Single-Shot Coherent X-ray Imaging Instrument at PAL-XFEL
Abstract
:1. Introduction
2. Materials and Methods
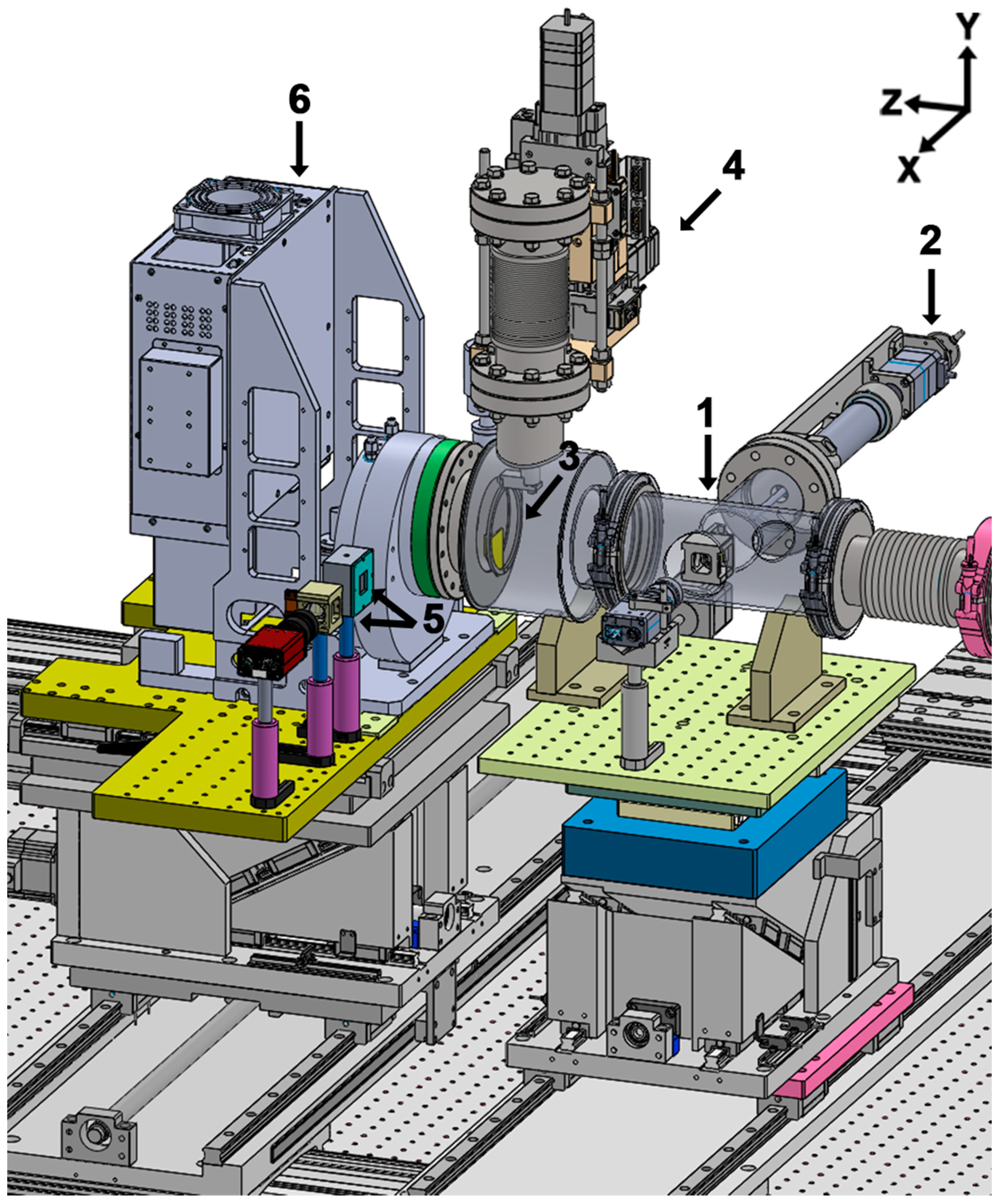
2.1. Overview of the Whole System
2.1.1. Sample Chamber and Loadlock Chamber
2.1.2. Sample Delivery Using a Thin Si3N4 Membrane
2.1.3. Detector Part
2.2. Pump Laser System
Timing Synchronization
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, C.; Jiang, H.; Mancuso, A.; Amirbekian, B.; Peng, L.; Sun, R.; Shah, S.S.; Zhou, Z.H.; Ishikawa, T.; Miao, J. Quantitative imaging of single, unstained viruses with coherent X-rays. Phys. Rev. Lett. 2008, 101, 158101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, M.M.; Ekeberg, T.; Maia, F.R.; Svenda, M.; Andreasson, J.; Jönsson, O.; Odić, D.; Iwan, B.; Rocker, A.; Westphal, D. Single mimivirus particles intercepted and imaged with an X-ray laser. Nature 2011, 470, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Bostedt, C.; Eremina, E.; Rupp, D.; Adolph, M.; Thomas, H.; Hoener, M.; de Castro, A.R.B.; Tiggesbäumker, J.; Meiwes-Broer, K.H.; Laarmann, T.; et al. Ultrafast X-ray Scattering of Xenon Nanoparticles: Imaging Transient States of Matter. Phys. Rev. Lett. 2012, 108, 093401. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.N.; Beitra, L.; Xiong, G.; Higginbotham, A.; Fritz, D.M.; Lemke, H.T.; Zhu, D.; Chollet, M.; Williams, G.J.; Messerschmidt, M.; et al. Ultrafast Three-Dimensional Imaging of Lattice Dynamics in Individual Gold Nanocrystals. Science 2013, 341, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Schot, G.; Svenda, M.; Maia, F.R.N.C.; Hantke, M.; DePonte, D.P.; Seibert, M.M.; Aquila, A.; Schulz, J.; Kirian, R.; Liang, M.; et al. Imaging single cells in a beam of live cyanobacteria with an X-ray laser. Nat. Commun. 2015, 6, 5704. [Google Scholar] [CrossRef] [Green Version]
- Ekeberg, T.; Svenda, M.; Abergel, C.; Maia, F.R.N.C.; Seltzer, V.; Claverie, J.-M.; Hantke, M.; Jönsson, O.; Nettelblad, C.; van der Schot, G.; et al. Three-Dimensional Reconstruction of the Giant Mimivirus Particle with an X-ray Free-Electron Laser. Phys. Rev. Lett. 2015, 114, 098102. [Google Scholar] [CrossRef] [Green Version]
- Hosseinizadeh, A.; Mashayekhi, G.; Copperman, J.; Schwander, P.; Dashti, A.; Sepehr, R.; Fung, R.; Schmidt, M.; Yoon, C.H.; Hogue, B.G.; et al. Conformational landscape of a virus by single-particle X-ray scattering. Nat. Methods 2017, 14, 877–881. [Google Scholar] [CrossRef]
