Cranial Implant Design Applying Shape-Based Interpolation Method via Open-Source Software
Abstract
:1. Introduction
2. Methods
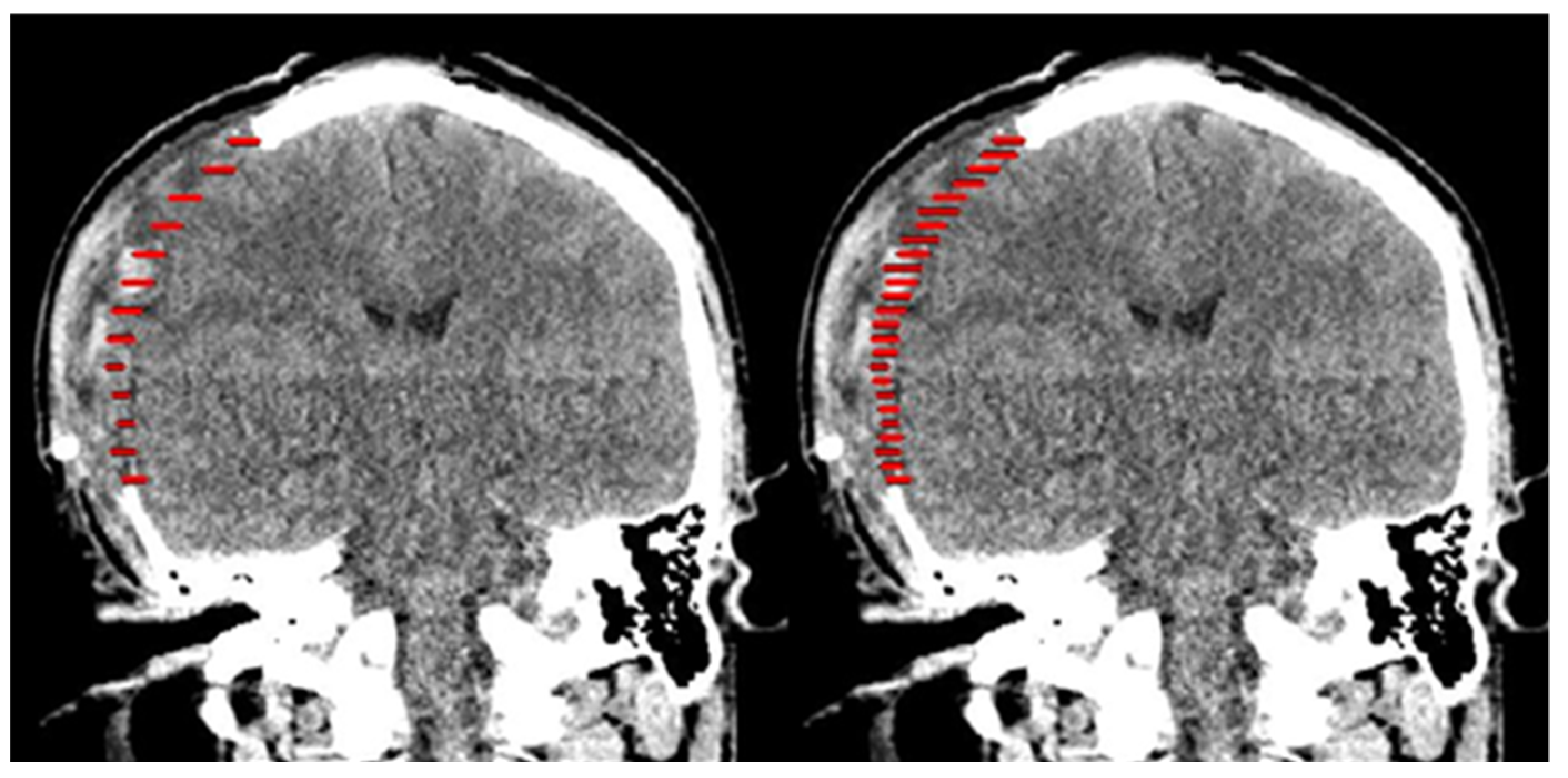
2.1. Design of Cranial Implants Using 3-Matic Software
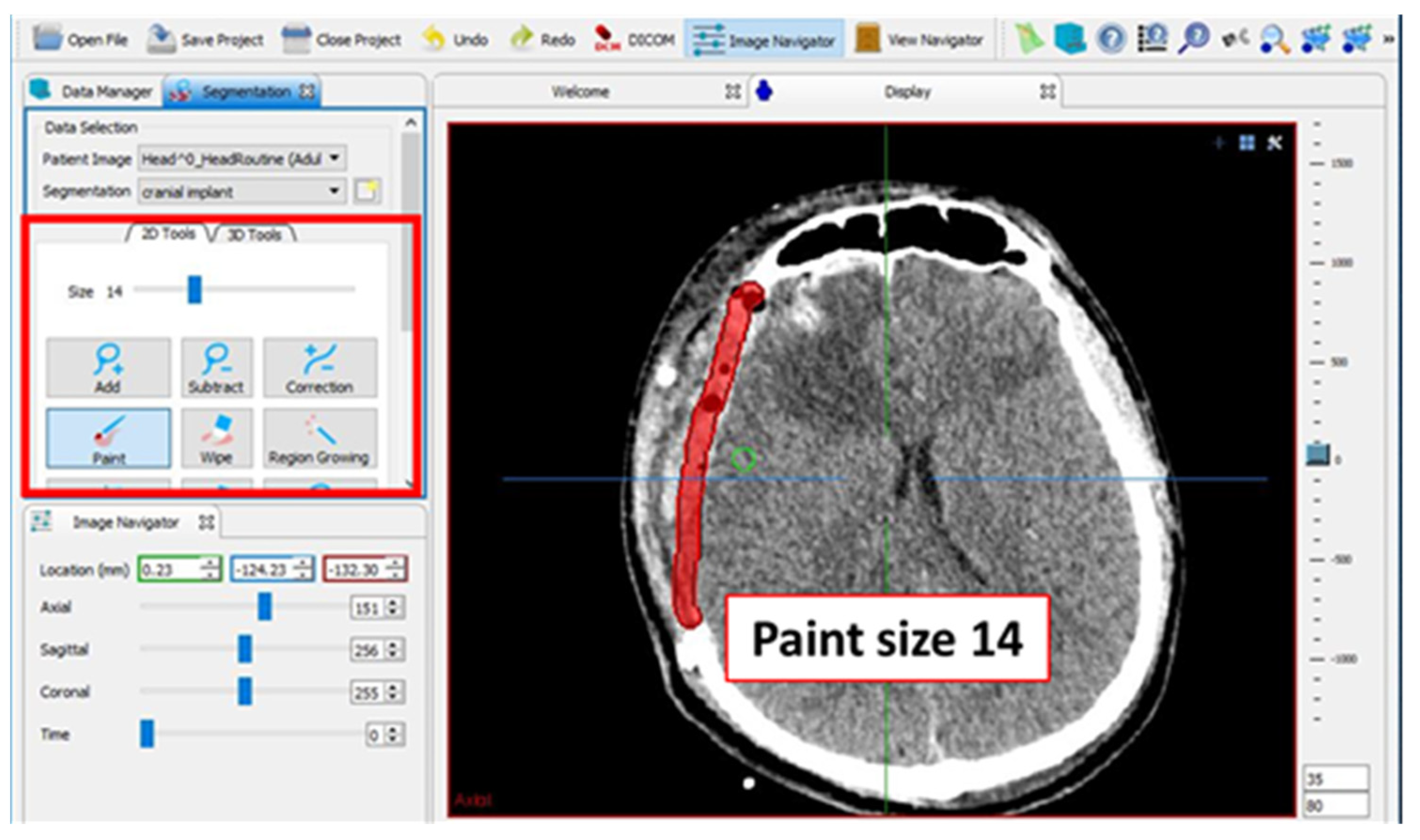
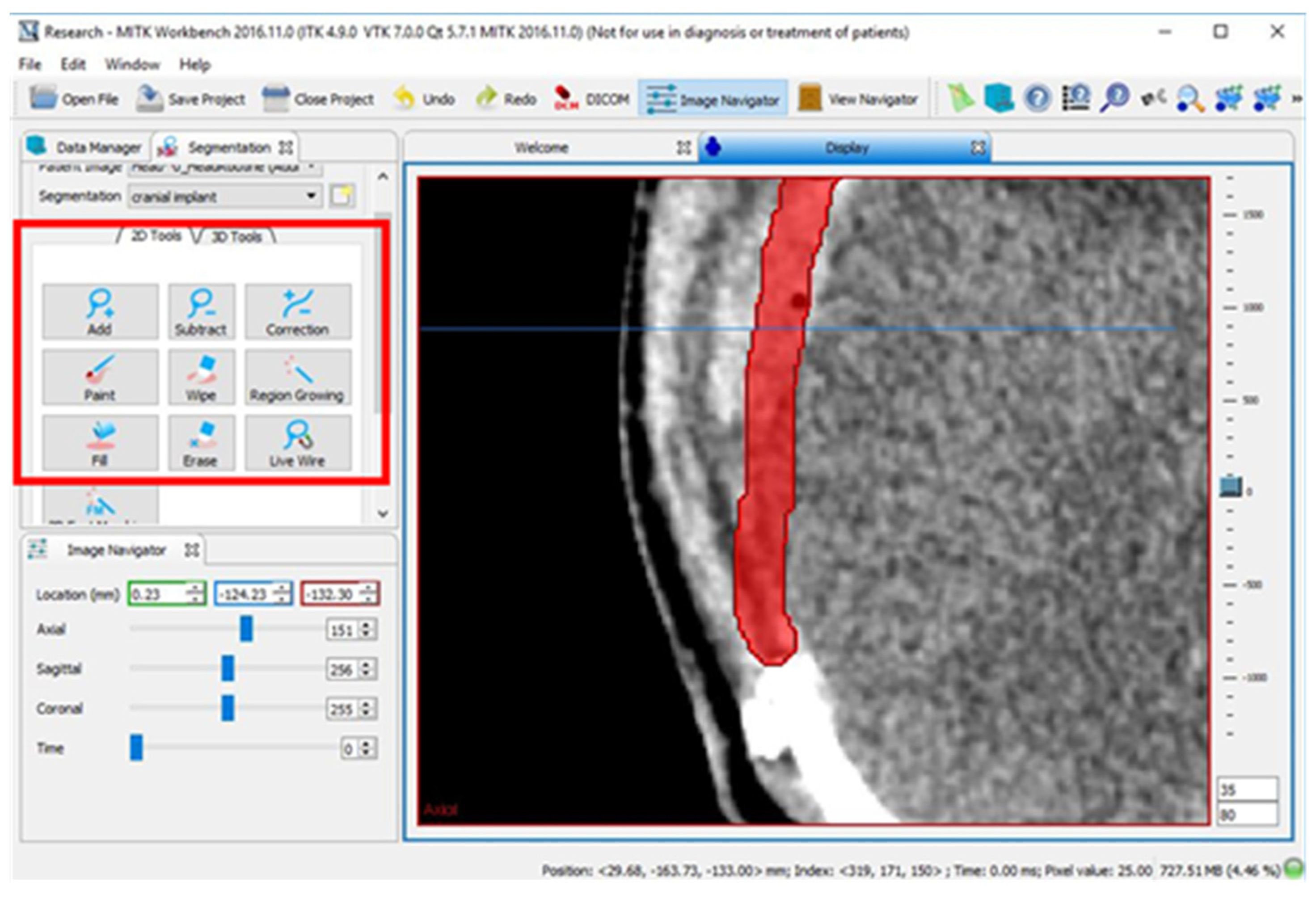
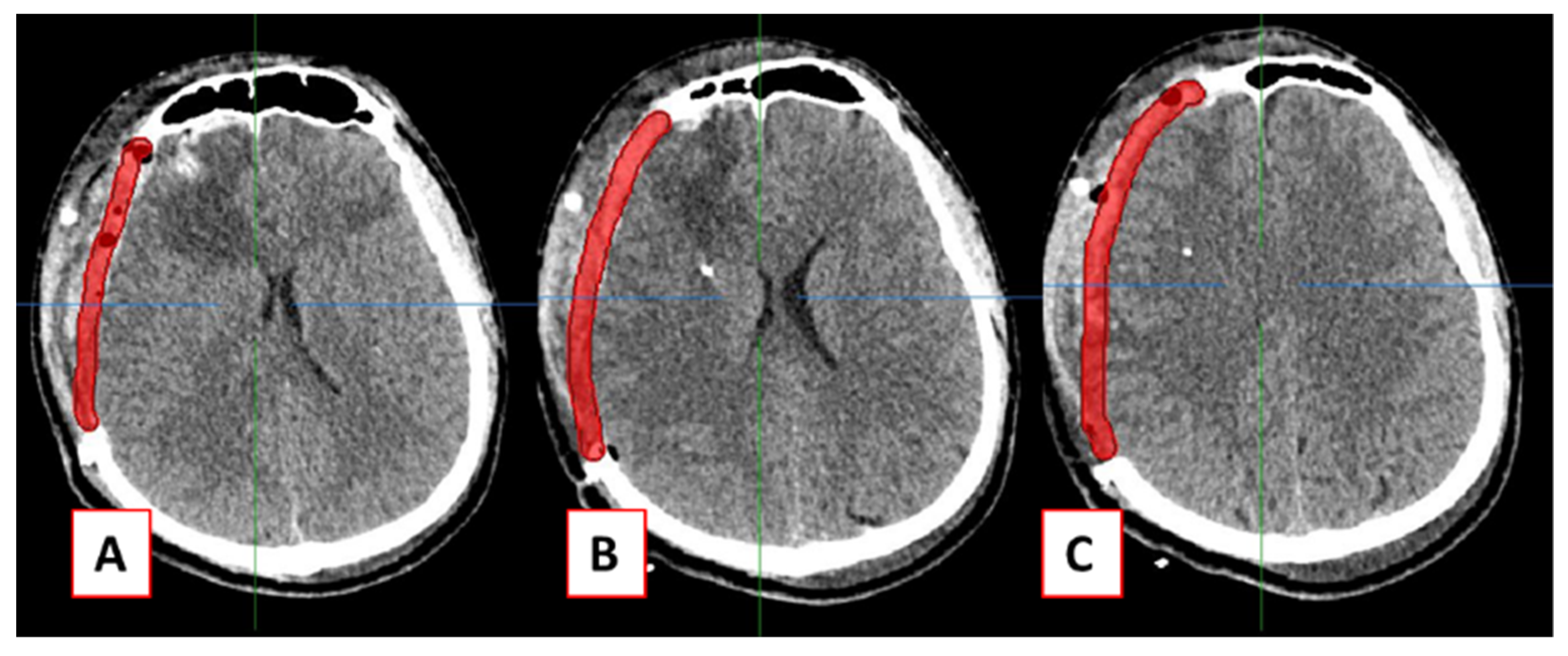
2.2. Design of Cranial Implants Using MITK Software
2.3. Statistical and Geometric Analyses
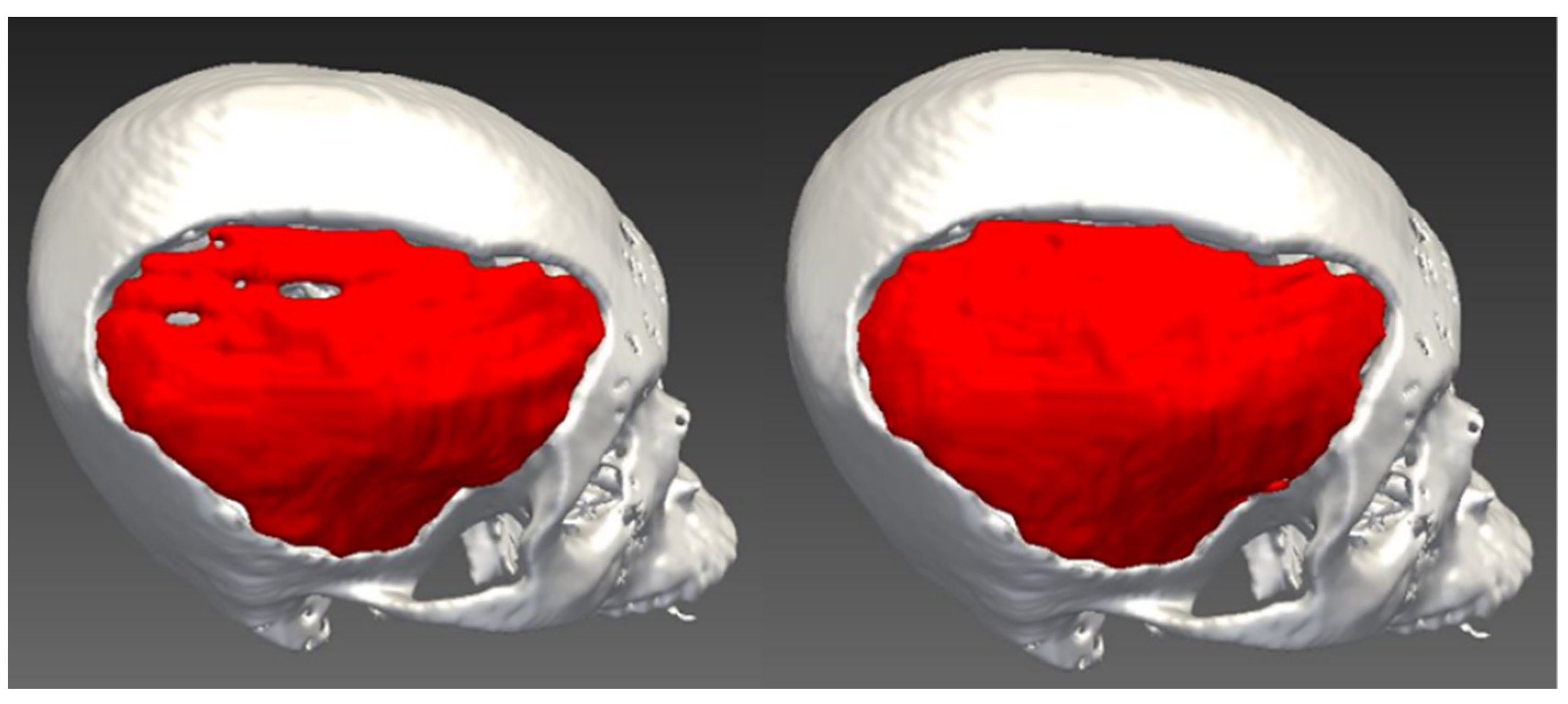
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmi, M.; Tuomi, J.; Bjorkstrand, R.; Kontio, R.; Paloheimo, M.; Makitie, A.A.; Mesimaki, K.; Salo, J.; Paloheimo, K.S. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Drstvensek, I.; Hren, N.I.; Strojnik, T.; Brajlih, T.; Valentan, B.; Pogacar, V.; Hartner, T.Z. Applications