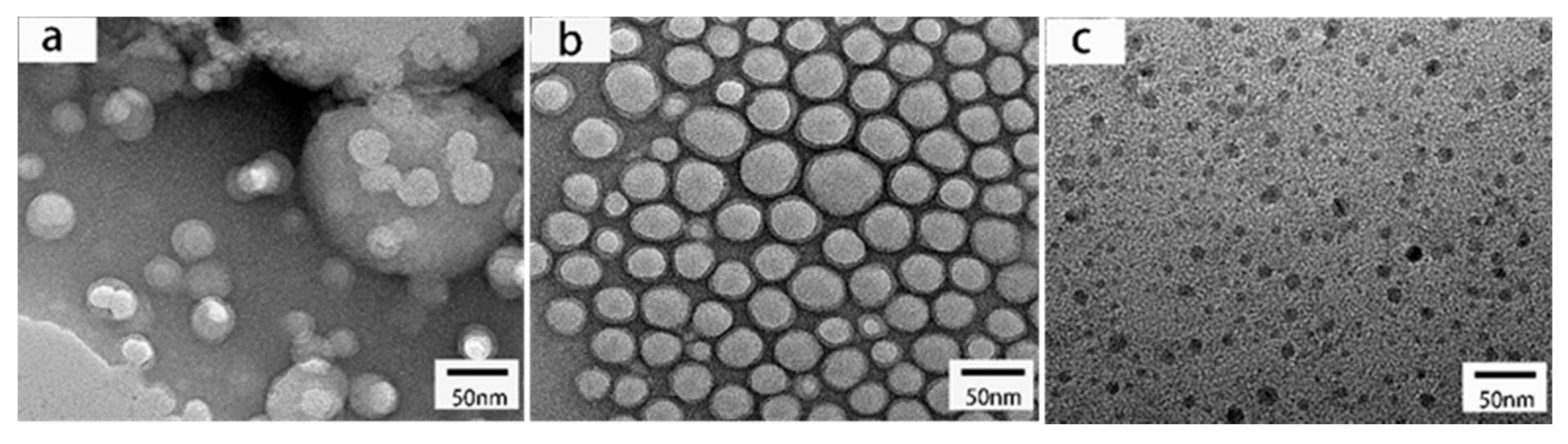


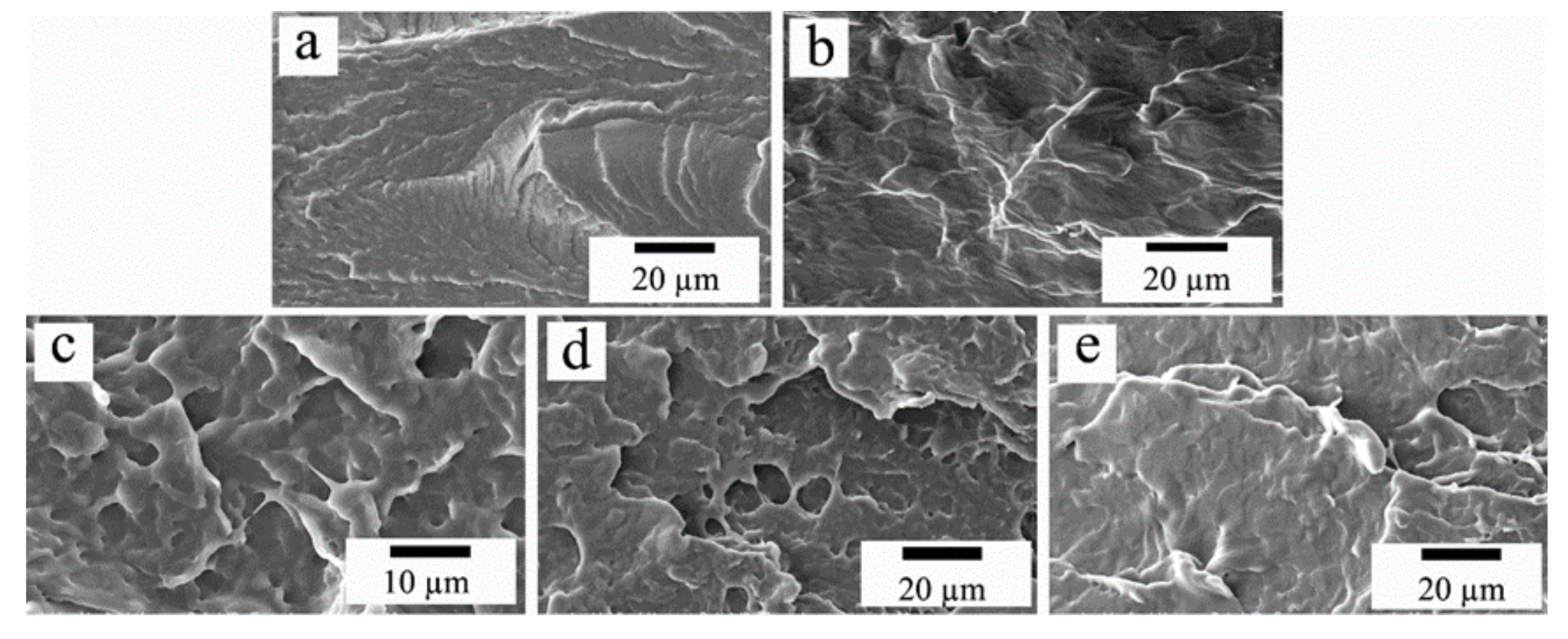
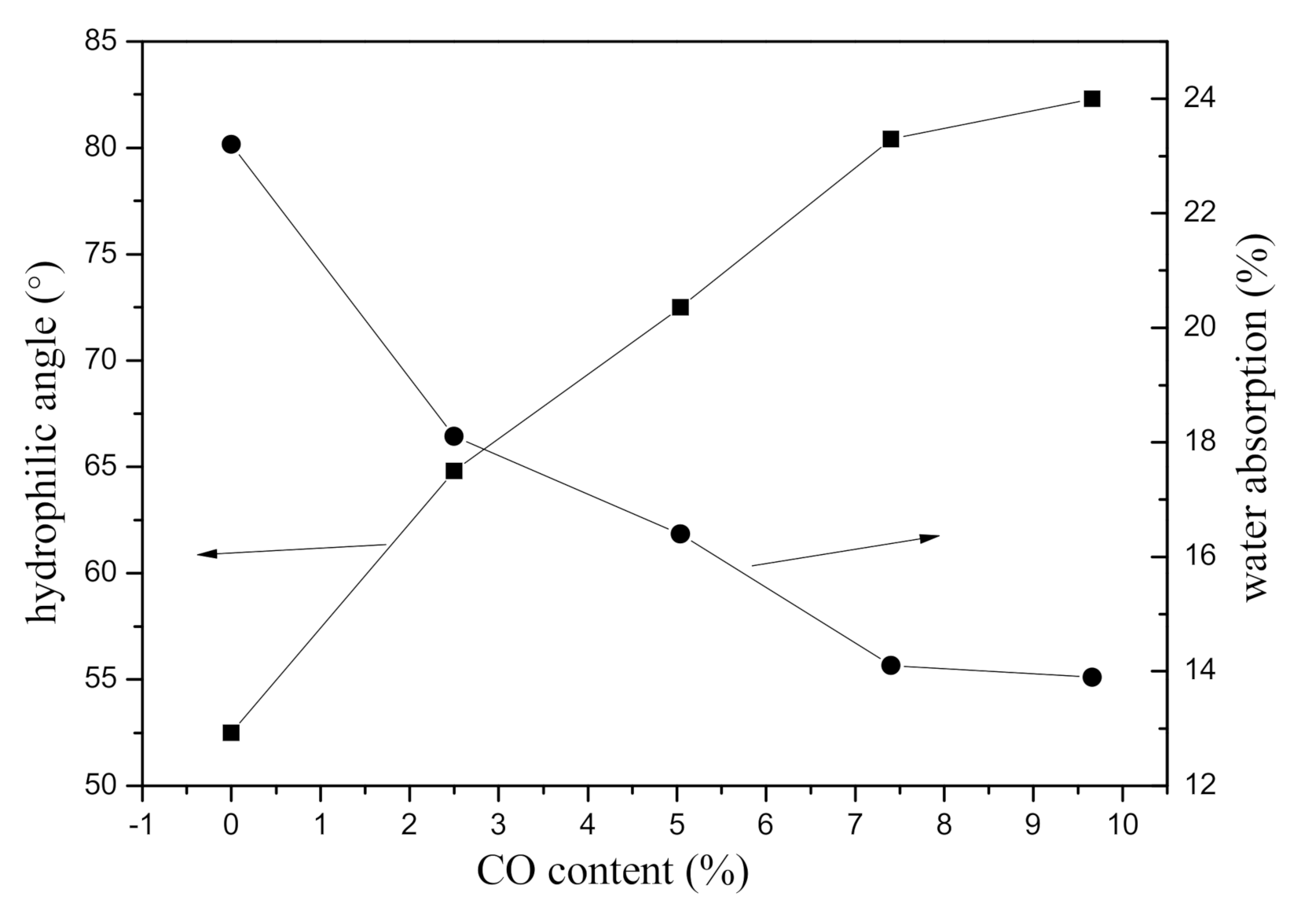
| Samples | PCL-OH (Molar) | CO (Molar) | MDEA (wt%) | Hard Segment (wt%) | CO (wt%) | T-Peel Strength (N/25 mm) | Zeta Poterntial (mV) |
|---|---|---|---|---|---|---|---|
| CO0%–WPU | 1 | 0 | 3.8 | 37.5 | 0 | 17.2 | 39 |
| CO2.50%–WPU | 1 | 0.095 | 3.3 | 38.9 | 2.5 | 23.4 | 45 |
| CO5.04%–WPU | 1 | 0.190 | 3.2 | 40.3 | 5.0 | 29.5 | 50 |
| CO7.40%–WPU | 1 | 0.285 | 3.2 | 41.6 | 7.4 | 36.8 | 54 |
| CO9.66%–WPU | 1 | 0.380 | 3.1 | 42.8 | 9.7 | 26.8 | 55 |
| Sample | CO0%–WPU | CO2.50%–WPU | CO5.04%–WPU | CO7.40%–WPU | CO9.66%–WPU |