- Gorkhover, T.; Ulmer, A.; Ferguson, K.; Bucher, M.; Maia, F.R.N.C.; Bielecki, J.; Ekeberg, T.; Hantke, M.F.; Daurer, B.J.; Nettelblad, C.; et al. Femtosecond X-ray Fourier holography imaging of free-flying nanoparticles. Nat. Photonics 2018, 12, 150–153. [Google Scholar] [CrossRef]
- Ihm, Y.; Cho, D.H.; Sung, D.; Nam, D.; Jung, C.; Sato, T.; Kim, S.; Park, J.; Kim, S.; Gallagher-Jones, M.; et al. Direct observation of picosecond melting and disintegration of metallic nanoparticles. Nat. Commun. 2019, 10, 2411. [Google Scholar] [CrossRef] [Green Version]
- Lehmkühler, F.; Dallari, F.; Jain, A.; Sikorski, M.; Möller, J.; Frenzel, L.; Lokteva, I.; Mills, G.; Walther, M.; Sinn, H.; et al. Emergence of anomalous dynamics in soft matter probed at the European XFEL. Proc. Natl. Acad. Sci. USA 2020, 117, 24110. [Google Scholar] [CrossRef]
- Roseker, W.; Lee, S.; Walther, M.; Lehmkühler, F.; Hankiewicz, B.; Rysov, R.; Hruszkewycz, S.O.; Stephenson, G.B.; Sutton, M.; Fuoss, P.H.; et al. Double-pulse speckle contrast correlations with near Fourier transform limited free-electron laser light using hard X-ray split-and-delay. Sci. Rep. 2020, 10, 5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmkühler, F.; Hankiewicz, B.; Schroer, M.A.; Müller, L.; Ruta, B.; Sheyfer, D.; Sprung, M.; Tono, K.; Katayama, T.; Yabashi, M.; et al. Slowing down of dynamics and orientational order preceding crystallization in hard-sphere systems. Sci. Adv. 2020, 6, eabc5916. [Google Scholar] [CrossRef] [PubMed]
- Büttner, F.; Pfau, B.; Böttcher, M.; Schneider, M.; Mercurio, G.; Günther, C.M.; Hessing, P.; Klose, C.; Wittmann, A.; Gerlinger, K.; et al. Observation of fluctuation-mediated picosecond nucleation of a topological phase. Nat. Mater. 2021, 20, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-S.; Min, C.-K.; Heo, H.; Kim, C.; Yang, H.; Kim, G.; Nam, I.; Baek, S.Y.; Choi, H.-J.; Mun, G.; et al. Hard X-ray free-electron laser with femtosecond-scale timing jitter. Nat. Photonics 2017, 11, 708–713. [Google Scholar] [CrossRef]
- Nam, I.; Min, C.-K.; Oh, B.; Kim, G.; Na, D.; Suh, Y.J.; Yang, H.; Cho, M.H.; Kim, C.; Kim, M.-J.; et al. High-brightness self-seeded X-ray free-electron laser covering the 3.5 keV to 14.6 keV range. Nat. Photonics 2021, 15, 435–441. [Google Scholar]