of Rapid Prototyping in CranioMaxilofacial Surgery Procedures. Int. J. Biol. Biomed. Eng. 2008, 2, 29–38. [Google Scholar]
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192. [Google Scholar] [CrossRef]
- Marreiros, F.M.; Heuze, Y.; Verius, M.; Unterhofer, C.; Freysinger, W.; Recheis, W. Custom implant design for large cranial defects. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Chang, C.J.; Su, W.C.; Chang, L.W.; Chu, I.H.; Lin, M.S. 3-D titanium mesh reconstruction of defective skull after frontal craniectomy in traumatic brain injury. Injury 2015, 46, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Patel, P.K.; Cohen, M. Application of Virtual Surgical Planning with Computer Assisted Design and Manufacturing Technology to Cranio-Maxillofacial Surgery. Arch. Plast. Surg. 2012, 39, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J. Recent advances in the reconstruction of cranio-maxillofacial defects using computer-aided design/computer-aided manufacturing. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 2. [Google Scholar] [CrossRef] [Green Version]
- Jardini, A.L.; Larosa, M.A.; Filho, R.M.; Zavaglia, C.A.; Bernades, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef]
- van der Meer, W.J.; Bos, R.R.M.; Vissink, A.; Visser, A. Digital planning of cranial implants. Br. J. Oral Maxillofac. Surg. 2013, 51, 450–452. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Khechoyan, D.Y.; Phillips, J.H.; Forrest, C.R. Custom CAD/CAM implants for complex craniofacial reconstruction in children: Our experience based on 136 cases. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1609–1617. [Google Scholar] [CrossRef]