|---|---|---|---|---|---|
| Tm (°C) | 48.8 | 48.2 | 47.5 | 46.8 | 45.2 |
| ΔH (J/g) | 47.7 | 44.6 | 42.2 | 39.1 | 38.0 |
| CO0%–WPU | CO2.50%–WPU | CO5.04%–WPU | CO7.40%–WPU | CO9.66%–WPU | |
|---|---|---|---|---|---|
| σm(MPa) | 24.1 | 18.7 | 17.8 | 17.0 | 15.3 |
| εb (%) | 5.0 | 110 | 189 | 241. | 391 |
| σb (MPa) | - | 10.8 | 11.5 | 11.3 | 12.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, S.; Shen, J.; Zhang, S.; Liu, M.; Lv, R.; Xu, W. Preparation and Properties of Biobased, Cationic, Waterborne Polyurethanes Dispersions from Castor Oil and Poly (Caprolactone) Diol. Appl. Sci. 2021, 11, 4784. https://doi.org/10.3390/app11114784
Li Y, Chen S, Shen J, Zhang S, Liu M, Lv R, Xu W. Preparation and Properties of Biobased, Cationic, Waterborne Polyurethanes Dispersions from Castor Oil and Poly (Caprolactone) Diol. Applied Sciences. 2021; 11(11):4784. https://doi.org/10.3390/app11114784
Chicago/Turabian StyleLi, Ying, Sichong Chen, Jun Shen, Siqi Zhang, Ming Liu, Ruixue Lv, and Wang Xu. 2021. "Preparation and Properties of Biobased, Cationic, Waterborne Polyurethanes Dispersions from Castor Oil and Poly (Caprolactone) Diol" Applied Sciences 11, no. 11: 4784. https://doi.org/10.3390/app11114784