- Yun, K.; Kim, S.; Kim, D.; Chung, M.; Jo, W.; Hwang, H.; Nam, D.; Kim, S.; Kim, J.; Park, S.-Y.; et al. Coherence and pulse duration characterization of the PAL-XFEL in the hard X-ray regime. Sci. Rep. 2019, 9, 3300. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.H.; Shen, Z.; Ihm, Y.; Wi, D.H.; Jung, C.; Nam, D.; Kim, S.; Park, S.-Y.; Kim, K.S.; Sung, D.; et al. High-Throughput 3D Ensemble Characterization of Individual Core–Shell Nanoparticles with X-ray Free Electron Laser Single-Particle Imaging. ACS Nano 2021, 15, 4066–4676. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-Y.; Park, J.; Kim, S.; Kim, S.; Rah, S.; Lim, J.; Nam, K.H. Focusing X-ray free-electron laser pulses using Kirkpatrick-Baez mirrors at the NCI hutch of the PAL-XFEL. J. Synchrotron Radiat. 2018, 25, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Barty, A.; Caleman, C.; Aquila, A.; Timneanu, N.; Lomb, L.; White, T.A.; Andreasson, J.; Arnlund, D.; Bajt, S.; Barends, T.R.M.; et al. Self-terminating diffraction gates femtosecond X-ray nanocrystallography measurements. Nat. Photonics 2012, 6, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Kim, S.; Kim, M.-J.; Kim, S. Development of a Fast Sample-exchange System for Fixed Target Single-shot Imaging at PAL-XFEL. J. Korean Phys. Soc. 2020, 76, 448–452. [Google Scholar] [CrossRef]
- Hunter, M.S.; Segelke, B.; Messerschmidt, M.; Williams, G.J.; Zatsepin, N.A.; Barty, A.; Benner, W.H.; Carlson, D.B.; Coleman, M.; Graf, A.; et al. Fixed-target protein serial microcrystallography with an X-ray free electron laser. Sci. Rep. 2014, 4, 6026. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.; Kim, C.; Kim, Y.; Ebisu, T.; Gallagher-Jones, M.; Park, J.; Kim, S.; Kim, S.; Tono, K.; Noh, D.Y.; et al. Fixed target single-shot imaging of nanostructures using thin solid membranes at SACLA. J. Phys. B Atom. Mol. Phys. 2016, 49, 034008. [Google Scholar] [CrossRef]
- Kameshima, T.; Ono, S.; Kudo, T.; Ozaki, K.; Kirihara, Y.; Kobayashi, K.; Inubushi, Y.; Yabashi, M.; Horigome, T.; Holland, A.; et al. Development of an X-ray pixel detector with multi-port charge-coupled device for X-ray free-electron laser experiments. Rev. Sci. Instrum. 2014, 85, 033110. [Google Scholar] [CrossRef]
- Clark, J.N.; Beitra, L.; Xiong, G.; Fritz, D.M.; Lemke, H.T.; Zhu, D.; Chollet, M.; Williams, G.J.; Messerschmidt, M.M.; Abbey, B.; et al. Imaging transient melting of a nanocrystal using an X-ray laser. Proc. Natl. Acad. Sci. USA 2015, 112, 7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orville, A.M. Recent results in time resolved serial femtosecond crystallography at XFELs. Curr. Opin. Struct. Biol. 2020, 65, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.G.; Nozawa, S.; Sato, T.; Oang, K.Y.; Kim, T.W.; Ki, H.; Jo, J.; Park, S.; Song, C.; et al. Direct observation of bond formation in solution with femtosecond X-ray scattering. Nature 2015, 518, 385–389. [Google Scholar] [CrossRef]