- Moiduddin, K.; Darwish, S.; Al-Ahmari, A.; ElWatidy, S.; Mohammad, A.; Ameen, W. Structural and mechanical characterization of custom design cranial implant created using additive manufacturing. Electron. J. Biotechnol. 2017, 29, 22–31. [Google Scholar] [CrossRef]
- Lo, L.J.; Chen, Y.R.; Tseng, C.S.; Lee, M.Y. Computer-aided reconstruction of traumatic fronto-orbital osseous defects: Aesthetic considerations. Chang. Gung Med. J. 2004, 27, 283–291. [Google Scholar] [PubMed]
- Senck, S.; Coquerelle, M.; Weber, G.W.; Benazzi, S. Virtual reconstruction of a very large skull defects featuring partly and completely missing midsagittal planes. Anat. Rec. 2013, 296, 745–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolden, M.; Zelzer, S.; Seitel, A.; Wald, D.; Muller, M.; Franz, A.M.; Maleike, D.; Fangerau, M.; Baumhauer, M.; Maier-Hein, L.; et al. The Medical Imaging Interaction Toolkit: Challenges and advances: 10 years of open-source development. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Monroe, J.I.; Lo, S.; Yao, M.; Harari, P.M.; Machtay, M.; Sohn, J.W. Quantitative evaluation of image segmentation incorporating medical consideration functions. Med. Phys. 2015, 42, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.A.; Liang, T.; Hittle, B.; Stredney, D.; Kerwin, T.; Wiet, G.J. Atlas-Based Segmentation of Temporal Bone Anatomy. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- CloudCompare. (version 2.10.1) [GPL Software]. Available online: https://www.danielgm.net/cc/ (accessed on 2 December 2018).