- Buzzi, M.; Först, M.; Mankowsky, R.; Cavalleri, A. Probing dynamics in quantum materials with femtosecond X-rays. Nat. Rev. Mater. 2018, 3, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Min, C.-K.; Eom, I. Laser systems for time-resolved experiments at the Pohang Accelerator Laboratory X-ray Free-Electron Laser beamlines. J. Synchrotron Radiat. 2019, 26, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gonzalez, A.; Johnson, A.S.; Fitzpatrick, A.; Hutchison, C.D.M.; Fare, C.; Cordon-Preciado, V.; Dorlhiac, G.; Ferreira, J.L.; Morgan, R.M.; Marangos, J.P.; et al. Coincidence timing of femtosecond optical pulses in an X-ray free electron laser. J. Appl. Phys. 2017, 122, 203105. [Google Scholar] [CrossRef]
- Sato, T.; Glownia, J.M.; Ware, M.R.; Chollet, M.; Nelson, S.; Zhu, D. A simple instrument to find spatiotemporal overlap of optical/X-ray light at free-electron lasers. J. Synchrotron Radiat. 2019, 26, 647–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamaly, E.G. Femtosecond Laser-Matter Interaction: Theory, Experiments and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pham, M.; Yin, P.; Rana, A.; Osher, S.; Miao, J. Generalized proximal smoothing (GPS) for phase retrieval. Opt. Express 2019, 27, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Redford, S.; Andrä, M.; Barten, R.; Bergamaschi, A.; Brückner, M.; Dinapoli, R.; Fröjdh, E.; Greiffenberg, D.; Lopez-Cuenca, C.; Mezza, D.; et al. First full dynamic range calibration of the JUNGFRAU photon detector. J. Instrum. 2018, 13, C01027. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, D.; Nam, D.; Kim, M.-j.; Kim, S.; Kim, K.S.; Park, S.-Y.; Hwang, S.M.; Jung, C.; Lee, H.; Cho, D.H.; et al. Single-Shot Coherent X-ray Imaging Instrument at PAL-XFEL. Appl. Sci. 2021, 11, 5082. https://doi.org/10.3390/app11115082
Sung D, Nam D, Kim M-j, Kim S, Kim KS, Park S-Y, Hwang SM, Jung C, Lee H, Cho DH, et al. Single-Shot Coherent X-ray Imaging Instrument at PAL-XFEL. Applied Sciences. 2021; 11(11):5082. https://doi.org/10.3390/app11115082
Chicago/Turabian StyleSung, Daeho, Daewoong Nam, Myong-jin Kim, Seonghan Kim, Kyung Sook Kim, Sang-Youn Park, Sun Min Hwang, Chulho Jung, Heemin Lee, Do Hyung Cho, and et al. 2021. "Single-Shot Coherent X-ray Imaging Instrument at PAL-XFEL" Applied Sciences 11, no. 11: 5082. https://doi.org/10.3390/app11115082