- Hvid, C.A.; Elstrom, U.V.; Jensen, K.; Alber, M.; Grau, C. Accuracy of software-assisted contour propagation from planning CT to cone beam CT in head and neck radiotherapy. Acta Oncol. 2016, 55, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polan, D.F.; Brady, S.L.; Kaufman, R.A. Tissue segmentation of Computed Tomography images using a Random Forest algorithm: A feasibility study. Phys. Med. Biol. 2016, 61, 6553–6569. [Google Scholar] [CrossRef] [PubMed]




| Variable | n | * Cranial Implant Surface (mm2) | ** Cranial Implant Volume (mm3) |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| 3-matic | 10 | 26211.8 (2944.9) | 68823.9 (21778.4) |
| MITK: 10th slice | 10 | 22616.0 (6614.0) | 57242.3 (27463.4) |
| MITH: 5th slice | 10 | 24102.9 (8351.3) | 68655.1 (23758.5) |
| Cranial Implant | 3-Matic vs. MITK Tenth Slice | 3-Matic vs. MITK Fifth Slice |
|---|---|---|
| 1 | 0.456 | 0.355 |
| 2 | 0.423 | 0.271 |
| 3 | 0.148 | 0.097 |
| 4 | 0.480 | 0.342 |
| 5 | 0.106 | 0.002 |
| 6 | 0.333 | 0.145 |
| 7 | 0.425 | 0.260 |
| 8 | 0.116 | 0.016 |
| 9 | 0.020 | 0.024 |
| 10 | 0.301 | 0.017 |
| Mean (SD) | 0.281 (0.17) | 0.152 (0.14) |
| Cranial Implant | 3-Matic vs. MITK Tenth Slice | 3-Matic vs. MITK Fifth Slice |
|---|---|---|
| 1 | 0.856 | 0.888 |
| 2 | 0.776 | 0.891 |
| 3 | 0.879 | 0.894 |
| 4 | 0.788 | 0.825 |
| 5 | 0.898 | 0.922 |
| 6 | 0.884 | 0.907 |
| 7 | 0.882 | 0.912 |
| 8 | 0.794 | 0.871 |
| 9 | 0.916 | 0.931 |
| 10 | 0.837 | 0.925 |
| Mean (SD) | 0.851 (0.05) | 0.897 (0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, J.Y.; Abdullah, A.M.; Hueh, L.P.; Husein, A.; Hadi, H.; Rajion, Z.A. Cranial Implant Design Applying Shape-Based Interpolation Method via Open-Source Software. Appl. Sci. 2021, 11, 7604. https://doi.org/10.3390/app11167604
Abdullah JY, Abdullah AM, Hueh LP, Husein A, Hadi H, Rajion ZA. Cranial Implant Design Applying Shape-Based Interpolation Method via Open-Source Software. Applied Sciences. 2021; 11(16):7604. https://doi.org/10.3390/app11167604
Chicago/Turabian StyleAbdullah, Johari Yap, Abdul Manaf Abdullah, Low Peh Hueh, Adam Husein, Helmi Hadi, and Zainul Ahmad Rajion. 2021. "Cranial Implant Design Applying Shape-Based Interpolation Method via Open-Source Software" Applied Sciences 11, no. 16: 7604. https://doi.org/10.3390/app11167604






